Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3320
Revised: January 14, 2014
Accepted: January 19, 2014
Published online: March 28, 2014
Processing time: 134 Days and 5.3 Hours
AIM: To evaluate the protective effects on kidney tissue of frequently used intravenous anesthetics (ketamine, propofol, thiopental, and fentanyl) in rats with obstructive jaundice.
METHODS: There is an increased incidence of postoperative acute renal failure in patients with obstructive jaundice. Thirty-two Wistar-albino rats were randomly divided into four equal groups. Laparatomy was performed on each animal in the four groups and common bile ducts were ligated and severed on day 0. After 7 d, laparotomy was again performed using ketamine, propofol, thiopental, or fentanyl anesthesia whose antioxidative properties are well known in oxidative stress in a rat liver model of obstructive jaundice. After 2 h, the rats were sacrificed. Renal tissue specimens were analyzed for catalase, superoxide dismutase and malondialdehyde enzymes activities. All values are expressed as the mean ± SD. P values less than 0.05 were considered statistically significant.
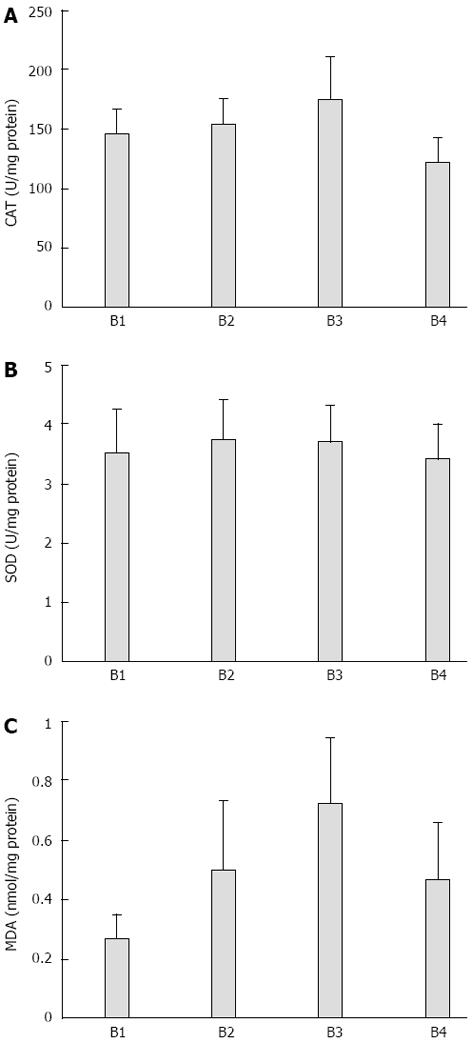
RESULTS: All animals survived without complications until the end of the study. Enlargement in the bile duct and obstructive jaundice were observed in all rats. Catalase was found to be significantly lower in the fentanyl group than in the ketamine (P = 0.039), propofol (P = 0.012), and thiopental (P = 0.001) groups. Superoxide dismutase activities were similar in all groups (P > 0.05). Malondialdehyde was found to be significantly lower in the ketamine group than in the propofol (P = 0.028), thiopental (P = 0.002) and fentanyl (P = 0.005) groups. Malondialdehyde was also lower in the fentanyl group than in the thiopental group (P = 0.001). The results showed that obstructive jaundice sensitizes renal tissue to damage under the different anesthetics.
CONCLUSION: Among the agents tested, ketamine and propofol generated the least amount of oxidative stres on renal tissues in this rat model of obstructive jaundice created by common bile duct ligation. The importance of free radical injury in renal tissue in obstructive jaundice under different intravenous anesthetics during hepatobiliary and liver transplant surgery should be considered for prevention of postoperative acute renal failure.
Core tip: There is an increased incidence of postoperative acute renal failure in patients with obstructive jaundice. Recent studies suggested that the free oxygen radicals produced in obstructive jaundice may play a major role in the etiopathogenesis of acute renal failure. We evaluated the protective effects on kidney tissue of frequently used intravenous anesthetics, whose antioxidative properties are well known, in a rat model of obstructive jaundice. Among the agents tested, ketamine and propofol generated the least amount of oxidative stres on renal tissues in this rat model of obstructive jaundice created by common bile duct ligation.
- Citation: Hatipoglu S, Yildiz H, Bulbuloglu E, Coskuner I, Kurutas EB, Hatipoglu F, Ciralik H, Berhuni MS. Protective effects of intravenous anesthetics on kidney tissue in obstructive jaundice. World J Gastroenterol 2014; 20(12): 3320-3326
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3320.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3320
Today, palliative and curative operations are performed on many patients with obstructive jaundice (OJ) under anesthesia. As a result of improvements in liver transplant surgery in the last 50 years, more complicated and prolonged operations are being conducted. Patients with severe OJ usually have a number of metabolic disorders and one or multiple organ function failure. Renal dysfunction is one of the serious complications in patients with OJ[1-3].
An association between OJ and acute renal failure (ARF) has been recognized for well over a century. The renal damage is due to biliary disorders either present on admission to hospital or which develop postoperatively. One third of patients with OJ have deterioration of renal function before surgical intervention[4]. On the other hand, surgery on patients with OJ is known to be associated with increased risk of postoperative renal failure[5-8]. Early diagnosis and prevention of spontaneous evolution of the disease can improve prognosis.
Patients with intra- or extra-hepatic bile duct obstruction are susceptible to ARF especially after major surgery[9]. Surgical treatment for the relief of OJ is still complicated by postoperative ARF in almost 10%of patients[3]. Patients with OJ are often subjected to either general or sedation anesthesia, usually using drugs which are metabolized by the liver and/or are eliminated by the kidney and the liver. Some intravenous anesthetic agents have been shown to increase production of reactive oxygen species and cause tissue damage[9-13]. In contrast, some intravenous anesthetic drugs are capable of reducing oxidative stress[13,14].
To date, there have been no reports of the effects of intravenous anesthetic agents on oxidative stress in renal tissues in rats with OJ. Biliary obstruction is associated with an intense state of oxidative stress. Antioxidant defenses [as demonstrated by superoxide dismutase (SOD) and catalase (CAT) activities] are decreased and lipid peroxidation [as demonstrated by malondialdehyde (MDA) levels] are increased in rat models with OJ[13,15]. In this study, we therefore investigated the effects on renal tissues of frequently used intravenous anesthetics (ketamine, propofol, thiopental, and fentanyl), in a rat model of oxidative stress caused by OJ through common bile duct ligation. We used these intravenous anesthetics as their antioxidative properties are well known.
The experimental protocol was approved by the Animal Ethics Review Committee of the Faculty of Medicine, University of Kahramanmaras and adhered to the National Institutes of Health Guidelines for the Use of Experimental Animals. Thirty-two male Wistar rats (300-375 g) were subjected to controlled conditions of temperature (about 22 °C) and illumination (12 h light:12 h dark cycle), and were provided with food and water ad libitum. They were fed a commercial diet. Rats were placed in individual metabolic cages and acclimatized for 1 wk before the study commenced.
In this prospective experimental study, rats were divided randomly into four groups, each group containing eight animals. Food was withdrawn for 12 h before the operation, with water available ad libitum during this period. Each rat was weighed during each anesthetic and anesthetized with ketamine (50 mg/kg) intramuscularly. As described by Lee et al[16], experimental jaundice was created by ligation of the common bile duct[13,15]. The abdominal cavity was opened with a midline incision after disinfection of the skin. The common bile duct was located and OJ induced by double ligation with 5/0 silk and transsection of the common bile duct in the supraduodenal part between the lowermost tributary of the bile duct and the uppermost tributary of the pancreatic duct. The abdominal wall was then closed with 3-0 silk in two layers. Cages were examined daily.
After 7 d, Group I rats received intramuscular single-dose ketamine (50 mg/kg), Group II received intramuscular single-dose propofol (10 mg/kg), Group III received intramuscular single-dose thiopental (20 mg/kg), and Group IV received intramuscular single-dose fentanyl (50 mcg/kg). Two hours later, the rats were sacrificed.
The animals were anesthetized and a second laparotomy was performed through a similar incision. The left and right kidneys of each rat were carefully removed in all groups and stored in iced 0.9% NaCl solution for a short time. A 0.5 cm × 0.5 cm sample of kidneys (left or right) which contain both renal cortical and medullar tissue were washed with physiological saline to remove hematoma and blotted on filter paper. The renal tissue was immediately frozen in liquid nitrogen and stored at -80 °C for later measurement of MDA, SOD and CAT activities.
In order to determine tissue antioxidant levels, the renal tissue samples were removed from the freezer, brought to room temperature, then homogenized with three volumes of ice-cold 1.15% KCl. Activities of antioxidant enzymes and levels of lipid peroxidation were measured in the supernatant after centrifugation at 14000 rpm. SOD activity was measured by the method described by Fridovich[17]. CAT activity was determined by measuring the decrease in hydrogen peroxide concentration at 230 nm by the method of Beutler[18]. Lipid peroxidation was reflected by MDA levels, which were measured by the method described by Ohkawa et al[19]. All enzyme activities are expressed as units per milligram protein (U/mg protein).
All values are expressed as mean ± SD. The Kolmogorov-Smirnov statistic was used to test the normality of the distribution. Differences between SOD groups were evaluated by Kruskal-Wallis variance analysis followed by a post hoc (Bonferroni correction) Mann-Whitney U test. Differences between MDA and CAT groups were evaluated by one way analysis of variance (ANOVA) for continuous variables with post hoc procedures (Bonferroni correction). P-values < 0.05 were considered statistically significant. Data were analyzed using SPSS 9.05 for Windows® statistical package (Chicago, IL, United States).
All animals survived without complications until the end of the study. Enlargement of the bile duct and OJ were observed in all rats. The mean values of the parameters studied are presented in Table 1. The results showed that the presence of OJ sensitized the renal tissue to damage under the different anesthetics.
CAT was found to be significantly lower in the fentanyl group than in the ketamine (P = 0.039), propofol (P = 0.012), and thiopental (P = 0.001) groups. Although CAT was higher in the thiopental group than in the ketamine and propofol groups, this difference was not statistically significant (Table 1, Figure 1A).
SOD activity was similar between all groups and intergroup differences were not found (P > 0.05) (Table 1, Figure 1B).
MDA was found to be significantly lower in the ketamine group than in the propofol (P = 0.028), thiopental (P = 0.002) and fentanyl (P = 0.005) groups. MDA was also lower in the fentanyl group than in the thiopental group (P = 0.001). MDA was similar between the propofol and thiopental groups and no other significant intergroup difference was found (Table 1, Figure 1C).
Many clinical observations and experimental studies point to the frequent occurrence of different organ complications in patients with OJ. One of the main consequences of biliary obstruction is its effect on renal function, which markedly increases patient morbidity and mortality. Acute renal failure is a life-threatening complication of OJ which continues to be a significant challenge, involving 6%-18% of patients, and is associated with high mortality (20%-100%)[20-23]. Patients with intra- or extra-hepatic bile duct obstruction are susceptible to ARF especially when undergoing major surgery, and postoperative ARF in patients with OJ remains a clinically significant complication[9,24]. ARF occurs in approximately 9% of patients requires surgery for relief of OJ, and contributes to eventual mortality in 76% of those who develop it. Postoperative mortality has been directly attributed to ARF in approximately 5%-16% of patients after surgery for OJ[25,26].
When mechanical biliary obstruction is diagnosed, surgical, endoscopic or radiologic intervention is usually recommended. On the other hand, despite advances in preoperative evaluation and postoperative care, surgical intervention for relief of obstructive jaundice still carries high morbidity and mortality rates, mainly due to sepsis and renal dysfunction[25,27-30]. The presence of OJ (total bilirubin < 8 mg/dL) is an independent risk factor for the development of postoperative renal dysfunction[31].
Although the association between OJ and ARF has been recognized since 1910 when Clairmont and Von Haberer[22] first postulated that jaundice might predispose to postoperative renal failure, surprisingly few reports or series have appeared in the literature[20,32]. Antifibrinolytic agents, OJ, prostaglandin inhibitors, cyclosporine A, radiocontrast dyes and volatile anesthetic agents contribute to ARF[31,33,34]. There is a lack of knowledge about the oxidative effects of intravenous anesthetic agents in renal tissue during surgery for OJ patients.
Postoperative ARF may be precipitated in patients with OJ either by surgery or septicemia or a combination of both. Effective plasma volume depletion, systemic endotoxemia, and myocardial dysfunction contribute to hemodynamic and renal disturbance in patients with acute OJ[32]. Intrarenal vasoconstriction, attributable to a decrease in effective arterial blood volume and induced by peripheral arterial vasodilation, is proposed to play a causative role in OJ[35]. Decreased creatinine clearance and urine osmolality are the parameters which point to the probability of renal dysfunction occurrence in OJ[4]. Although the etiology of renal dysfunction is multifactorial, it is strongly associated with hemodynamic and body fluid disturbances. However, the etiology of this clinical status is still unclear.
Oxidative stress occurs during many pathological processes in an organism. Free oxygen radicals are neutralized by the antioxidant system and a balance is maintained. A major protective mechanism against reactive oxygen metabolites is also the antioxidant enzyme cascade[36-38]. Antioxidant defenses (as demonstrated by CAT and SOD activities) are decreased and lipid peroxidation (as demonstrated by MDA levels) are increased during extrahepatic OJ in rat models[13,15]. When this balance is impaired, however, tissue damage may result. Oxidant injury is considered to be an important mechanism in the pathophysiology of ARF[36-40] and severe oxidative stress has been implicated in the renal dysfunction associated with experimental OJ[41]. Ischemia and nephrotoxicity are important factors in the pathogenesis of ARF and their effects on renal cells can be loss of SOD and superoxide radical accumulation[36]. Oxygen free radicals produce damage to the renal arteriolar endothelial cells, glomerular cells, and renal tubular epithelial cells[36-38].
Renal tissues in OJ appear to be susceptible to ischemia-reperfusion injury[42]. Tissue injury induced by OJ involves lipid peroxidation[43]. Experimental animals with OJ have been shown to be deficient in fat-soluble vitamins, such as vitamins A and E[13,43]. Because these vitamins have potential to ameliorate secondary tissue damage induced by lipid peroxidation, enhanced oxidative stress could exacerbate secondary tissue damage. Moreover, OJ could alter the activities of antioxidant enzymes resulting in increased production of superoxide and hydrogen peroxide[44]. Tissue damage associated with OJ may be caused by accelerated generation of hydroxyl radicals[43-46]. Oxidative stress seems to be a cardinal feature of cholestasis, implicated in the pathophysiology of organ injury not only in the liver, but also in renal tissues[47]. Superoxide radicals may play an important role in the pathophysiology of cholestatic liver injury, intestinal barrier failure and ARF[47].
Commonly used intravenous agents have been shown to increase oxygen production and generate tissue damage[9-13,48]. Intravenous anesthetic agents generate free radicals by altering intracellular cytochrome p450, peroxisomes, and enzymatic systems in the mitochondria[9]. Moreover, they consume and inhibit enzymatic and non-enzymatic systems that protect the cells via scavenging free radicals. They cause lipid peroxidation, DNA damage and changes in proteins by inducing oxidative damage, which eventually may lead to alterations in cellular functions such as reduced gap junction-mediated transmission, activation of transcription factors, intracellular calcium and pH changes, and/or cell death[9-13,49].
Transient functional impairment of renal cation and water transport in the proximal convoluted tubule occurred 3 to 4 d following bile duct ligation in rats[6]. Maximum plasma concentrations and renal clearance of bile acids occurred between the 3rd or 4th postoperative day following common bile duct ligation. This peak coincided with maximal disruption of the proximal convoluted tubule architecture and postoperative changes in renal function such as increased urine flow rate and decreased urine osmolality and sodium excretion[6]. Because of these results, we chose to sacrifice our rats on the 7th postoperative day and specimens of renal tissues were resected.
Ketamine has been extensively studied as a safe and reliable dissociative sedative/anesthetic agent in various clinical situations. Ketamine’s properties as a protective agent against oxidative stress and ischemia/reperfusion injury of the brain, kidney, skeletal muscle, heart, and intestine have been reported[50-54]. In our experiment, MDA levels were lower in the ketamine group compared with the other groups, confirming ketamine as an agent which protects against oxidative stress. MDA is one of the fairly reactive metabolic products resulting from the effect of free oxygen radicals on tissues and from a series of reactions during lipid peroxidation. The tissue MDA level is a sensitive indicator of lipid peroxidation and thus of oxidative stress[55]. Since ketamine lowered MDA levels more than the other agents used, we can conclude that it has an influence over the antioxidant defense system, while reducing lipid peroxidation.
Propofol and thiopental are another type of highly lipid-soluble anesthetic which have demonstrated antioxidant properties by inhibiting lipid peroxidation[56-58]. Both are often used to reduce cerebral edema during liver transplantation in fulminant hepatic failure patients[13]. Propofol is widely used for the induction and maintenance of general anesthesia, as well as for sedation of intubated postoperative patients on mechanical ventilation. Propofol has been proven to ameliorate ischemic/reperfusion injury in several organs, including the heart[59], lungs[60], brain[61], and kidney[62]. Propofol has been found to limit oxidative injury in the liver and other tissues[63]. According to our literature searches, the oxidative effects of propofol and thiopental on renal tissue injury due to OJ have not been studied before now. Regarding the markers of oxidative stress, MDA was highest in the thiopental group, and was significantly higher than in the ketamine or fentanyl groups. Although CAT was higher in the thiopental group than in the ketamine and propofol groups, this difference was not statistically significant. SOD catalyzes the produced superoxide radicals into H2O2, whereas CAT prevents oxidative damage by dissociating H2O2 and inhibiting lipid peroxidation[64]. In our experiment, SOD activity was similar amongst all groups and no significant intergroup difference was found.
Fentanyl is one of many opioid receptor agonists and has effects on the brain, heart, and liver[65]. Regarding its oxidative effects on renal tissue in OJ however, little is known. In our experiment, CAT was found to be significantly lower in the fentanyl group than in the ketamine, propofol, and thiopental groups.
The association between ARF and OJ is well established. However, despite the substantial number of clinical reviews, animal studies, and various pathogenic mechanisms and therapeutic strategies proposed, the main pathophysiological mechanisms are still obscure. Therefore, postoperative ARF remains a major challenge in hepatobiliary and liver transplant surgery. It is important to recognize ARF early and take adequate measures to prevent its occurrence. As free oxygen radicals appear to play a significant role in ARF etiopathogenesis, one option is preoperative and postoperative antioxidant treatment to prevent ARF in OJ. According to our experiment, ketamine and propofol generated the least amount of oxidative stress in renal tissues in this rat model of OJ created by common bile duct ligation. In addition, close collaboration of clinicians, especially hepatobiliary and liver transplant surgeons and anesthesiologists, is very important during the preoperative, perioperative, and postoperative process to prevent ARF.
The association between acute renal failure and obstructive jaundice is well established and there is an increased incidence of postoperative acute renal failure in patients with obstructive jaundice. Recent studies suggest that the free oxygen radicals produced in obstructive jaundice may play a major role in the etiopathogenesis of acute renal failure. The authors evaluated the protective effects on kidney tissue of frequently used intravenous anesthetics whose antioxidative properties are well known in oxidative stress in a rat liver model of obstructive jaundice.
The importance of free radical injury on renal tissue in obstructive jaundice under difference intravenous anesthetics should be considered during hepatobiliary surgery for prevention of postoperative acute renal failure.
To date, no one has reported the effects on renal tissues of intravenous anesthetic agents on oxidative stress in a rat model of obstructive jaundice. Biliary obstruction is associated with an intense state of oxidative stress. Antioxidant defenses (as demonstrated by superoxide dismutase and catalase activities) are decreased and lipid peroxidation (as demonstrated by malondialdehyde levels) are increased in rat models with obstructive jaundice. Ketamine and propofol generated the least amount of oxidative stress in renal tissues in this rat model of obstructive jaundice created by common bile duct ligation.
The paper describes how different anesthetics could potentially reduce the risk of acute renal failure in patients with obstructive jaundice by reducing the oxidative stress inflicted by jaundice in combination with acute surgery. So that, it is of interest and should be of interest to the readers.
P- Reviewers: Boyuk A, Coelho AMM, Sandblom G S- Editor: Gou SX L- Editor: Cant MR E- Editor: Zhang DN
| 1. | Lazzara S, Pergolizzi FP, Melita G, Cavaleri A, Tigano D, Riso F. [Alpha-glucosidase and alanine-amino-peptidase in the early diagnosis of renal failure in obstructive jaundice]. Chir Ital. 1997;49:51-52. [PubMed] |
| 2. | Sural S, Sharma RK, Gupta A, Sharma AP, Gulati S. Acute renal failure associated with liver disease in India: etiology and outcome. Ren Fail. 2000;22:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Cahill CJ, Pain JA, Bailey ME. Bile salts, endotoxin and renal function in obstructive jaundice. Surg Gynecol Obstet. 1987;165:519-522. [PubMed] |
| 4. | Raicević Sibinović S, Nagorni A, Brzacki V, Radisavljević M. [Prediction of renal dysfunction in patients with obstructive icterus]. Med Pregl. 2011;64:503-506. [PubMed] |
| 5. | Govil D, Anand AC, Mishra MC, Kapur BM, Tandon RK. Renal functions in obstructive jaundice: a pre and post operative assessment. J Assoc Physicians India. 1993;41:151-153. [PubMed] |
| 6. | Kaler B, Karram T, Morgan WA, Bach PH, Yousef IM, Bomzon A. Are bile acids involved in the renal dysfunction of obstructive jaundice? An experimental study in bile duct ligated rats. Ren Fail. 2004;26:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Bouillot JL, Ledorner G, Alexandre JH. [Risk factors in surgery of obstructive jaundice. Retrospective studies apropos of 176 patients]. Gastroenterol Clin Biol. 1985;9:238-243. [PubMed] |
| 8. | Acalovschi I, Chirileanu T. Acute renal failure in obstructive diseases of the extrahepatic biliary ducts. Med Interne. 1984;22:203-208. [PubMed] |
| 9. | Kramer HJ. Impaired renal function in obstructive jaundice: roles of the thromboxane and endothelin systems. Nephron. 1997;77:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Fassoulaki A, Andreopoulou K, Williams G, Pateras C. The effect of single and repeated doses of thiopentone and fentanyl on liver function in the rat. Anaesth Intensive Care. 1986;14:145-147. [PubMed] |
| 11. | Okutomi T, Nomoto K, Nakamura K, Goto F. Autogenous production of hydroxyl radicals from thiopental. Acta Anaesthesiol Scand. 1995;39:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Abidova SS. [Effect of propofol and ketamine on lipid metabolism and lipid peroxidation in rats]. Eksp Klin Farmakol. 2002;65:46-48. [PubMed] |
| 13. | Yildiz H, Coskuner I, Bulbuloglu E, Silay E, Kurutas EB, Dogan Z, Kantarceken B, Oksuz H, Senoglu N, Yuzbasioglu MF. The protective effects of ketamine and propofol in obstructive jaundice: an experimental study. Bratisl Lek Listy. 2012;113:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005;101:1275-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Singh S, Shackleton G, Ah-Sing E, Chakraborty J, Bailey ME. Antioxidant defenses in the bile duct-ligated rat. Gastroenterology. 1992;103:1625-1629. [PubMed] |
| 16. | Lee E. The effect of obstructive jaundice on the migration of reticulo-endothelial cells and fibroblasts into early experimental granulomata. Br J Surg. 1972;59:875-877. [PubMed] |
| 17. | Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 771] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. 2nd ed. New York: Grune & Stratton, Inc 1975; 66-69. |
| 19. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18816] [Article Influence: 409.0] [Reference Citation Analysis (0)] |
| 20. | Fogarty BJ, Parks RW, Rowlands BJ, Diamond T. Renal dysfunction in obstructive jaundice. Br J Surg. 1995;82:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Clarke DL, Pillay Y, Anderson F, Thomson SR. The current standard of care in the periprocedural management of the patient with obstructive jaundice. Ann R Coll Surg Engl. 2006;88:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Coratelli P, Passavanti G. Pathophysiology of renal failure in obstructive jaundice. Miner Electrolyte Metab. 1990;16:61-65. [PubMed] |
| 23. | Allison MEM. The kidney and the liver. Pr6- and postoperative factors. Surgery of Liver and Biliary Tract. 1st ed. London: Churchill Livingstone 1990; 405-421. |
| 24. | Yamamoto T, Hishida A. [Renal damage in liver cirrhosis: pathophysiology and management]. Nihon Rinsho. 1994;52:159-164. [PubMed] |
| 25. | Wait RB, Kahng KU. Renal failure complicating obstructive jaundice. Am J Surg. 1989;157:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Thompson JN, Edwards WH, Winearls CG, Blenkharn JI, Benjamin IS, Blumgart LH. Renal impairment following biliary tract surgery. Br J Surg. 1987;74:843-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Nanji AA, Scudamore CH, Filipenko JD, Owen DA. Hepatorenal syndrome associated with obstructive jaundice. J Clin Gastroenterol. 1985;7:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in obstructive jaundice. World J Gastroenterol. 2007;13:6458-6464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Pain JA, Cahill CJ, Bailey ME. Perioperative complications in obstructive jaundice: therapeutic considerations. Br J Surg. 1985;72:942-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Greig JD, Krukowski ZH, Matheson NA. Surgical morbidity and mortality in one hundred and twenty-nine patients with obstructive jaundice. Br J Surg. 1988;75:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Byers J, Sladen RN. Renal function and dysfunction. Curr Opin Anaesthesiol. 2001;14:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Naranjo A, Cruz A, López P, Chicano M, Martín-Malo A, Sitges-Serra A, Muntané J, Padillo J. Renal function after dopamine and fluid administration in patients with malignant obstructive jaundice. A prospective randomized study. J Gastrointestin Liver Dis. 2011;20:161-167. [PubMed] |
| 33. | Kramer HJ, Schwarting K, Bäcker A, Meyer-Lehnert H. Renal endothelin system in obstructive jaundice: its role in impaired renal function of bile-duct ligated rats. Clin Sci (Lond). 1997;92:579-585. [PubMed] |
| 34. | Kucuk C, Sozuer E, Ikizceli I, Avsarogullari L, Keceli M, Akgun H, Muhtaroglu S. Role of oxygen free radical scavengers in acute renal failure complicating obstructive jaundice. Eur Surg Res. 2003;35:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | O’Neill PA, Wait RB, Kahng KU. Role of renal sympathetic nerve activity in renal failure associated with obstructive jaundice in the rat. Am J Surg. 1991;161:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 795] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 37. | Burke TJ, Schrier RW. Pathophysiology of Cell Ischemia. Diseases of the Kidney. 5 ed. Boston: Little Brown and Co 1993; 1257-1286. |
| 38. | Yoshioka T, Ichikawa I. Cellular defence mechanisms against ischaemic and toxic injury. Nephrol Dial Transplant. 1994;9 Suppl 4:34-36. [PubMed] |
| 39. | Schrier RW, Burke TJ. New aspects in pathogenesis of acute renal failure. Nephrol Dial Transplant. 1994;9 Suppl 4:9-14. [PubMed] |
| 40. | Yoshioka T, Fogo A, Beckman JK. Reduced activity of antioxidant enzymes underlies contrast media-induced renal injury in volume depletion. Kidney Int. 1992;41:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Cruz A, Padillo FJ, Túnez I, Muñoz C, Granados J, Pera-Madrazo C, Montilla P. Melatonin protects against renal oxidative stress after obstructive jaundice in rats. Eur J Pharmacol. 2001;425:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Tajiri K, Miyakawa H, Liu J, Kamiyama T, Marumo F, Sato C. Enhanced renal susceptibility to ischemia-reperfusion injury in the rat with obstructive jaundice. Hepatogastroenterology. 1997;44:789-795. [PubMed] |
| 43. | Tsai LY, Lee KT, Tsai SM, Lee SC, Yu HS. Changes of lipid peroxide levels in blood and liver tissue of patients with obstructive jaundice. Clin Chim Acta. 1993;215:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Tsai LY, Lee KT, Lu FJ. Biochemical events associated with ligation of the common bile duct in Wistar rats. J Formos Med Assoc. 1997;96:17-22. [PubMed] |
| 45. | Sikuler E, Buchs AE, Yaari A, Keynan A. Hemodynamic characterization of conscious and ketamine-anesthetized bile duct-ligated rats. Am J Physiol. 1991;260:G161-G166. [PubMed] |
| 46. | Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1-14. [PubMed] |
| 47. | Assimakopoulos SF, Mavrakis AG, Grintzalis K, Papapostolou I, Zervoudakis G, Konstantinou D, Chroni E, Vagianos CE, Georgiou C. Superoxide radical formation in diverse organs of rats with experimentally induced obstructive jaundice. Redox Rep. 2008;13:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Murphy PG, Bennett JR, Myers DS, Davies MJ, Jones JG. The effect of propofol anaesthesia on free radical-induced lipid peroxidation in rat liver microsomes. Eur J Anaesthesiol. 1993;10:261-266. [PubMed] |
| 49. | Bachowski S, Kolaja KL, Xu Y, Ketcham CA, Stevenson DE, Walborg EF, Klaunig JE. Role of oxidative stress in the mechanism of dieldrin’s hepatotoxicity. Ann Clin Lab Sci. 1997;27:196-209. [PubMed] |
| 50. | Reeker W, Werner C, Möllenberg O, Mielke L, Kochs E. High-dose S(+)-ketamine improves neurological outcome following incomplete cerebral ischemia in rats. Can J Anaesth. 2000;47:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Salman AE, Dal D, Salman MA, Iskit AB, Aypar U. The effect of ketamine on acute muscular ischaemia reperfusion in rats. Eur J Anaesthesiol. 2005;22:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Kato R, Foëx P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002;49:777-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Cámara CR, Guzmán FJ, Barrera EA, Cabello AJ, Garcia A, Fernández NE, Caballero E, Ancer J. Ketamine anesthesia reduces intestinal ischemia/reperfusion injury in rats. World J Gastroenterol. 2008;14:5192-5196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209-1214. [PubMed] |
| 56. | Almaas R, Saugstad OD, Pleasure D, Rootwelt T. Effect of barbiturates on hydroxyl radicals, lipid peroxidation, and hypoxic cell death in human NT2-N neurons. Anesthesiology. 2000;92:764-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Demopoulos HB, Flamm ES, Seligman ML, Jorgensen E, Ransohoff J. Antioxidant effects of barbiturates in model membranes undergoing free radical damage. Acta Neurol Scand Suppl. 1977;64:152-153. [PubMed] |
| 58. | Smith DS, Rehncrona S, Siesjö BK. Inhibitory effects of different barbiturates on lipid peroxidation in brain tissue in vitro: comparison with the effects of promethazine and chlorpromazine. Anesthesiology. 1980;53:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Lim KH, Halestrap AP, Angelini GD, Suleiman MS. Propofol is cardioprotective in a clinically relevant model of normothermic blood cardioplegic arrest and cardiopulmonary bypass. Exp Biol Med (Maywood). 2005;230:413-420. [PubMed] |
| 60. | Balyasnikova IV, Visintine DJ, Gunnerson HB, Paisansathan C, Baughman VL, Minshall RD, Danilov SM. Propofol attenuates lung endothelial injury induced by ischemia-reperfusion and oxidative stress. Anesth Analg. 2005;100:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Ergün R, Akdemir G, Sen S, Taşçi A, Ergüngör F. Neuroprotective effects of propofol following global cerebral ischemia in rats. Neurosurg Rev. 2002;25:95-98. [PubMed] |
| 62. | Wang HH, Zhou HY, Chen CC, Zhang XL, Cheng G. Propofol attenuation of renal ischemia/reperfusion injury involves heme oxygenase-1. Acta Pharmacol Sin. 2007;28:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Lin LN, Wang WT, Wu JZ, Hu ZY, Xie KJ. [Protective effect of propofol on liver during ischemia-reperfusion injury in patients undergoing liver surgery]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:42-44. [PubMed] |
| 64. | Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751-5754. [PubMed] |
| 65. | Chinev S, Bakalova R, Peneva V, Uzunova P, Galabova T, Sokolova Z, Ribarov S. Nitrous oxide with fentanyl and droperidol minimizes lipid peroxidation in the liver. Eur J Anaesthesiol. 1995;12:155-162. [PubMed] |