Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3312
Revised: November 27, 2013
Accepted: January 3, 2014
Published online: March 28, 2014
Processing time: 175 Days and 21 Hours
AIM: To study the relation between collagen 1, α-smooth muscle actin (α-SMA) and CD34 expression and the most essential portoenterostomy (PE) outcomes.
METHODS: Liver specimens were obtained at PE from 33 biliary atresia (BA) patients for immunohistochemical analysis of collagen 1, α-SMA and CD34. Liver biopsies from 35 organ donors were used as controls. Expression patterns were related to clinical data including age at PE, serum total and conjugated bilirubin concentration at the time of PE and during follow-up, incidence of esophageal varices in follow-up upper gastrointestinal endoscopies, and native liver survival as well as to detailed histopathological findings.
RESULTS: Collagen 1 (16.4% vs 4.5%, P < 0.0001), α-SMA (17.9% vs 4.6%, P < 0.0001) and CD34 (4.9% vs 3.8%, P = 0.017) were markedly overexpressed in BA patients compared with controls. Patients who underwent liver transplantation by age of two years had significantly higher expression of collagen 1 (18.6% vs 13.7%, P = 0.024), α-SMA (20.4% vs 15.4%, P = 0.009) and CD34 (5.9% vs 4.0%, P = 0.029) at PE compared with native liver survivors. CD34-positive microvessels were identified in the centrizonal region close to central vein in every BA patient. In majority of BA cases (56%) neovascularization was frequent as CD34-positive microvessels were observed in over half of the hepatic lobules. In controls, the CD34-positive microvessels were rare as they were completely absent in 40 % and were found in less than 5 % of the hepatic lobules in the rest. The difference between BA patients and controls was significant (P < 0.0001). Patients who developed esophageal varices by two years had significantly higher expression of CD34 at PE compared with patients without varices (5.6% vs 4.0%, P = 0.019). Expression of α-SMA (r = 0.758, P < 0.0001) and collagen 1 (r = 0.474, P = 0.016), and the amount of CD34-positive microvessels (r = 0.356, P = 0.047) were related to patient age at PE.
CONCLUSION: Hepatic myofibroblastic cell activation, fibrogenesis and neovascularization are enhanced in BA, progress with increasing PE age and relate to native liver survival and development of esophageal varices.
Core tip: The majority of biliary atresia (BA) patients require liver transplantation (LTx) due to progressive hepatic fibrosis and associated portal hypertension. We aimed to relate expression of collagen 1, α-smooth muscle actin (α-SMA) and CD34 to the most essential portoenterostomy (PE) outcomes. Collagen 1, α-SMA and CD34 were markedly overexpressed in BA patients compared with controls and centrizonal neovascularization was increased in BA. Patients who underwent LTx by age of two years had significantly higher expression of collagen 1, α-SMA and CD34 at PE compared with native liver survivors. Fibrogenesis and neovascularization are enhanced in BA, progress with increasing PE age and relate to native liver survival and development of esophageal varices.
- Citation: Suominen JS, Lampela H, Heikkilä P, Lohi J, Jalanko H, Pakarinen MP. Myofibroblastic cell activation and neovascularization predict native liver survival and development of esophageal varices in biliary atresia. World J Gastroenterol 2014; 20(12): 3312-3319
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3312
Biliary atresia (BA) is a rare cholestatic liver disease of infancy leading, if untreated, to cirrhosis, liver failure and death within the first two years of life[1]. Kasai portoenterostomy (PE) aims to re-establish bile flow and is considered the first line treatment of BA[2]. However, BA still remains the most common indication for paediatric liver transplantation (LTx) due to progressive liver fibrosis and associated complications such as esophageal varices following PE[3].
Although the pathogenesis of BA remains unclear, virus-induced fibroinflammatory process affecting the biliary tree is marked[4,5]. The process of hepatic fibrogenesis and development of cirrhosis is multifactorial and multitude of involving proteins has been identified[6]. In liver fibrosis extracellular matrix proteins, mainly collagens accumulate excessively. Majority of collagens are deposited by myofibroblasts that differentiate from hepatic stellate cells (HSCs) and portal fibroblasts[7,8]. In normal liver tissue HSCs are inconspicuous but upon activation these cells transform into α-smooth muscle actin (α-SMA) producing contractile myofibroblastic cells[9]. In two previous studies with a smaller number of patients, increased α-SMA expression was found to associate with histological fibrosis scores of the liver and PE outcomes in infants with BA[10,11].
CD34 is a cell surface glycoprotein expressed in vascular endothelium. The relationship between progressive fibrosis and angiogenesis is unclear but neovascularization is found in a variety of liver diseases. This vascular reorganization may represent a response to liver injury including alterations in liver microcirculation associated with portal hypertension and development of esophageal varices[12,13].
In the present study we explored the hepatic expression of collagen 1, α-SMA and CD34 at the time of PE in a controlled manner, and related the results to the most essential PE outcomes including clearance of jaundice, native liver survival and development of esophageal varices.
This study was performed at the Children’s Hospital in Helsinki, Finland. The national paediatric LTx program has been running since 1987 in our hospital and the treatment of BA was nationally centralized to our unit in 2005[14]. All available perioperative liver biopsies obtained at the time of PE were collected for immunohistological analysis and medical records were reviewed. A total of 33 BA patients born between 1990 and 2012 were enrolled. Collected clinical data included age at PE, serum total and conjugated bilirubin concentration at the time of PE and during follow-up, detection of esophageal varices in follow-up upper gastrointestinal endoscopies, and indications as well as the age at LTx. All patients underwent endoscopic surveillance for esophageal varices as described previously[15]. A multidisciplinary team (paediatric hepatologists, transplant paediatricians, paediatric gastrointestinal, and transplant surgeons, neurologists) decided on listing for liver transplantation based on the following parameters: (1) original liver disease, quality of life (cholangitis and septic episodes), growth, nutrition, neurology, kidney function, bone health; (2) portal hypertension (portal flow, spleen size, hypersplenism, varices, ascites); (3) cholestasis (bilirubin, bile acid levels, itching); (4) liver biochemistry (ALT, AST, GT, clotting factors, prealbumin, albumin, cholesterol, Galactose half-life); (5) imaging findings (liver and spleen size, parenchymal heterogeneity, biliary lakes, focal lesions); and (6) liver histology (fibrosis, cirrhosis, bile ducts). Clearance of jaundice was defined as a decrease in serum bilirubin concentration below 20 µmol/L. As controls we examined 35 liver biopsies obtained from deceased donors at organ recovery.
The ethics committee of the hospital district of Helsinki and Uusimaa approved this study a priori and the study conforms to the principles of the 1975 Declaration of Helsinki.
Altogether 33 biopsies were taken at the time of Kasai PE, including 6 core needle biopsies and 27 surgical wedge biopsies. The biopsies were fixed in formalin, embedded in paraffin, sliced, and stained with conventional stains. The representativeness of the biopsy material was considered good: > 8 (wedge) or > 10 (needle) portal areas were present in 29 (88%) biopsies. Histological liver fibrosis was assessed by Metavir (0-4) and Ishak (0-6) fibrosis scores by two experienced pediatric liver pathologists, blinded to the clinical patient data, until consensus was reached[16,17].
Immunostaining for collagen 1 was performed with COL1A2/COL1A1 monoclonal antibody, clone I-8H5 (Abnova Corporation, Taiwan), for α-SMA using Monoclonal Mouse Anti-Human Smooth Muscle Actin, clone 1A4 (Dako, Denmark) and for CD34 using Monoclonal Mouse Anti-Human CD34 Class II, clone QBEnd-10 (Dako, Denmark) and NovoLink Polymer Detection System (Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, United Kingdom).
A Leica DM RXA microscope was used to obtain images of stained sections. A total of five random portal areas were chosen from each section of wedge biopsies (× 100 magnification) and all core needle biopsies were photographed as a whole. The proportion of the antibody-positive area (area fraction) was measured by using ImageJ Image Analysis Software[18]. To evaluate neovascularization CD34-positive endothelial cell clusters forming centrizonal microvessels were counted with semi-quantitative scoring system. Each biopsy was reviewed and scored for the presence of microvessels on scale of 1-4 (Table 1). Imaging and all immunohistochemical expression analyses were performed without knowledge of the clinical patient data.
| Number of patients | BA | Controls |
| Patients | ||
| Grade 1 | 0 | 14 |
| Grade 2 | 5 | 21 |
| Grade 3 | 9 | 0 |
| Grade 4 | 18 | 0 |
| P < 0.0001 |
The data are reported as means and standard deviation or medians and range. Comparisons between groups were performed with Mann-Whitney U-test and multiple comparisons with Kruskal-Wallis test. Correlations were calculated with Spearman’s rank correlation. A P-value < 0.05 was considered significant. All of the analyses were made with Statview software (Statview 5.0.1; SAS Institute Inc., CA, United States).
Table 2 illustrates the patient characteristics at the time of PE. No type I atresias were found and in four patients with type III atresia perioperative cholangiography showed patent passage from gallbladder to bowel (type IIIa). The median (range) age at PE among all BA patients was 64 (7-141) days and median serum bilirubin at the time of PE and at three months following PE was 174 (98-470) μmol/L and 21 (2-627) μmol/L, respectively. Of the 33 patiens, 30 had been followed-up over two years. Overall, 58% (19/33) of the patients cleared their jaundice and 57% (17/30) survived with their native liver beyond two years following PE. By two years after PE, 47% (14/30) of the patients had developed endoscopically verified esophageal varices.
| BA type | n(%) | Age at PE (d) | Bilirubin (μmol/L) | Conjugated bilirubin |
| II | 3 (9.1) | 102 (78-141) | 186 (98-247) | 162 (86-207) |
| III | 26 (78.8) | 64 (7-140) | 172 (103-470) | 117 (45-224) |
| IIIa | 4 (12.1) | 51 (37-81) | 171 (103-201) | 85 (69-109) |
| P = 0.092 | P = 0.838 | P = 0.183 |
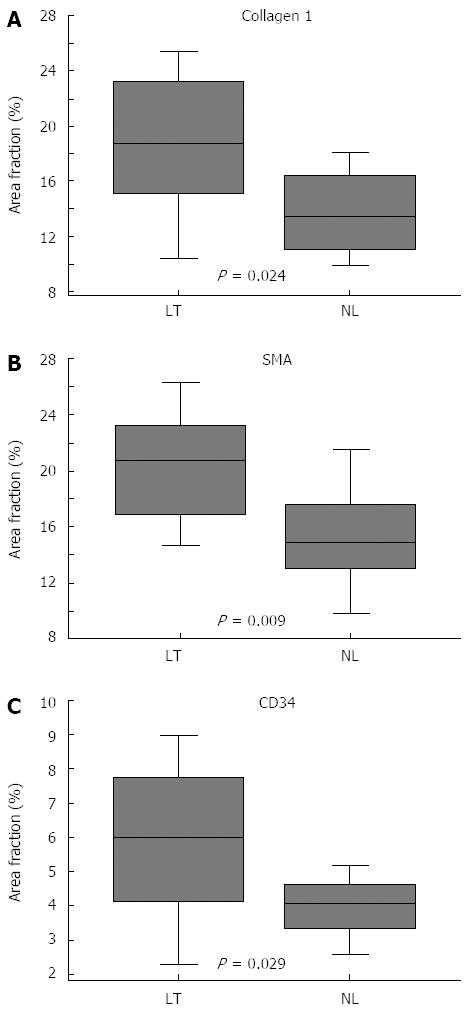
As shown in Figure 1, expression of collagen 1, α-SMA and CD34 was markedly increased in BA when compared with controls (16.4% vs 4.5%, P < 0.0001, 17.9% vs 4.6%, P < 0.0001, 4.9% vs 3.8%, P = 0.017, respectively). The increase was most striking for collagen 1 and α-SMA, stained area fractions being about four fold higher than in controls. The portal tracts stained intensively with collagen 1 and marked collagen 1 positive septae protruded towards the central vein. α-SMA staining showed strong immunoreactivity both on portal areas and along perisinusoidal spaces. The expression of collagen 1, α-SMA and CD34 did not differ significantly between different subtypes (II, III, IIIa) of BA (P = 0.360, P = 0.345 and P = 0.736, respectively for collagen 1, α-SMA and CD34).
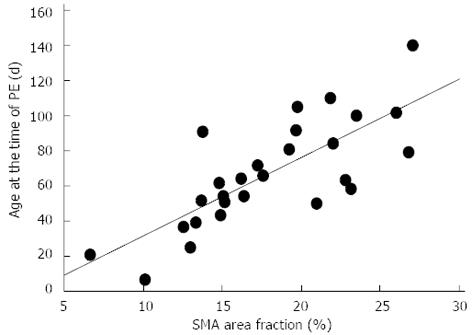
Patients who underwent LTx by age of two years showed significantly higher expression of collagen 1, α-SMA and CD34 in liver biopsies obtained at PE when compared to patients who survived with their native livers beyond two years (Figure 2 and Table 3). Children who cleared their jaundice tended to have lower expression of collagen 1 and α-SMA at PE (Table 3), although this did not reach statistical significance, (P = 0.079 and P = 0.097, respectively). The degree of α-SMA staining correlated with the level of preoperative conjugated bilirubin (r = 0.448, P = 0.023), bilirubin level at three months (r = 0.446, P = 0.029) and relative change of bilirubin level by three months (r = -0.395, P = 0.044). Patients who underwent LTx by age of two years were older at the time of PE than native liver survivors, but the difference was not quite statistically significant (79.1 d vs 65.8 d, P = 0.054). The age at the time of PE correlated positively with the expression of α-SMA (r = 0.758, P < 0.0001) (Figure 3), collagen 1 (r = 0.474, P = 0.016), and the amount of CD34-positive microvessels (r = 0.356, P = 0.047).
| Clearance of jaundice | 2-yr native liver survival | Esophageal varices | All BA patients | Controls | ||||
| Yes | No | NL | LT | Yes | No | |||
| Collagen 1 | 14.7 (4.9) | 18.5 (5.4) | 13.7 (3.5) | 18.6 (5.4) | 17.2 (4.9) | 14.6 (5.0) | 16.4 (5.4) | 4.5 (2.2) |
| Area, % (SD) | P = 0.079 | P = 0.024 | P = 0.217 | P < 0.0001 | ||||
| α-SMA | 16.4 (5.1) | 19.8 (4.8) | 15.4 (4.9) | 20.4 (4.2) | 18.2 (4.3) | 17.1 (6.1) | 17.9 (5.2) | 4.6 (1.9) |
| Area, % (SD) | P = 0.097 | P = 0.009 | P = 0.537 | P < 0.0001 | ||||
| CD34 | 4.4 (1.4) | 5.6 (2.4) | 4.0 (0.9) | 5.9 (2.4) | 5.6 (2.0) | 4.0 (1.6) | 4.9 (2.0) | 3.8 (1.0) |
| Area, % | P = 0.213 | P = 0.029 | P = 0.019 | P = 0.017 | ||||
Patients who developed esophageal varices by two years following PE had significantly higher expression of CD34 at the time of PE compared with patients who remained free of varices (Table 3). Of note, expression of collagen 1 or α-SMA were not associated with development of esophageal varices.
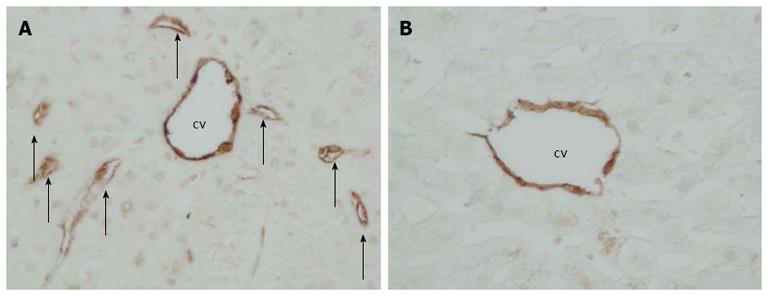
CD34-positive microvessels were identified in the centrizonal region close to central vein in every BA patient. In 18 BA cases (56%) neovascularization was frequent as CD34-positive microvessels were observed in over half of the hepatic lobules. In controls, the CD34-positive microvessels were rare as they were absent in 40 % and were found in less than 5 % of the hepatic lobules in the rest (Figure 4 and Table 1). The difference between BA patients and controls was significant (P < 0.0001). The amount of CD34-positive microvessels did not differ significantly between different subtypes (II, III, IIIa) of BA (P = 0.426).
Expression of α-SMA was closely related with the expression of collagen 1 (r = 0.731, P = 0.0002). It also correlated positively with CD34 (r = 0.502, P = 0.011) expression and histological fibrosis scores (Metavir and Ishak, r = 0.515, P = 0.010 and r = 0.511, P = 0.011, respectively). Collagen 1 expression correlated with fibrosis scores (Metavir and Ishak, r = 0.503, P = 0.012 and r = 0.513, P = 0.010, respectively) and CD34 expression (r = 0.625, P = 0.0014).
Early diagnosis and successful PE establishing sufficient bile flow are the key events for extended native liver survival in BA[1]. Even though the majority of BA patients end up with LTx due to progressive hepatic fibrosis and associated portal hypertension it is essential to increase the proportion of BA patients, who survive with their own liver into adolescence or even adulthood. LTx carries well-known risks of allograft ageing and the side effects of long-term immunosuppression[19]. Several cytokines have been identified to play an important role in the regulation of liver fibrogenesis. Transforming growth factor β1 (TGF-β1) is one of the most powerful profibrotic cytokines and the family of matrix metalloproteases is recognized as another key mediator in this process[20,21]. Despite intensive research, understanding of mechanisms that regulate disease progression following PE is scarce. Several candidate genes have been proposed to act as a prognostic markers when predicting outcomes[22,23], but further understanding of pathogenesis of BA is critical concerning development of more effective treatment strategies.
Hepatic stellate cells have a crucial role in the pathogenesis of hepatic fibrosis. After transformation into myofibroblasts in response to a liver injury, stellate cells start to produce increased amounts of collagen and express an intracellular microfilament protein α-SMA, a marker of activated HSC phenotype[24]. Increased α-SMA expression has been shown to associate with increased liver fibrosis as assessed by histological scoring and PE outcomes in infants with BA[11,25]. We found a 4-fold increase in α-SMA and collagen 1 expression in BA patients compared with controls. Overexpression of α-SMA staining was related to clearance of jaundice as it correlated with serum bilirubin concentration and relative decrease of bilirubin three months after PE. Patients who survived with their native liver for the first two years had significantly lower expression of α-SMA and collagen 1 at the time of PE than patients who underwent LTx. Those who underwent LTx were older at the time of PE and, although the age difference was not quite statistically significant, it accords well with the improved clinical outcomes of PE performed at early age[26]. Furthermore, age at PE had a strong positive correlation with the expression of α-SMA and collagen 1. Collectively, these findings suggest that myofibroblastic cell activation associated liver fibrosis progresses with advancing age after birth before PE, being a major predictor of native liver survival.
Besides activated stellate cells, portal myofibroblasts are another cell type expressing α-SMA, and similarly to the present study, increase in α-SMA expression around portal tracts has been previously shown to occur in BA[27]. Here, an intense increase in α-SMA staining was observed also in perisinusoidal spaces in addition to portal regions. Although definitive source of α-SMA expressing cells can not be ascertained by immunohistochemistry alone, it seems likely that these cells originated from portal myofibroblasts and activated stellate cells. As expected, expression pattern of accumulating collagen 1 followed that of α-SMA expressing myofibroblastic cells producing extracellular matrix proteins[7].
CD34 is a glycoprotein expressed on vascular endothelium and CD34 immunostaining can be used to elucidate neovascularization in response to a liver injury[11,12]. We assessed neoangiogenesis by CD34 staining using both quantitative and qualitative approaches. The degree of CD34 staining was significantly increased in BA patients compared with controls when measured as an immunopositive area on histological liver sections. We then analysed centrizonal areas in hepatic lobules to explore possible neovascularization in the region around the central vein, theoretically most prone to ischemic injury. Only distinct CD34-positive endothelial cell clusters with a vessel lumen were considered as microvessels to avoid misinterpretation of faint diffuse sinusoidal expression of CD34 as revascularization. Microvessels around the central vein were more frequent among BA patients compared with controls, which is a novel finding in BA. Interestingly, patients who developed endoscopically verified esophageal varices during follow-up had significantly higher expression level of CD34 already at the time of PE compared to those who did not. Regarding pathogenesis of esophageal varices, myofibroblastic differentation of portal fibroblasts and HSC activation lead to increased hepatic vascular resistance by accumulation of extracellular matrix proteins, but also dynamic changes in hepatic vasculature occuring in chronic liver injury are important as evidenced by activation of the renin-angiotensin system[28]. Experimental studies on portal hypertension and liver cirrhosis further imply that not only altered hemodynamics but also active neovascularization contributes to formation of collateral vessels and that hypoxia and angiogenesis progress along with fibrogenesis in response to chronic liver injury[29,30]. Hepatopulmonary syndrome (HPS) represents an important cause of pulmonary disease in children with BA. Corresponding animal models suggest involvement of pulmonary neovascularization and activation of angiogenic signalling pathways also in HPS[31].
This study has certain limitations such as the relatively small number of patients. It is also impossible to obtain liver biopsies from matched healthy infants. However, considering rarity of BA and current literature our series of 33 BA patients is reasonably good in size.
In conclusion, hepatic expression of α-SMA, collagen 1 and CD34 was increased in BA, suggesting myofibroblastic cell activation associated fibrogenesis and neovascularization. Low expression of α-SMA and collagen 1 at the time of PE was related to improved bile flow and native liver survival. Microvessels were common in the centrizonal region of the hepatic lobules among BA patients already at the time of PE and increased expression of CD34 predicted development of esophageal varices.
Biliary atresia is a potentially lethal disease affecting extrahepatic biliary tract. It is associated with progressive hepatic fibrosis despite successful primary repair with Kasai portoenterostomy and the pathogenesis of biliary atresia (BA) has remained largely obscure.
Various biochemical and haematological values at presentation have been shown to relate with the prognosis and the research hotspot has been to understand the factors that predict the outcome after Kasai portoenterostomy.
Low expression of α-SMA and collagen 1 at the time of portoenterostomy (PE) was related to improved bile flow and native liver survival suggesting that myofibroplastic cell activation is an early event in the cascade leading to hepatic fibrosis. Microvessels around the central vein were more frequent among BA patients compared with controls, which is a novel finding in BA.
The study results suggest that myofibroblastic cell activation associated liver fibrosis progresses with advancing age after birth before PE, being a major predictor of native liver survival.
α-SMA is a marker for activated hepatic stellate cells that have a crucial role in the pathogenesis of hepatic fibrosis. CD34 is a glycoprotein expressed on vascular endothelium and CD34 immunostaining can be used to elucidate neovascularization in response to a liver injury.
The author examined liver tissue of BA infants at the time of Kasai PE. They found that degree of myofibroblastic cell activation could be a predictive factor for transplant-free survival and that of CD34-positive neovascularization for incidence of esophageal varices. They have prepared quite large number of patients for their retrospective study regarding the incidence of BA. The results seem quite reasonable, especially the positive relationship between hepatic neovascularization and incidence of esophageal varices is novel although the relationship between liver fibrogenesis and clinical outcome of PE has already widely known as well as that between age performed PE and liver fibrogenesis.
P- Reviewers: Wong GLH, Yoshiji H S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 638] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 2. | Kasai M. Advances in treatment of biliary atresia. Jpn J Surg. 1983;13:265-276. [PubMed] |
| 3. | Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Dillon P, Belchis D, Tracy T, Cilley R, Hafer L, Krummel T. Increased expression of intercellular adhesion molecules in biliary atresia. Am J Pathol. 1994;145:263-267. [PubMed] |
| 5. | Davenport M, Gonde C, Redkar R, Koukoulis G, Tredger M, Mieli-Vergani G, Portmann B, Howard ER. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36:1017-1025. [PubMed] |
| 6. | Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9:646-651. [PubMed] |
| 7. | Perepelyuk M, Terajima M, Wang AY, Georges PC, Janmey PA, Yamauchi M, Wells RG. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605-G614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci USA. 2013;110:2324-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193-203. [PubMed] |
| 10. | Dong R, Luo Y, Zheng S. α-SMA overexpression associated with increased liver fibrosis in infants with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Shteyer E, Ramm GA, Xu C, White FV, Shepherd RW. Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr. 2006;42:93-99. [PubMed] |
| 12. | Aiad HA, Kandil MA, Samaka RM, Sultan MM, Badr MT, Nada GE. The role of CK7, Ki-67, CD34 and vimentin in the differentiation between biliary atresia and idiopathic neonatal hepatitis in Egyptian cholestatic neonates. APMIS. 2012;120:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Gill RM, Belt P, Wilson L, Bass NM, Ferrell LD. Centrizonal arteries and microvessels in nonalcoholic steatohepatitis. Am J Surg Pathol. 2011;35:1400-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Lampela H, Ritvanen A, Kosola S, Koivusalo A, Rintala R, Jalanko H, Pakarinen M. National centralization of biliary atresia care to an assigned multidisciplinary team provides high-quality outcomes. Scand J Gastroenterol. 2012;47:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Lampela H, Kosola S, Koivusalo A, Lauronen J, Jalanko H, Rintala R, Pakarinen MP. Endoscopic surveillance and primary prophylaxis sclerotherapy of esophageal varices in biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] |
| 17. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 18. | Rasband WS, Image J. U. S. National Institutes of Health, Bethesda, Maryland, United States, 1997-2012. Available from: http://imagej.nih.gov/ij/. |
| 19. | Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152-160. [PubMed] |
| 20. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [PubMed] |
| 21. | Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21 Suppl 3:S88-S91. [PubMed] |
| 22. | Zhao R, Li H, Shen C, Zheng S. RRAS: A key regulator and an important prognostic biomarker in biliary atresia. World J Gastroenterol. 2011;17:796-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Wu JF, Kao PC, Chen HL, Lai HS, Hsu HY, Chang MH, Ni YH. A high serum interleukin-12p40 level prior to Kasai surgery predict a favourable outcome in children with biliary atresia. Liver Int. 2012;32:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol. 1998;153:527-535. [PubMed] |
| 25. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [PubMed] |
| 26. | Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, Gottrand F, Broué P, Dabadie A, Gauthier F. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Farrington C, Novak D, Liu C, Haafiz AB. Immunohistochemical localization of transforming growth factor β-1 and its relationship with collagen expression in advanced liver fibrosis due to biliary atresia. Clin Exp Gastroenterol. 2010;3:185-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117-125. [PubMed] |
| 29. | Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886-894. [PubMed] |
| 30. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [PubMed] |
| 31. | Zhang J, Fallon MB. Hepatopulmonary syndrome: update on pathogenesis and clinical features. Nat Rev Gastroenterol Hepatol. 2012;9:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |