Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3265
Revised: December 27, 2013
Accepted: February 20, 2014
Published online: March 28, 2014
Processing time: 181 Days and 13.2 Hours
To improve the clinical outcomes of cancer patients, early detection and accurate monitoring of diseases are necessary. Numerous genetic and epigenetic alterations contribute to oncogenesis and cancer progression, and analyses of these changes have been increasingly utilized for diagnostic, prognostic and therapeutic purposes in malignant diseases including gastric cancer (GC). Surgical and/or biopsy specimens are generally used to understand the tumor-associated alterations; however, those approaches cannot always be performed because of their invasive characteristics and may fail to reflect current tumor dynamics and drug sensitivities, which may change during the therapeutic process. Therefore, the importance of developing a non-invasive biomarker with the ability to monitor real-time tumor dynamics should be emphasized. This concept, so called “liquid biopsy”, would provide an ideal therapeutic strategy for an individual cancer patient and would facilitate the development of “tailor-made” cancer management programs. In the blood of cancer patients, the presence and potent utilities of circulating tumor cells (CTCs) and cell-free nucleic acids (cfNAs) such as DNA, mRNA and microRNA have been recognized, and their clinical relevance is attracting considerable attention. In this review, we discuss recent developments in this research field as well as the relevance and future perspectives of CTCs and cfNAs in cancer patients, especially focusing on GC.
Core tip: The potent utilities of circulating tumor cells and cell-free nucleic acids have recently attracted attention toward their clinical application in therapeutic management of cancer patients. The concept of “liquid biopsy” can allow for repeated samplings and real-time monitoring of tumor dynamics in each individual patient and consequently would facilitate the development of “tailor-made” cancer management programs. Before translating this novel diagnostic and prognostic assay into the clinical settings, further large-scale studies with well-established methods are required to validate its clinical relevance.
- Citation: Tsujiura M, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Otsuji E. Liquid biopsy of gastric cancer patients: Circulating tumor cells and cell-free nucleic acids. World J Gastroenterol 2014; 20(12): 3265-3286
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3265.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3265
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related death in the world[1]. Although recent improvements in diagnostic techniques and peri-operative management have resulted in an increase in the early detection of GC and a decrease in its mortality in the past decades, a total of 986600 new GC cases and 738000 deaths are estimated to have occurred in 2008 worldwide[1]. Several factors seem to restrict diagnostic and therapeutic strategy for treatment of GC and, consequently, to incur the insufficient survival rate: (1) a lack of satisfactory diagnostic assays for early detection of GC; (2) an absence of valuable prognostic indicators; (3) the insufficient effectiveness of current treatments including surgery and chemotherapy for GC patients with advanced stages; and (4) poorly understood mechanisms of tumor progression and resistance to treatments, and a consequent deficiency of targeted therapy. Therefore, the importance of developing useful diagnostic and monitoring tools should be emphasized to improve the clinical outcome of patients with GC.
In the past few decades, numerous studies have demonstrated the potential utility of blood-based biomarkers such as circulating tumor cells (CTCs) and cell-free nucleic acids (cfNAs)[2-5]. These promising markers are considered to possess great potential and could facilitate therapeutic strategies for cancer including the following: early detection of diseases, predication of prognostic outcome, monitoring of tumor dynamics and development of novel targeted treatments.
Generally, tumor-linked genetic alterations are investigated using tissue samples from surgical or biopsy specimens. These procedures cannot be conducted routinely owing to their invasive nature especially in recurrent and/or metastatic cases with anatomical and/or clinical difficulties. Moreover, a result acquired from a single biopsy can provide only spatiotemporally restricted information and may fail to reflect its heterogeneity and inconsistent tumor characteristics. Detecting CTCs and cfNAs could serve as a “liquid biopsy” for cancer patients, which would be less invasive compared to surgical or endoscopic biopsy and allow us to have repeated samplings and to track the current status of tumor characteristics, such as therapeutic efficiency and resistance. From these viewpoints, the concept of “liquid biopsy” may lead to a better understanding of the genetic landscape in both primary and metastatic lesions as well as the opportunity for tracing genomic evolution.
In this article, we review the historical backgrounds, characterizations and recent developments of both CTCs and cfNAs in cancer research including GC and discuss future perspectives.
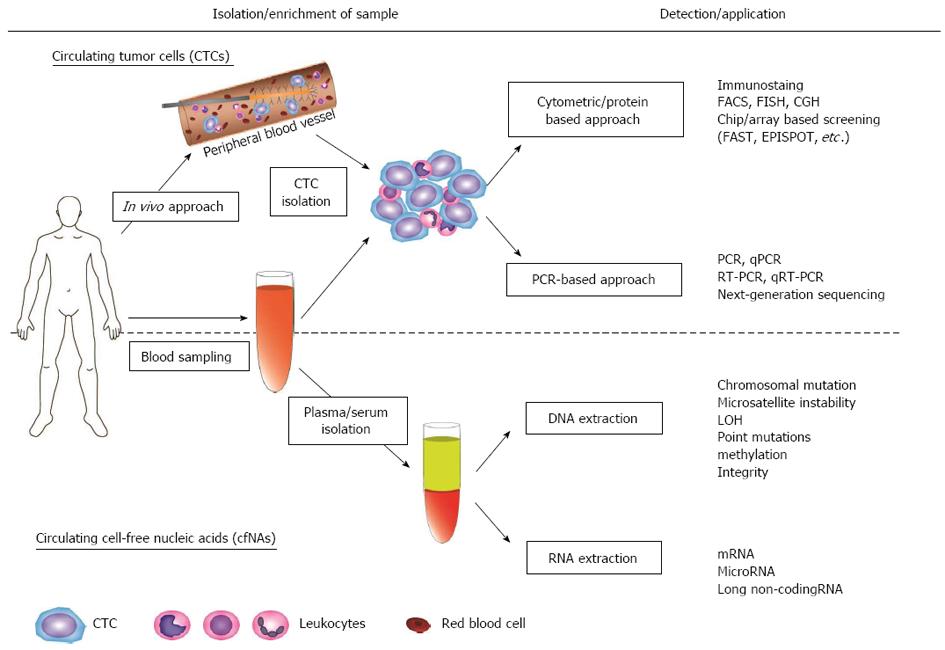
In 1869, Ashworth reported the presence of CTCs for the first time in a case of a metastatic cancer patient, in whom cells similar to those in the primary tumors were found in the blood at autopsy[6]. Since then, various studies have demonstrated the identification and characterization of CTCs in peripheral blood of patients with various malignancies, validating Ashworth’s previous remarks. Generally, CTCs are considered to appear at very low concentrations in the peripheral blood of cancer patients, usually a single tumor cell in a background of millions of blood cells[7,8]. Thus, the accurate detection of CTCs with sufficient sensitivity and specificity has been a major technical challenge for researchers (Figure 1).
The approaches of CTC isolation/enrichment can be mainly categorized into two groups: (1) physical methods; and (2) biological methods. Isolation based on physical properties does not require the immunological labeling of CTCs because it depends on the characteristics of CTCs, such as size, density, electric charge, migratory capacity and deformability. These methods include density gradient centrifugation, filtration, and dielectrophoresis. Several filtration-based approaches have been developed based on the concept that the majority of CTCs derived from epithelial cancers are generally larger in diameter than other blood cells[9,10]. However, significant variations in cell size within an individual patient as well as in different types of tumor cells have been reported[11-13]. Therefore, new approaches using multiple filters have been investigated to resolve those issues and achieve the accurate enrichment of CTCs[14,15]. While those new approaches are likely to possess great promise in isolating CTCs, further validation studies should be conducted to verify their significance.
Biological methods are another popular approach for the isolation of CTCs, which rely on immunological antibody-based capture of CTCs. In general, this assay involves positive selection with antibodies against tumor-associated antigens, such as epithelial cell adhesion (EpCAM) and cytokeratins (CKs), as well as negative selection with antibodies against the common leukocyte antigen CD45. In particular, EpCAM has been demonstrated to be over expressed and to function as an oncogene in human epithelial cancers including GC[16-18]. Among several technologies based on antibody-based isolation, the CellSearch system (Veridex) is the most widely used separation system. In this platform, immunomagnetic beads coated with anti-EpCAM antibodies capture CTCs, followed by immunostaining with both positive markers, which are CK8/18/19 for cytoplasmic epithelial markers and 4',6'-diamidino-2-phenylindole hydrochloride for nucleic acids, and a negative marker, leukocyte-specific marker CD45. Accumulating studies have demonstrated the usefulness of the CellSearch system as diagnostic and prognostic indicator in patients with metastatic disease. To date, it is the only technology that has been approved by the United States Food and Drug Administration for the detection of CTCs in the peripheral blood of patients with metastatic breast, prostate and colon cancers[19-24].
CTCs are generally thought to be quite heterogeneous in both phenotype and genotype, and only a few cells with malignant features could develop into metastatic tumors. During the journey toward the development of a metastatic lesion, some CTCs might undergo the epithelial-to-mesenchymal transition (EMT), which is characterized by decreased expression of epithelial markers and the acquisition of mesenchymal features[25]. The EMT has been proposed to be frequently related with cancer aggressiveness and might increase the ability of tumor cells to migrate. Although the identification of EMT-like cancer cells in the bloodstream and its relevance to cancer dissemination is currently under evaluation, assays targeting only epithelial cells may miss the most invasive and potentially significant subpopulation with respect to cancer progression. Therefore, alternative enrichment approaches with different epithelial antigens or negative selection methods aimed to avoid the biased selection of CTC population might be advantageous.
Tumor-specific markers, such as human epidermal growth factor 2 (HER2), prostate-specific antigen (PSA), mucin-1/2 (MUC1/2) and carcinoembryonic antigen (CEA), have also been implemented to capture CTCs more specifically and adapt to the heterogeneity of CTCs in immunological approaches. More recently, a microfluidic-based device, called the “CTC-Chip”, has been developed for CTC cell detection strategies with a significant increase in yield and purity. Using this new technology, in which whole blood is flowing through chips with automatically optimized flow kinetics, microposts coated with anti-EpCAM antibodies capture CTCs directly from small volumes of blood samples. The CTCs are then stained with secondary antibodies against either CKs or tissue-specific markers, such as PSA in prostate cancer or HER2 in breast cancer, followed by automated scanning of the microposts. Several studies have demonstrated the potent usefulness of this method due to its enhanced sensitivity and specificity, in that the higher number of isolated CTCs facilitates dynamic monitoring during a time course of cancer therapies[26-28]. Moreover, recent technological progress has allowed for the isolation and analysis of single intact CTCs[29-31]. These remarkable approaches should have major impacts and further understanding of the biology and significance of those heterogeneous populations.
Although most of these technologies have used blood samples in vitro, a new revolutionary in vivo approach allows the enrichment of CTCs directly from a peripheral vein of patients[32] (Figure 1). In this system, a structured medical Seldinger guidewire is functionalized with the attachment of EpCAM antibodies. The device is inserted into a peripheral vein, which enables the capture of a large number of CTCs from up to 1.5 L of blood over the duration of 30 min. Despite its potent utility, a large-scale study is required to verify its relevance and to eliminate the possibility of adverse effects.
After enrichment of CTCs, identification procedures are conducted to investigate their genetic and biological profiles in detail. Various methodologies for this process have been advocated and developed in the past few decades, ranging from cytometric/protein-based approaches to polymerase chain reaction (PCR)-based approaches. The former approaches involve conventional methods, such as immunostaining for specific markers, fluorescence in situ hybridization (FISH) and comparative genomic hybridization, and newly developed methods, such as fiber-optic array scanning technology with high throughput in CTC screening[33,34] and epithelial immunospot, which can detect proteins secreted from CTCs[35-37].
PCR-based detection of CTCs has evolved remarkably, especially after the introduction of the quantitative RT-PCR (qRT-PCR) technique, which can minimize possible false-positive results by using a certain “cutoff value” during the analysis process. Identification of appropriate DNA/RNA-based markers expressed by CTCs is considered critical in order to enhance the specificity and reliability of its detection. Therefore, conventional markers for CTCs, such as CKs and CEA, and other diverse markers have been investigated towards their possible clinical application in several malignancies[38]. CTC-related markers and the introduction of profile analysis including microRNAs (miRNAs) features also might be useful to resolve these issues[39-41].
To date, many researchers have tried to detect CTCs in patients with GC and demonstrated its relevance to biological and oncogenic functions using various approaches. Table 1 represents a summary of previous reports, especially focusing on methodologies, targeted molecules and detection rates. Since its introduction, RT-PCR technology has become the most widely used approach to achieve a satisfactory detection rate despite the extremely low concentration of CTCs in the bloodstream. However, a high sensitivity of RT-PCR may cause an increase in false positive detection even in healthy controls. Therefore, some researchers have utilized multiple detection markers in an mRNA-based assay and suggested its potent usefulness[42-44]. Of particular note, Wu et al[44] have developed a sensitive assay using a high-throughput colorimetric membrane array, in which multiple markers, such as human telomerase reverse transcriptase (TERT), cytokeratin 19 (CK19), CEA and MUC1, are measured simultaneously and the combination of four markers serves as a prognostic indicator for overall survival and postoperative recurrence/metastasis in GC. Recently, non-coding RNAs, such as miRNAs and Piwi-interacting RNAs (piRNAs), have been proven to alter their expression in carcinogenesis and tumor progression[45-47], so these cancer-specific alterations have been reported to be useful for the detection of CTCs in GC[48-52]. However, some of those reports, in which a mononuclear cell layer was used to isolate total RNA, may not reflect miRNAs originating only from CTCs because the possibility of contamination by leukocyte-originated RNAs cannot be excluded. The presence of miRNAs originating from peripheral blood cells has been demonstrated in the blood of both cancer patients and normal individuals, and furthermore, contamination from those miRNAs has been observed even for circulating cell-free miRNA analysis[53,54]. Those issues should be addressed before proceeding to clinical practice, and moreover, exhaustive exploration to identify more sensitive miRNA/piRNA-related markers might be desirable to achieve an accurate assay.
| Characteristic and number of patients | Control (n) | Detection method | Detection rate/statistic value | Ref. | |||
| Pre or post treatment | 9 (PB) | 4 | RT-PCR | CEA mRNA | 22.2% | (Pt.) | Funaki et al[133] |
| 0% | (Ctrl.) | ||||||
| I-IV | 20 (PB) | 22 | RT-PCR | CEA mRNA | 35% | (Pt.) | Mori et al[134] |
| 0% | (Ctrl.) | ||||||
| I-IV | 49 (PB) | 50 | RT-PCR | CK19 mRNA | 0% | (PB) | Aihara et al[135] |
| 21 (PV) | 0% | (PV) | |||||
| 0% | (Ctrl.) | ||||||
| I-IV | 30 (PB) | 58 | RT-PCR | CK20 mRNA | 16.7% | (Pt.) | Soeth et al[136] |
| 3.4% | (Ctrl.) | ||||||
| Inoperable/metastatic | 34 (PB) | 33 | RT-PCR | CK19 mRNA | 20.6% | (Pt.) | Yeh et al[137] |
| 0% | (Ctrl.) | ||||||
| I, III, IV | 35 (PB) | 9 | RT-PCR | CEA mRNA | 45.7% | (Pt.) | Noh et al[138] |
| 0% | (Ctrl.) | ||||||
| I-IV | 52 (PB) | 14 | RT-PCR | CK19 mRNA | 9.6% | (Pt.) | Majima et al[139] |
| 0% | (Ctrl.) | ||||||
| CK20 mRNA | 9.6% | (Pt.) | |||||
| 1% | (Ctrl.) | ||||||
| I-IV | 41 (PB) | RT-PCR | CEA mRNA | 22.2% | (before curative surgery) | Nishida et al[60] | |
| (36 with curative surgery) | 33.3% | (during curative surgery) | |||||
| (5 with inoperable) | 80% | (inoperable Pt.) | |||||
| I-IV | 57 (PA) | 30 | RT-PCR | CEA mRNA | PA: 17.5% | Miyazono et al[59] | |
| 49 (PV) | PV: 18.4% | ||||||
| 51 (SVC) | SVC: 21.6% | ||||||
| 8.8% | (before surgery) | At least either one positive in PA/PV/SVC | |||||
| 33.3% | (during surgery) | ||||||
| 0% | (Ctrl.) | ||||||
| EGC, III | 29 (PB) | 15 | RT-PCR | CEA mRNA | EGC: 22.2% | (after surgery) | Noh et al[140] |
| (paired, after surgery during surgery and follow-up) | IIIa: 20% | ||||||
| IIIb: 26.7% | |||||||
| Total: 24.1% | |||||||
| EGC: 22.2% | (during follow-up) | ||||||
| IIIa: 20% | |||||||
| IIIb: 34.4% | |||||||
| Total: 34.4% | |||||||
| 0% | (Ctrl.) | ||||||
| I-IV | 106 (PB) | RT-PCR | CEA mRNA | 40.6% | Sumikura et al[141] | ||
| (during surgery) | |||||||
| I-IV | 41 (PB) | 10 | RT-PCR | CEA mRNA | 24.4% | (Pt.) | Koike et al[142] |
| 0% | (Ctrl.) | ||||||
| I-IV | 46 (PB) (18 EGJ caner)(28 with GC) | 10 (with benign GI disease)100 (tumor-free Ctrl.) | qRT-PCROncoquick density gradient centrifugation | CK20 mRNA | 27.8% | (Pt. with EGJ cancer) | Friederichs et al[143] |
| 21.4% | (Pt. with GC) | ||||||
| 0% | (with benign GI disease) | ||||||
| 1% | (tumor-free Ctrl.) | ||||||
| I-IV | 59 (PB) | 15 | qRT-PCR | CEA mRNA | 0% | (before surgery) | Ikeguchi et al[58] |
| 45.8% | (after surgery) | ||||||
| 0% | (Ctrl.) | ||||||
| I-IV | 70 (PB) | RT-PCR | CK20 mRNA | 36.6% | (Pt. with curative resection) | Illert et al[144] | |
| (41 with curative resection) | 44.8% | (Pt. with residual tumor) | |||||
| (29 with residual tumor) | Total: 40% | ||||||
| I-III | 46 (PB) | 13 | RT-PCR | CEA mRNA | 52.2% | (before surgery) | Seo et al[145] |
| (with curative resection) | 19.6% | (after surgery) | |||||
| (paired, before and after surgery) | 0% | (Ctrl.) | |||||
| I-IV | 52 (PB) | 36 | RT-PCR | c-Met mRNA | 61.5% | (Pt.) | Uen et al[42] |
| 5.6% | (Ctrl.) | ||||||
| MUC1 mRNA | 71.2% | (Pt.) | |||||
| 8.3% | (Ctrl.) | ||||||
| I-IV | 42 (PB) | 30 | RT-PCR | hTERT mRNA | 61.9% | (Pt.) | Wu et al[43] |
| 0% | (Ctrl.) | ||||||
| CK19 mRNA | 69.0% | (Pt.) | |||||
| 3.3% | (Ctrl.) | ||||||
| CK20 mRNA | 61.9% | (Pt.) | |||||
| 3.3% | (Ctrl.) | ||||||
| CEA mRNA | 78.6% | (Pt.) | |||||
| 0% | (Ctrl.) | ||||||
| I-IV | 64 (PB) | 80 | MAH | hTERT mRNA | 81.3% | Wu et al[44] | |
| CK19 mRNA | 78.1% | ||||||
| CK20 mRNA | 82.8% | ||||||
| MUC1 mRNA | 84.4% | ||||||
| No detection in controls | |||||||
| I-IV | 32 (PB) | FACS/ICC | CK8/18/19 | 21.9% | (before chemotherapy) | Kolodziejczyk et al[146] | |
| (paired, before and after chemotherapy) | 15.6% | (after chemotherapy) | |||||
| I-IV | 57 (PB) | FACS/ICC | CK8/18/19 | 54.4% | (before surgery) | Pituch-Noworolska et al[61] | |
| (before surgery) | 21.2% | (after surgery) | |||||
| 52 (PB) | |||||||
| (after surgery) | 26.8% | (TDB sample) | |||||
| 56 (TDB) | |||||||
| I-IV | 52 (PB) | 20 | qRT-PCR | CEA mRNA | 5.0% | (before surgery) | Tani et al[147] |
| (40 pre-ope) | 16.7% | (after surgery) | |||||
| (12 post-ope) | |||||||
| I-IV | 41 (PB) | 41 | Cell search system | EpCAM | 14.3% | (Nonmetastatic GC) | Hiraiwa et al[55] |
| CK8/18/19 | 55.6% | (metastatic GC) | |||||
| 0% | (Ctrl.) | ||||||
| I-IV | 101 (PB) | 14 | qRT-PCR | CK19 mRNA↑ | P = 0.0127 | Curative resection (n = 69) vs Ctrl. (n = 14) | Koga et al[148] |
| (69 with curative ope) | P = 0.0087 | Non-curative resection (n = 32) vs Ctrl. (n = 14) | |||||
| (32 with non-curative ope) | CK20 mRNA↑ | P = 0.0022 | Non-curative resection (n = 32) vs Ctrl. (n = 14) | ||||
| I-IV | 810 (PB) | 29 | RT-PCR | MT1-MMP mRNA | 22.8% | Mimori et al[57] | |
| No data for Ctrl. | |||||||
| I-IV | 55 (PB) | 86 | RT-PCR ELISA | Survivin mRNA↑ | 45.5% | (Pt.) | Yie et al[149] |
| AUC = 0.772 | Pt. (n = 55) vs Ctrl. (n = 86) | ||||||
| I-IV | 70 (PB) | 20 | qRT-PCR | CEA mRNA | 45.7% | Bertazza et al[150] | |
| CK19 mRNA | 97.1% | ||||||
| VEGF mRNA | 38.6% | ||||||
| Survivin mRNA | 98.6% | ||||||
| (Control samples were used the calibrator source) | |||||||
| I-IV | 846 (PB) | 25 | qRT-PCR | uPAR mRNA↑ | 404/846 47.8% | Kita et al[151] | |
| P < 0.0001 | Pt. (n = 846) vs Ctrl. (n = 25) | ||||||
| Advanced | 52 (PB) | Cell search system | EpCAM | 32.7% | (baseline) | Matsusaka et al[56] | |
| (paired, before and during chemotherapy) | CK8/18/19 | 13.7% | (2 wk after chemotherapy) | ||||
| 18.8% | (4 wk after chemotherapy) | ||||||
| I-IV | 123 (PB) | 30 | qRT-PCR | CEA mRNA | 36.6% | (Pt.) | Qiu et al[152] |
| 30% | (Ctrl.) | ||||||
| I-IV | 30 (PB) | qRT-PCR | CK18 mRNA | I/II: 81.8% | Saad et al[153] | ||
| (after curative surgery) | III/IV: 31.6% | ||||||
| Total: 50% | |||||||
| N/A | 90 (PB) | miR-106a↑ | P = 0.006 | Pre-ope vs Ctrl. | Zhou et al[48] | ||
| (90 before surgery) | AUC = 0.684 | ||||||
| (41 preoperative) | P = 0.016 | Post-ope vs Ctrl. | |||||
| (49 postoperative) | miR-17↑ | P = 0.001 | Pre-ope vs Ctrl. | ||||
| AUC = 0.743 | |||||||
| P = 0.019 | Post-ope vs Ctrl. | ||||||
| I-IV | 95 (PB) | 21 | qRT-PCR | B7-H3 mRNA↑ | 50.5% | Arigami et al[154] | |
| P < 0.0001 | Pt. (n = 95) vs Ctrl. (n = 21) | ||||||
| AUC = 0.86 | |||||||
| I-IV | 98 (PB) | 30 | RT-PCR ELISA | Survivin mRNA | I/II: 25% | Cao et al[155] | |
| III/IV: 56.1% | |||||||
| I-IV: 45.9% | |||||||
| I-IV | 93 (PB) | 32 | qRT-PCR | piR-651↓ | P < 0.001 | Pt. (n = 93) vs Ctrl. (n = 32) | Cui et al[49] |
| (42 preo-ope) | AUC = 0.841 | ||||||
| (51 post-ope) | piR-823↓ | P < 0.001 | |||||
| AUC = 0.822 | |||||||
| II-IV | 35 (PB) | 50 | qRT-PCR | CEA mRNA | 22.9% | (Pt.) | Dardaei et al[156] |
| CK20 mRNA | 37.1% | (Pt.) | |||||
| TFF1 mRNA | 31.4% | (Pt.) | |||||
| MUC2 mRNA | 22.9% | (Pt.) | |||||
| No detection in controls | |||||||
| I-IV | 53 (PB) | 20 | qRT-PCR | miR-21↑ | P < 0.0001 | Pt. (n = 53) vs Ctrl. (n = 20) | Zheng et al[50] |
| AUC = 0.853 | |||||||
| I-IV | 52 (PB) | 15 | qRT-PCR | miR-200c↑ | P = 0.018 | Pt. (n = 52) vs Ctrl. (n = 15) | Valladares-Ayerbes et al[51] |
| AUC = 0.715 | |||||||
| I-IV | 40 (PB) | 17 | qRT-PCR | miR-421↑ | P < 0.01 | Pt. (n = 40) vs Ctrl. (n = 17) | Zhou et al[52] |
| AUC = 0.773 | |||||||
Recurrence and metastasis are the most critical factors not only for predicting clinical outcome but also for the quality of life in patients with GC. As summarized in Table 2, accumulating reports have suggested the significance of CTC detection as a prognostic indicator by various approaches, including both the CellSearch System and RT-PCR/qRT-PCR methods. Hiraiwa et al[55] examined CTCs in 130 gastrointestinal cancer patients involving 44 GC patients using the CellSearch System. Their results demonstrated that the metastatic GC patients with ≥ 2 CTCs (n = 15) had a significantly shorter overall survival rate than the metastatic GC patients with < 2 CTCs (n = 12) (P = 0.039). In a prospective study, Matsusaka et al[56] also evaluated the relevance of CTCs to chemotherapy and clinical outcome using the CellSearch System. Their results showed that GC patients with ≥ 4 CTCs at 2 and 4 wk after the initiation of chemotherapy had significantly shorter overall survival and progression-free survival in comparison with GC patients with < 4 CTCs, whereas CTC status at baseline (i.e., before the initiation of chemotherapy) had no statistical association with clinical outcomes. These findings may imply the close relationship of CTC status and treatment response.
| Characteristic and number of patients | Detection method | Statistic value | Ref. | |||||
| 17 | RT-PCR | CK19 mRNA | OS | P = 0.014 | CK19 (+) vs (-) | Yeh et al[137] | ||
| (non-responsive to chemotherapy) | ||||||||
| I-IV | 57 | RT-PCR | CEA mRNA | Liver metastasis recurrence | P = 0.03 | CEA (+) vs (-) | (a) | Miyazono et al[59] |
| I-IV | 106 | RT-PCR | CEA mRNA | Recurrence/metastasis | P = 0.02 | CEA (+) vs (-) | (a) | Sumikura et al[141] |
| I-IV | 46 | qRT-PCR | CK20 mRNA | 2-year-survival | P < 0.05 | CK20 (+) vs (-) | N/A | Friederichs et al[143] |
| I-IV | 41 | RT-PCR | CK20 mRNA | OS | P = 0.0363 | CK20 (+) vs (-) | (b) | Illert et al[144] |
| (with curative resection) | ||||||||
| I-III | 46 | RT-PCR | CEA mRNA | Recurrence | P ≤ 0.00022 | CEA after sugery (+) vs (-) | (a) | Seo et al[145] |
| Recurrence | P = 0.015 | (c) | ||||||
| I-IV | 52 | RT-PCR | C-Met mRNA | OS | P = 0.0178 | C-Met (+) vs (-) | (b) | Uen et al[42] |
| MUC1 mRNA | OS | P = 0.0352 | MUC1 (+) vs (-) | (b) | ||||
| I-IV | 42 | RT-PCR | CEA mRNA | Recurrence/metastasis | P = 0.032 | CEA (+) vs (-) | (c) | Wu et al[43] |
| I-IV | 64 | MAH | hTERT/CK19/CEA/MUC1 | Recurrence/metastasis | P = 0.009 | All marker (+) vs the others | (c) | Wu et al[44] |
| OS | P = 0.0223 | (b) | ||||||
| Metastatic | 27 | CellSearch | EpCAM | OS | P = 0.039 | CTC ≥ 2 vs < 2 | (b) | Hiraiwa et al[55] |
| System | CK8/18/19 | |||||||
| I-IV | 69 | qRT-PCR | CK19 mRNA | OS | P = 0.0347 | CK19 (+) vs (-) | (b) | Koga et al[148] |
| (with curative resection) | CK20 mRNA | OS | P = 0.049 | CK20 (+) vs (-) | (b) | |||
| I-IV | 810 | RT-PCR | MT1-MMP | Recurrence/metastasis | P = 0.0018 | MT1-MMP (+) vs (-) | (c) | Mimori et al[57] |
| I-IV | 55 | RT-PCR ELISA | Survivin mRNA | RFS | P = 0.026 | Survivin (+) vs (-) | (b) | Yie et al[149] |
| P = 0.026 | (d) | |||||||
| I-IV | 70 | qRT-PCR | Survivin mRNA | OS | P = 0.036 | Survivin high vs low | (b) | Bertazza et al[150] |
| P < 0.001 | (d) | |||||||
| Advanced | 51 (2 wk after chemotherapy) | Cell search system | EpCAM | PFS (2 wk after chemotherapy) | P < 0.001 | CTC ≥ 4 vs < 4 | (b) | Matsusaka et al[56] |
| CK8/18/19 | P < 0.001 | (d) | ||||||
| 48 (4 wk after chemotherapy) | OS (2 wk after chemotherapy) | P < 0.001 | (b) | |||||
| P < 0.001 | (d) | |||||||
| PFS (4 wk after chemotherapy) | P < 0.001 | (b) | ||||||
| P < 0.001 | (d) | |||||||
| OS (4 wk after chemotherapy) | P < 0.001 | (b) | ||||||
| P = 0.004 | (d) | |||||||
| I-IV | 123 | qRT-PCR | CEA mRNA | Recurrence | P = 0.001 | CEA (+) vs (-) | (a) | Qiu et al[152] |
| DFS | P = 0.001 | (b) | ||||||
| P = 0.02 | (d) | |||||||
| I-IV | 30 | qRT-PCR | CK18 mRNA | RFS | P < 0.001 | CK18 (+) vs (-) | (b) | Saad et al[153] |
| (after curative surgery) | P = 0.04 | (d) | ||||||
| OS | P = 0.001 | (b) | ||||||
| P = 0.06 | (d) | |||||||
| I-IV | 95 | qRT-PCR | B7-H3 mRNA | OS | P = 0.02 | B7-H3 high vs low | (b) | Arigami et al[154] |
| P = 0.046 | (d) | |||||||
| I-IV | 98 | RT-PCR ELISA | Survivin mRNA | DFS | P < 0.001 | Survivin (+) vs (-) | (b) | Cao et al[155] |
| P < 0.001 | (d) | |||||||
| I-IV | 52 | qRT-PCR | miR-200c | OS | P = 0.016 | miR-200c high vs low | (b) | Valladares-Ayerbes et al[51] |
| P = 0.028 | (d) | |||||||
| RFS | P = 0.044 | miR-200c high vs low | (b) | |||||
| P = 0.028 | (d) | |||||||
The majority of the studies using RT-PCR/qRT-PCR methods, which are also widely used for prognosis analysis, have relatively small numbers of cases. Under such circumstances, Mimori et al[57] focused on one candidate marker, membrane type 1 matrix metalloproteinase (MT1-MMP) mRNA levels, based on results from cDNA microarray analysis, and consequently validated its relevance in a subsequent qRT-PCR based study involving more than 800 GC patients. As a result, MT1-MMP mRNA levels in peripheral blood were indicated to be an independent factor for determining recurrence and distant metastasis of GC (P = 0.0018).
Intriguingly, some groups have reported time-dependent changes in the detection rate of CTCs during the peri-operative time course[58-61]. Those changes may imply the possibility of monitoring the tumor dynamics; however, the biological and clinical meaning of CTCs still remains unknown and controversial. In fact, incompatible events, including both increase and decrease in CTC detection rates during surgical maneuvers, have been proposed so far[58-61]. This discrepancy might be partially explained by a wide variety of measurement parameters, from the methodology itself to targeted markers, patient background/properties and sample conditions.
In summary, recent technological advances have provided considerable progress and interest in the detection of CTCs in various cancers, including GC. Although previous studies have shown a potent usefulness of CTC detection as a novel diagnostic and prognostic assay in cancer patients, little remains known about the biological features and fundamental roles of these cells. Detailed characterization of CTCs and well-designed experiments should resolve current underlying issues and provide the opportunity for clinical impact in cancer therapy.
The study of cfNAs has a considerably long history since it was first reported in 1948 by Mandel and Metais[62], who successfully detected nucleic acids in human plasma. Unfortunately, their work attracted little attention at that time owing to a lack of sufficient understanding of that innovative concept. Regarding malignant disease, in 1977, Leon et al[63] first reported the presence of cell-free DNA (cfDNA) in the serum of cancer patients. Furthermore, they also mentioned its potent function as a clinical indicator, showing decreased cfDNA levels in response to radiotherapy. In 1989, Vasioukhin et al[64] successfully detected cfDNA with neoplastic characteristics and proposed the first evidence suggesting that tumors can shed DNA into the circulation. This hypothesis was further strengthened by two studies in 1994, in which NRAS mutations in the plasma of patients with myelodysplastic syndrome or acute myelogenous leukemia, and KRAS mutations in the plasma or serum of patients with pancreatic cancer[65], were detected. Those findings opened up a new field in the exploration of circulating nucleic acids, and many meritorious studies have demonstrated the biological function of cfNAs and their potential as novel biomarkers regarding DNA, mRNA and miRNAs (Figure 1).
In regard to the origin of circulating nucleic acids, two main potent release mechanisms, called “passive” and “active”, are advocated to date. The passive mechanism involves the release of nucleic acids originated from apoptotic and necrotic cells into the bloodstream. Macrophages and phagocytes play an important role in phagocytosis of necrotic and apoptotic cells and can release digested nucleic acids into the microenvironment[66,67]. In contrast, it is reported that fragments of cellular nucleic acids can be actively released[68,69]. Although the active secretion into the circulation remains enigmatic, one potential explanation is that cancer cells would release nucleic acids to transform the targeted recipient cells at distant locations[70-72]. In addition to those two mechanisms, cfNAs might be released by CTCs, however, there appears to be a huge gap between the amount of cfNAs and the rarity of CTCs in the bloodstream as described in the previous section. Thus, this hypothesis has been controversial so far.
The study of circulating cfDNA in the plasma/serum involves the measurement of the total volume of circulating DNA as well as the detection of cancer-related genetic/epigenetic aberrations, which include microsatellite instability, loss of heterozygosity, genetic polymorphisms, point mutations, methylation, deletion/amplification/translocation of chromosome and integrity (i.e., the ratio of longer DNA fragment to shorter one based on the different cleavage process between apoptosis and necrosis[73]). The latter approach is generally recognized to be able to cover a wider range of oncogenic alterations in various cancers and to possess more potent application in the clinical setting than the former one, partly because cfDNA can be released into the bloodstream and is detectable in the plasma/serum in healthy humans[69,74]. In fact, numerous reports have demonstrated the detection of genetic and epigenetic alterations in circulating DNA in the plasma/serum in cancer patients[75-79]. Furthermore, in colorectal cancer, recent two reports clearly demonstrated a correlation between acquired resistance to the anti-EGFR antibody drugs, such as cetuximab and panitumumab, and the emergence of KRAS mutations, which was successfully detected and monitored in the blood of patients under treatment[80,81]. Misale et al[81] also indicated the potential of cfDNA to monitor tumor dynamics more sensitively compared to conventional assays, showing that KRAS mutant alleles were confirmed in the blood of a cetuximab-treated patient 10 mo earlier than radiographic examinations. Moreover, Leary et al[82] have recently analyzed individual tumor-specific DNA translocations in paired solid tumor and circulating cfDNA samples using next-generation sequencing technology and consequently demonstrated the feasibility of personalized biomarkers, enabling a so-called “tailor-made” therapeutic strategy. In summary, moving toward the development and future application in the clinical setting, the accumulated evidence has proven the potent usefulness of cfDNA for the detection of disease as well as for the assessment of residual disease, recurrence, and secondary resistance.
Previous reports regarding circulating cfDNA in GC patients are summarized in Table 3. Among those reports, a few studies with respect to the concentration of circulating cfDNA are found, in which a housekeeping gene, beta-actin[83], and a non-coding genomic DNA repeat sequence, ALU[84], were evaluated. In contrast, the detection of methylated DNA in plasma/serum appears to be the most widely used approach in GC, which was usually investigated by methylation specific-PCR (MSP) or quantitative methylation specific-PCR (qMSP) assays. In 2002, Lee et al[85] first reported the potent application of detecting methylated DNA of death-associated protein-kinase, E-cadherin, GSTP1, p15 and p16 in the serum of GC patients. Thereafter, technological advances and the exploration of more sensitive and specific genes have provided the accumulated evidence in this field. In detail, comprehensive analyses by methylation CpG island microarray have suggested the possibility of more significant genes for detecting methylated DNA[86,87]. Most recently, Ling et al[88] clearly demonstrated the potent usefulness of detecting methylated XAF1 DNA as a diagnostic as well as prognostic biomarker with satisfactory degrees of specificity and sensitivity. Specifically, methylated XAF1 DNA in serum was detected in 69.8% (141/202) of the GC patients and none of the healthy individuals (0/88) with an area under the receiver operating characteristic curve (AUC) of 0.909 in a receiver operating characteristic (ROC) curve analysis for discrimination of the two groups and was significantly correlated with poorer prognosis in GC (P < 0.001, disease-free survival, Kaplan-Meier survival curves, Log-rank test).
| Characteristic and number of patients | Controls (n) | Plasma/serum | Detection method | Detection rate/statistic value | Ref. | ||||
| Unresectable | 198 | 78 (peptic ulcer) | Serum | Immuno-PCR | MG7-Ag | 82.8% | (GC) | Ren et al[157] | |
| 118 (chronic gastritis) | Semi quantitative | 7.7% | (peptic ulcer) | ||||||
| PCR | |||||||||
| 236 (healthy donors) | 5.9% | (chronic gastritis) | |||||||
| 0.8% | (healthy donors) | ||||||||
| P < 0.01 | Correlation with metastasis | (a) | |||||||
| N/A | 51 | 30 (gastritis) | Serum | qPCR | EBV DNA | 100% | (Pt. with EBER (+) | Lo et al[158] | |
| in primary tumor) | |||||||||
| 197 (healthy controls) | 92.9% | (Pt. with EBER (+) | |||||||
| in filtrating lymphocytes) | |||||||||
| 0% | (Pt. with EBER (-) | ||||||||
| in primary tumor) | |||||||||
| 23.3% | (gastritis) | ||||||||
| 3.6% | (Ctrl.) | ||||||||
| I-IV | 54 | 30 | Serum | MSP | DAP-kinase | 48.1% | Lee et al[85] | ||
| E-cadherin | 57.4% | ||||||||
| GSTP1 | 14.8% | ||||||||
| p15 | 55.6% | ||||||||
| p16 | 51.9% | ||||||||
| No detection in controls | |||||||||
| I-IV | 60 | 16 | Serum | MSP | p16 | 26.1% | With p16 methylation | Kanyama et al[159] | |
| in primarily tumor | |||||||||
| 0% | Without p16 methylation | ||||||||
| in primarily tumor | |||||||||
| 0% | (Ctrl.) | ||||||||
| I-IV | 109 | 10 | Serum | MSP | p16 | 18.3% | Ichikawa et al[160] | ||
| E-cadherin | 23.9% | ||||||||
| p16 + E-cadherin | 36.7% | ||||||||
| No detection in controls | |||||||||
| I-IV | 41 | 10 | Serum | MSP | p16 | 22.0% | Koike et al[142] | ||
| E-cadherin | 22.0% | ||||||||
| RARb | 14.6% | ||||||||
| p16 + E-cadherin + RARb | 24.4% | ||||||||
| No detection in controls | |||||||||
| I-IV | 63 | 10 | Serum | MSP | p16 | 27.0% | Koike et al[161] | ||
| E-cadherin | 23.8% | ||||||||
| RARb | 17.5% | ||||||||
| p16 + E-cadherin + RARb | 50.8% | ||||||||
| No detection in controls | |||||||||
| I-IV | 60 | 22 | Serum | qMSP | APC | 16.7% | Leung et al[162] | ||
| E-cadherin | 13.3% | ||||||||
| hMLH1 | 41.7% | ||||||||
| TIMP3 | 16.7% | ||||||||
| Four markers combined | 55% | ||||||||
| 13.6% | (Ctrl.) | ||||||||
| APC + E-cadherin | OS: P = 0.006 | Methylation (+) vs (-) | (b) | ||||||
| I-IV | 109 | 10 | Serum | MSP | RARb | 23.8% | Ikoma et al[163] | ||
| p16 + E-cadherin + RARb | 47.7% | ||||||||
| I-IV | 53 | 21 | Plasma | qPCR | β-actin (102 bp) | P = 0.03 | Pt.(n = 53) vs Ctrl. (n = 21) | (c) | Sai et al[83] |
| β-actin (253 bp) | P < 0.0001 | (c) | |||||||
| AUC = 0.75 | |||||||||
| DNA integrity (253 bp/102 bp) | P = 0.07 | (c) | |||||||
| N/A | 4 | 10 | Serum | MSP | RUNX3 | 100% | Tan et al[164] | ||
| p16 | 50% | ||||||||
| RASSF1A | 25% | ||||||||
| CDH1 | 25% | ||||||||
| No detection in controls | |||||||||
| I-IV | 52 | 20 | Serum | MSP | p16 | 9.6% | Tani et al[147] | ||
| (40 pre-ope) | E-cadherin | 9.6% | |||||||
| (12 post-ope) | RARb | 3.8% | |||||||
| p16 + E-cadherin + RARb | 23.1% | ||||||||
| I-IV | 52 | 50 | Serum | MSP | p16 | 26.9% | (all Pt.) | Abbaszadegan et al[165] | |
| 60.9% | (Pt. with p16 methylation in primary tumor) | ||||||||
| 0% | (Ctrl.) | ||||||||
| I-IV | 20 | 22 | Plasma | Fluorescence-based assay | DNA concentration↑ | P < 0.005 | Pt.(n = 20) vs Ctrl. (n = 22) | (d) | Kolesnikova et al[166] |
| MSP | MGMT | 70% | (Pt.) | ||||||
| 36.4% | (Ctrl.) | ||||||||
| p15 | 50% | (Pt.) | |||||||
| 18.2% | (Ctrl.) | ||||||||
| hMLH1 | 25% | (Pt.) | |||||||
| 9.1% | (Ctrl.) | ||||||||
| I-IV | 47 | 30 (benign gastric disease) | Serum | MSP | RASSF1A | 24.0% | (Pt.) | Wang et al[167] | |
| 30 (healthy controls) | 3.3% | (benign gastric disease) | |||||||
| 0% | (healthy controls) | ||||||||
| I-IV | 20 | 21 | Serum | MSP | HSulf-1 | 55% | (Pt.) | Chen et al[168] | |
| 19.0% | (Ctrl.) | ||||||||
| I-IV | 57 | 79 | Plasma | qPCR | MYC/GAPDH↑ | P < 0.001 | Pt. (n = 57) vs Ctrl. (n = 79) | Park et al[91] | |
| AUC = 0.841 | |||||||||
| I-IV | 65 | 50 | Serum | qMSP | RUNX3 | 29.2% | (Pt.) | Sakakura et al[169] | |
| 10% | (Ctrl.) | ||||||||
| AUC = 0.8651 | Pt. (n = 65) vs Ctrl. (n = 50) | ||||||||
| I-IV | 65 | 40 (benign gastric disease) | Serum | MSP | DLEC1 | 33.8% | (Pt.) | Zhang et al[170] | |
| 20 (healthy controls) | 5% | (benign gastric disease) | |||||||
| 0% | (healthy controls) | ||||||||
| I-IV | 73 | 20 | Serum | qMSP | TFPI2 | 9.6% | (Pt.) | Hibi et al[171] | |
| 0% | (Ctrl.) | ||||||||
| P = 0.004 | Correlation with LN meta. | (a) | |||||||
| P = 0.0115 | Correlation with distant meta. | (a) | |||||||
| I-IV | 65 | 80 | Plasma | qMSP | SLC19A3 | P < 0.0001 | Pt.(n = 45) vs Ctrl. (n = 60) | Ng et al[172] | |
| (Validation 1) | |||||||||
| AUC = 0.82 | Pt. (n = 20) vs Ctrl. (n = 20) | ||||||||
| (Validation 2) | |||||||||
| I-IV | 46 | 30 (healthy controls) | Serum | Methylation CpG island microarray | BX141696 | 56.5% | Zheng et al[86] | ||
| 46 (benign gastric disease) | MSP | WT1 | 50% | ||||||
| CYP26B1 | 73.9% | ||||||||
| KCNA4 | 67.4% | ||||||||
| I-IV | 58 | 30 (healthy controls) | Serum | MeDIP | CHRM2 | 31.0% | Chen et al[87] | ||
| 46 (gastric precancerous lesions) | Methylation CpG island microarray | FAM5C | 31.0% | ||||||
| MSP | P < 0.001 | Pre- vs post-operation | |||||||
| MYLK | 70.7% | ||||||||
| P < 0.001 | Pre- vs post-operation | ||||||||
| FAM5C + MYLK | 77.6% | (GC Pt.) | |||||||
| 10% | (healthy controls) | ||||||||
| 30.4% | (gastric precancerous lesions) | ||||||||
| I-IV | 59 | 54 | Plasma | qPCR | ALU↑ | P < 0.001 | Pt. (n = 59) vs Ctrl. (n = 54) | (c) | Park et al[84] |
| AUC = 0.784 | |||||||||
| N/A | 25 | 9 | Plasma | MSP | ATP4B | 64% | (Pt.) | Raja et al[173] | |
| 0% | (Ctrl.) | ||||||||
| I-IV | 71 | 21 | Serum | qMSP | Vimentin | P = 0.018 | Pt. (n = 73) vs Ctrl. (n = 21) | (c) | Shirahata et al[174] |
| Operable | 73 | 20 | Serum | MSP | SOX17 | 58.9% | (Pt.) | Balgkouranidou et al[175] | |
| 0% | (Ctrl.) | ||||||||
| OS: P = 0.049 | Methylation (+) vs (-) | (b) | |||||||
| I-IV | 73 | Plasma | qPCR | HER2 | 64.4% | Pt. with 2+/3+ score in HER2 IHC assay | Lee et al[92] | ||
| Pt. with 2+/3+ score in HER2 IHC assay | |||||||||
| I-IV | 202 | 88 | Serum | qMSP | XAF1 | 69.8% | (Pt.) | Ling et al[88] | |
| 0% | (Ctrl.) | ||||||||
| AUC = 0.909 | Pt. (n = 202) vs Ctrl.(n = 88) | ||||||||
| DFS: P < 0.0001 | Methylation (+) vs (-) | (b) | |||||||
Concerning genetic alterations in other types of cancers, the relationships with tumor-specific gene alteration such as HER2 in breast cancer[89] and adenomatous polyposis coli in colorectal cancer[75,90] have been revealed even in circulating cfDNA. In GC, Park et al[91] investigated gene amplification of MYC in the plasma of GC patients and showed that the plasma MYC/GAPDH ratio was significantly higher in the GC patients than that in the healthy controls (P < 0.001) and correlated with the tissue MYC/GAPDH ratio (P = 0.009), and tissue MYC status by FISH (P = 0.024). In contrast, among GC patients with a 2+ or 3+ score in a HER2 IHC assay, Lee et al[92] reported that no significant association was observed between the HER2 level in plasma and the copy number variation in tumor tissue determined by FISH. Although it is unclear why there was a discrepancy between these two results, it may be partially explained by the inappropriate employment of reference genes and the heterogeneity of GC tissues. The investigation of circulating cfDNA relating to genetic aberration in GC remains in its infancy. Therefore, further evidence is expected to address current controversial issues and develop this field.
The presence of RNase in plasma/serum had long been known, and furthermore, the RNase concentration in serum was reported to be elevated in cancer patients in the 1970s[93,94]. Given that mRNA in plasma/serum might be more fragile than DNA and susceptible to degradation by RNase, it was not clear whether mRNA could exist in plasma/serum with sufficient integrity to allow amplification, although several reports had previously suggested the possible presence of RNA in serum forming a complex with proteolipids[95,96]. In 1999, two groups reported the successful detection of cell-free RNA such as tyrosinase mRNA in serum of patients with malignant melanoma[97] and epstein-barr virus-associated RNA associated in plasma of patients with nasopharyngeal carcinoma[98]. Subsequently, many studies have demonstrated the presence of specific mRNA in plasma/serum and its potent clinical relevance in patients with a variety of cancers[99-101]. At present, it is considered that mRNA in plasma/serum may be protected from degradation by packaging in secretory membrane vesicles, such as exosomes, microvesicles and multivesicles, which are released from cellular surfaces into the bloodstream[102,103].
In the past decade, circulating cell-free miRNAs in plasma/serum have attracted increasing attention among investigators in various types of research field including oncology. The discovery of miRNA dates back to 1993, when Lee et al[104] found that a short RNA product encoded by the lin-4 gene inhibited the translation of its putative target, lin-14 mRNA, with partial sequence complementarity during a study of Caenorhabditis elegans (C. elegans) development. In 2000, a second miRNA, let-7, was identified to repress the functions of multiple mRNAs in C. elegans[105]. Subsequently, let-7 was found to be widely conserved across species, implying the ubiquitous roles of miRNAs[106]. Since then, accumulated research has revealed their biological features in a variety of diseases.
In summary, miRNAs are a group of noncoding small RNAs, whose mature form generally consists of 19-25 nucleotides. miRNAs are involved in post-translational regulation of gene expression by inhibiting stability and translation of mRNAs[107]. To date, more than 1800 miRNAs have been characterized in Homo sapiens according to the miRNA database (miRBase), and the number of listed miRNAs is increasing. It is suggested that one miRNA can regulate multiple different mRNAs, and conversely one mRNA can be regulated by multiple miRNAs[108,109].
Concerning malignant diseases, miRNAs have been demonstrated to play essential roles in cell proliferation, cell differentiation, apoptosis, EMT, and metastasis[45,46]. Numerous studies have proven the aberrant expression of miRNA and its critical characteristics including both oncogenic and tumor suppressive functions in various cancers. Those findings have motivated us to accelerate this promising field to the next stage, such as development of miRNA-based biomarkers and therapies[110,111].
In 2008, the successful detection of circulating miRNAs and their significance in malignant diseases were first reported by several groups[112-115]. Notably, Mitchell et al[112] and Chen et al[113] clearly demonstrated the biological features and potent utility of circulating miRNA, showing their high stability and reproducibility with resistance to endogenous/exogenous RNase, prolonged incubation at room temperature, extreme pH conditions and multiple freeze-thawing processes. The stability of miRNA in plasma/serum, likely greater than that of mRNA, could be explained by some protective mechanisms, which involve packaging in secretory particles (apoptotic bodies, exosomes etc.)[72,116] and binding to certain proteins (Argonatute 2, High-density Lipoproteins, etc.)[117-119]. Furthermore, secretory vesicles including miRNAs have been shown to be able to function as intercellular transmitters[72,116,120], suggesting that circulating miRNAs in plasma/serum possess various roles in cancer development and metastasis. Those findings have provided new insight into the screening and monitoring of cancer patients, and emerging evidence has suggested the promising potential of circulating miRNA as a novel and non-invasive biomarker in clinical practice[121,122].
As summarized in Table 4, the number of previous reports regarding circulating mRNA in GC patients is small compared with those regarding other types of cancers and with those concerning circulating DNA in GC. However, as Kang et al[123] recently reported that the detection of plasma hTERT mRNA can serve as a potential marker for diagnosis and prognosis of GC patients, increased insight and evidence about circulating mRNA might facilitate the development of this field.
| Characteristic and number of patients | Controls (n) | Plasma/serum | Detection method | Detection rate/statistic value | Ref. | ||||
| I-IV | 52 | 20 | Plasma | qRT-PCR | hTERT | 7.5% | (preoperative) | Tani et al[147] | |
| (40 preoperative) | MUC1 | 1% | |||||||
| (12 postoperative) | hTERT + MUC1 | 15% | |||||||
| hTERT | 16.7% | (postoperative) | |||||||
| MUC1 | 8.3% | ||||||||
| hTERT + MUC1 | 16.7% | ||||||||
| No detection in controls | |||||||||
| I-IV | 89 | 42 | Plasma | qRT-PCR | CXCR4↑ | 41.6% | [before surgery (n = 89)] | Xu et al[176] | |
| [paired, before and | 23.2% | [after surgery (n = 69)] | |||||||
| after surgery (n = 69)] | 21.4% | [Ctrl. (n = 42)] | |||||||
| P < 0.05 | Before surgery (n = 89) vs Ctrl. (n = 42) | (a) | |||||||
| Bmi-1↑ | 57.3% | [before surgery (n = 89)] | |||||||
| 43.5% | [after surgery (n = 69)] | ||||||||
| 28.6% | [Ctrl. (n = 42)] | ||||||||
| P < 0.05 | Before surgery (n = 89) vs Ctrl. (n = 42) | (a) | |||||||
| P < 0.05 | Before (n = 89) vs after (n = 69) surgery | (a) | |||||||
| I-IV | 118 | 40 (gastritis) | Plasma | qRT-PCR | hTERT mRNA↑ | P < 0.05 | GC (n = 118) vs gastritis (n = 40) | (a) | Kang et al[123] |
| 58 (healthy controls) | P < 0.05 | GC (n = 118) vs Ctrl. (n = 58) | (a) | ||||||
| AUC = 0.891 | GC (n = 118) vs Ctrl. (n = 58) | ||||||||
| DFS: P < 0.001 | (b) | ||||||||
| DFS: P = 0.001 | (c) | ||||||||
| OS: P < 0.001 | (b) | ||||||||
| OS: P < 0.001 | (c) | ||||||||
Since the potent utility of determining miRNAs in the plasma of GC patients was first reported by our group in 2010[124], many studies have demonstrated the significance of circulating miRNAs as novel biomarkers (Table 5). Still, the immaturity of the field has led to several issues concerning its actual introduction in clinical settings. To date, there has been no consensus regarding how inter- and intra-individual variations can affect the results, which sample (i.e., plasma or serum) is more favorable for measuring circulating miRNA, or which molecule is the most appropriate for the sensitive detection and endogenous controls. Moreover, as mentioned before, one miRNA can regulate multiple mRNAs and the numbers of discovered miRNAs and targeted mRNAs are still increasing owing to recent advances in bioinformatic analysis, making it more difficult to obtain a meticulous understanding of each miRNA. Although comprehensive approaches by genome-wide profiling can address those problems to some extent, a further large-scale validation with well-established methods seems to be required in this area as well.
| Characteristic and number of patients | Controls (n) | Plasma/serum | Detection method | Detection rate/statistic value | Ref. | |||||
| microRNA | ||||||||||
| I-IV | 69 | 30 | Plasma | qRT-PCR | miR-17-5p↑ | P = 0.05 | Pt. (n = 69) vs Ctrl (n = 30) | (a) | Tsujiura et al[124] | |
| miR-21↑ | P = 0.006 | Pt. (n = 69) vs Ctrl (n = 30) | (a) | |||||||
| P = 0.013 | paired (n = 10), pre-op > post-op | (b) | ||||||||
| miR-106a↑ | P = 0.008 | Pt. (n = 69) vs Ctrl (n = 30) | (a) | |||||||
| miR-106b↑ | P < 0.001 | AUC = 0.721 | Pt. (n = 69) vs Ctrl (n = 30) | (a) | ||||||
| P = 0.022 | paired (n = 10), pre-op > post-op | (b) | ||||||||
| let-7a↓ | P = 0.002 | Pt. (n = 69) vs Ctrl (n = 30) | (a) | |||||||
| miR-106a/let-7a↑ | AUC = 0.879 | |||||||||
| I-IV | 164 | 127 | Serum | Solexa sequencing | miR-1↑ | P < 0.01 | Pt. (n = 164) vs Ctrl (n = 127) | (a) | Liu et al[177] | |
| qRT-PCR | miR-20a↑ | P < 0.01 | (a) | |||||||
| miR-27a↑ | P < 0.01 | (a) | ||||||||
| miR-34a↑ | P < 0.01 | (a) | ||||||||
| miR-423-5p↑ | P < 0.01 | (a) | ||||||||
| Five-serum miRNA signature↑ | AUC = 0.879 | Pt. (n = 22) vs Ctrl (n = 22) (test study) | ||||||||
| AUC = 0.831 | Pt. (n = 142) vs Ctrl (n = 105) (validation study) | |||||||||
| I-IV | 56 | 30 | Plasma | Microarray | miR-451↑ | P < 0.01 | AUC = 0.96 | Pt. (n = 56) vs Ctrl (n = 30) | (a) | Konishi et al[125] |
| qRT-PCR | P < 0.01 | paired (n = 29), pre-op > post-op | (b) | |||||||
| miR-486↑ | P < 0.01 | AUC = 0.92 | Pt. (n = 56) vs Ctrl (n = 30) | (a) | ||||||
| P < 0.01 | paired (n = 29), pre-op > post-op | (b) | ||||||||
| I-IV | 40 | 41 | Serum | Microarray | miR-187*↑ | P = 0.0016 | AUC = 0.704 | Pt. (n = 40) vs Ctrl (n = 41) | (a) | Liu et al[178] |
| qRT-PCR | miR-371-5p↑ | P = 0.0009 | AUC = 0.715 | (a) | ||||||
| miR-378↑ | P < 0.0001 | AUC = 0.861 | (a) | |||||||
| N/A | 82 | 82 | Serum | Microarray | miR-221↑ | AUC = 0.74 | Pt. (n = 68) vs Ctrl (n = 68) (second validation study) | Song et al[179] | ||
| qRT-PCR | miR-376c↑ | AUC = 0.71 | ||||||||
| miR-744↑ | AUC = 0.71 | |||||||||
| N/A | 20 | Serum | qRT-PCR | miR-196a | P = 0.012 | Pre-op (n = 20) > post-op (n = 20) | (a) | Tsai et al[180] | ||
| (pre-op, post-op and recurrent) | P = 0.002 | Post-op (n = 20) < at recurrent (n = 20) | (a) | |||||||
| I-IV | 30 | 39 | Serum | qRT-PCR | miR-21↑ | P < 0.001 | AUC = 0.81 | Pt. (n = 30) vs Ctrl (n = 39) | (a) | Wang et al[181] |
| I-IV | 87 | Plasma | qRT-PCR | miR-17-5p↑ | P < 0.001 | Pre-op (n = 65) > post-op (n = 16) | (a) | Wang et al[182] | ||
| (65 pre-op) | P = 0.003 | Post-op (n = 16) < recurrent (n = 6) | (a) | |||||||
| (16 post-op) | OS: P = 0.0003 | miR-17-5p high vs low | Pre-op (n = 65) | (c) | ||||||
| (6 recurrent) | miR-20a↑ | P = 0.006 | Pre-op (n = 65) > post-op (n = 16) | (a) | ||||||
| OS: P = 0.0003 | miR-20a high vs low | Pre-op (n = 65) | (c) | |||||||
| OS: P = 0.013 | miR-20a high vs low | pre-op (n = 65) | (d) | |||||||
| I-IV | 20 | 20 | Serum | miRNA microarray | miR-375↓ | P < 0.001 | AUC = 0.835 | Pt. (n = 20) vs Ctrl (n = 20) | (a) | Zhang et al[183] |
| qRT-PCR | ||||||||||
| 20 | 190 | Plasma | miRNA microarray | miR-195-5p↓ | P < 0.05 | Fold changes 13.3 | Pt. (n = 20) vs Ctrl (n = 130) | (a) | Gorur et al[184] | |
| I-III | 79 | miR-21↑ | P < 0.001 | Correlation with pN stage | (e) | Kim et al[185] | ||||
| (25 without LN meta) | miR-146a↑ | P = 0.001 | Correlation with pN stage | (e) | ||||||
| (54 with LN meta) | miR-148a↑ | P < 0.001 | Correlation with pN stage | (e) | ||||||
| I-IV | 69 | Plasma | qRT-PCR | miR-21↑ | CSS: P = 0.0451 | miR-21 high vs low | (c) | Komatsu et al[186] | ||
| CSS: P = 0.0133 | (d) | |||||||||
| I-II | 80 | 70 | Plasma | qRT-PCR | miRNA-199a-3p↑ | P < 0.001 | AUC = 0.818 | Pt. (n = 80) vs Ctrl. (n = 70) | (a) | Li et al[187] |
| (healthy controls) | P = 0.004 | Pt. (n = 80) vs pancreous disease (n = 20) | (a) | |||||||
| 20 | P = 0.012 | Pre-op > post-op (n = 30) | (b) | |||||||
| (precancerous disease) | ||||||||||
| Long non-coding RNA | ||||||||||
| I-IV | 43 | 33 | Plasma | qRT-PCR | H19 | P = 0.029 | Pt. (n = 43) vs Ctrl. (n = 33) | (a) | Arita et al[128] | |
| HOTAIR | P = 0.096 | (a) | ||||||||
| MALAT1 | P = 0.14 | (a) | ||||||||
To overcome obstacles due to inter-individual variations, our group tried to identify candidate miRNAs by comparing miRNA profiles between pre- and post-operative samples in the same individuals[125]. Because miRNAs are involved in various non-cancerous cell biology including physiological modulation and pathological disruption of basic pathways[126,127], the existence of inter-individual differences can be strongly suspected based on miRNA expression. As a result, two miRNAs, miR-451 and miR-486, were selected based on this strategy and their significant value in discriminating between GC patients and healthy controls was clearly demonstrated with an AUC of 0.96 and 0.92 in ROC curve analysis for miR-451 and miR-486, respectively. We suggest that the miRNAs isolated by these concepts could be valuable biomarkers for the effective detection of early cancer and recurrence because the change of these miRNAs can be affected by the reduction of the volume of cancer tissue and is therefore directly related to tumor existence.
Most recently, our group published new observations in which long non-coding RNAs (lncRNAs) in the plasma of GC patients were successfully detected[128]. Specifically, three lncRNAs (H19, HOX antisense intergenic RNA and metastasis associated lung adenocarcinoma transcript-1) stably exist in plasma from both GC patients and healthy controls. Plasma H19 levels were significantly higher in the patient group than the control group and decreased postoperatively, implying the possible use of H19 levels as a novel diagnostic marker in GC. LncRNAs are defined as non-protein coding transcripts longer than 200 nt lacking significant open reading frames and involved in fundamental cellular processes, such as RNA processing, gene regulation, chromatin modification, gene transcription, and post-transcriptional gene regulation based on RNA sequence complementary interactions[129,130]. Detailed investigations have shown that lncRNAs can exhibit developmental and tissue specific expression patterns as well as aberrant regulation in a variety of diseases, including GC[131,132]. Explorations of a novel type of RNA can provide more intriguing aspects in this research field.
Although the concept of “liquid biopsy” possesses great potential in detection and monitoring of diseases as previously described in detail, several hurdles should be overcome before translating it into clinical settings. One of the most important issues is the lack of consensus in technical approaches, which involves various aspects of the methodologies, such as preferable sample type, storage conditions, candidate molecules and suitable detection techniques. Moreover, technical errors may introduce contaminated cells or molecules into experimental samples, which could cause misunderstandings and statistical errors. Therefore, the standardization of techniques throughout all experimental steps should be emphasized.
Owing to recent remarkable technological developments, novel revolutionary approaches including an in vivo CTC isolation system[32] and multi-detectable array have been introduced into this research field. However, some issues raised by those advances should be addressed properly. Although multi-detection approaches can facilitate exhaustive screenings and provide us with various types of information, the important considerations are which molecules should be selected as a tumor marker and how the result of an individual patient obtained by multiple detection panels should be effectively utilized. Of course, the cost and practicality of each assay should also be taken into consideration to some extent.
In summary, the science of CTCs and circulating cfNAs remains in its infancy. Despite numerous approaches and techniques that have been advocated to accomplish the ultimate goal, that is, the development of a useful, sensitive and real-time monitoring system from the blood, few proposals have been translated into clinical practice. Large-scale studies and further understanding of their biology and significance could resolve those problems and enhance their utility as biomarkers. Consequently, the development of novel biomarkers based on CTCs and cfNAs could provide many benefits for cancer patients including the improvement of clinical outcomes in the near future.
P- Reviewers: Streba CT, Zhu YL S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 2. | Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 811] [Article Influence: 67.6] [Reference Citation Analysis (2)] |
| 3. | van de Stolpe A, Pantel K, Sleijfer S, Terstappen LW, den Toonder JM. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res. 2011;71:5955-5960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 5. | Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 2148] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 6. | Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death.. Aust Med J. 1869;14:146-149. |
| 7. | Ghossein RA, Bhattacharya S, Rosai J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin Cancer Res. 1999;5:1950-1960. [PubMed] |
| 8. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1954] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 9. | Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 414] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 801] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 11. | Coumans FA, van Dalum G, Beck M, Terstappen LW. Filter characteristics influencing circulating tumor cell enrichment from whole blood. PLoS One. 2013;8:e61770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Coumans FA, van Dalum G, Beck M, Terstappen LW. Filtration parameters influencing circulating tumor cell enrichment from whole blood. PLoS One. 2013;8:e61774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Marrinucci D, Bethel K, Bruce RH, Curry DN, Hsieh B, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi A. Case study of the morphologic variation of circulating tumor cells. Hum Pathol. 2007;38:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Tan SJ, Yobas L, Lee GY, Ong CN, Lim CT. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed Microdevices. 2009;11:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Mohamed H, Murray M, Turner JN, Caggana M. Isolation of tumor cells using size and deformation. J Chromatogr A. 2009;1216:8289-8295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627-5629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Wenqi D, Li W, Shanshan C, Bei C, Yafei Z, Feihu B, Jie L, Daiming F. EpCAM is overexpressed in gastric cancer and its downregulation suppresses proliferation of gastric cancer. J Cancer Res Clin Oncol. 2009;135:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3386] [Article Influence: 161.2] [Reference Citation Analysis (0)] |
| 20. | Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053-7058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 21. | Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218-4224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 776] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 22. | Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 1030] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 23. | Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, García-Saenz JA, Vidaurreta M, Martín M, Arroyo M. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Thompson EW, Haviv I. The social aspects of EMT-MET plasticity. Nat Med. 2011;17:1048-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1363] [Cited by in RCA: 1321] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 27. | Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3036] [Cited by in RCA: 2577] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 28. | Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 425] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 29. | Sakaizawa K, Goto Y, Kiniwa Y, Uchiyama A, Harada K, Shimada S, Saida T, Ferrone S, Takata M, Uhara H. Mutation analysis of BRAF and KIT in circulating melanoma cells at the single cell level. Br J Cancer. 2012;106:939-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, Lax S, Waldispuehl-Geigl J, Mauermann O, Lackner C. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013;73:2965-2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 31. | Cann GM, Gulzar ZG, Cooper S, Li R, Luo S, Tat M, Stuart S, Schroth G, Srinivas S, Ronaghi M. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS One. 2012;7:e49144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol. 2012;41:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB. A rare-cell detector for cancer. Proc Natl Acad Sci USA. 2004;101:10501-10504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Hsieh HB, Marrinucci D, Bethel K, Curry DN, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi A, Lazarus N. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Alix-Panabières C, Vendrell JP, Pellé O, Rebillard X, Riethdorf S, Müller V, Fabbro M, Pantel K. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin Chem. 2007;53:537-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Alix-Panabières C, Vendrell JP, Slijper M, Pellé O, Barbotte E, Mercier G, Jacot W, Fabbro M, Pantel K. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. 2009;11:R39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 517] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 39. | Sieuwerts AM, Mostert B, Bolt-de Vries J, Peeters D, de Jongh FE, Stouthard JM, Dirix LY, van Dam PA, Van Galen A, de Weerd V. mRNA and microRNA expression profiles in circulating tumor cells and primary tumors of metastatic breast cancer patients. Clin Cancer Res. 2011;17:3600-3618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 41. | Sieuwerts AM, Kraan J, Bolt-de Vries J, van der Spoel P, Mostert B, Martens JW, Gratama JW, Sleijfer S, Foekens JA. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res Treat. 2009;118:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Uen YH, Lin SR, Wu CH, Hsieh JS, Lu CY, Yu FJ, Huang TJ, Wang JY. Clinical significance of MUC1 and c-Met RT-PCR detection of circulating tumor cells in patients with gastric carcinoma. Clin Chim Acta. 2006;367:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Wu CH, Lin SR, Hsieh JS, Chen FM, Lu CY, Yu FJ, Cheng TL, Huang TJ, Huang SY, Wang JY. Molecular detection of disseminated tumor cells in the peripheral blood of patients with gastric cancer: evaluation of their prognostic significance. Dis Markers. 2006;22:103-109. [PubMed] |
| 44. | Wu CH, Lin SR, Yu FJ, Wu DC, Pan YS, Hsieh JS, Huang SY, Wang JY. Development of a high-throughput membrane-array method for molecular diagnosis of circulating tumor cells in patients with gastric cancers. Int J Cancer. 2006;119:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7370] [Article Influence: 368.5] [Reference Citation Analysis (0)] |
| 46. | Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2468] [Cited by in RCA: 2465] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 47. | Mei Y, Clark D, Mao L. Novel dimensions of piRNAs in cancer. Cancer Lett. 2013;336:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Zhou H, Guo JM, Lou YR, Zhang XJ, Zhong FD, Jiang Z, Cheng J, Xiao BX. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using microRNA as a marker. J Mol Med (Berl). 2010;88:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 49. | Cui L, Lou Y, Zhang X, Zhou H, Deng H, Song H, Yu X, Xiao B, Wang W, Guo J. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin Biochem. 2011;44:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Zheng Y, Cui L, Sun W, Zhou H, Yuan X, Huo M, Chen J, Lou Y, Guo J. MicroRNA-21 is a new marker of circulating tumor cells in gastric cancer patients. Cancer Biomark. 2011;10:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Haz M, Santamarina I, Blanco M, Fernández-Tajes J, Quindós M. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 52. | Zhou H, Xiao B, Zhou F, Deng H, Zhang X, Lou Y, Gong Z, Du C, Guo J. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers. 2012;17:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS One. 2011;6:e20769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 54. | Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila). 2012;5:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 723] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 55. | Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol. 2008;15:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Matsusaka S, Chìn K, Ogura M, Suenaga M, Shinozaki E, Mishima Y, Terui Y, Mizunuma N, Hatake K. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 2010;101:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Mimori K, Fukagawa T, Kosaka Y, Ishikawa K, Iwatsuki M, Yokobori T, Hirasaki S, Takatsuno Y, Sakashita H, Ishii H. A large-scale study of MT1-MMP as a marker for isolated tumor cells in peripheral blood and bone marrow in gastric cancer cases. Ann Surg Oncol. 2008;15:2934-2942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Ikeguchi M, Kaibara N. Detection of circulating cancer cells after a gastrectomy for gastric cancer. Surg Today. 2005;35:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Miyazono F, Natsugoe S, Takao S, Tokuda K, Kijima F, Aridome K, Hokita S, Baba M, Eizuru Y, Aikou T. Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann Surg. 2001;233:189-194. [PubMed] |
| 60. | Nishida S, Kitamura K, Ichikawa D, Koike H, Tani N, Yamagishi H. Molecular detection of disseminated cancer cells in the peripheral blood of patients with gastric cancer. Anticancer Res. 2000;20:2155-2159. [PubMed] |
| 61. | Pituch-Noworolska A, Kolodziejczyk P, Kulig J, Drabik G, Szczepanik A, Czupryna A, Popiela T, Zembala M. Circulating tumour cells and survival of patients with gastric cancer. Anticancer Res. 2007;27:635-640. [PubMed] |
| 62. | Mandel P, Metais P. [Les acides nucleiques du plasma sanguin chez l’homme]. C R Seances Soc Biol Fil. 1948;142:241-243. [PubMed] |
| 63. | Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646-650. [PubMed] |
| 64. | Vasioukhin V, Anker P, Maurice P, Lyautey J, Lederrey C, Stroun M. Point mutations of the N-ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br J Haematol. 1994;86:774-779. [PubMed] |
| 65. | Sorenson GD, Pribish DM, Valone FH, Memoli VA, Bzik DJ, Yao SL. Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol Biomarkers Prev. 1994;3:67-71. [PubMed] |
| 66. | Choi JJ, Reich CF, Pisetsky DS. The role of macrophages in the in vitro generation of extracellular DNA from apoptotic and necrotic cells. Immunology. 2005;115:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Pisetsky DS, Fairhurst AM. The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity. 2007;40:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Stroun M, Lyautey J, Lederrey C, Mulcahy HE, Anker P. Alu repeat sequences are present in increased proportions compared to a unique gene in plasma/serum DNA: evidence for a preferential release from viable cells? Ann N Y Acad Sci. 2001;945:258-264. [PubMed] |
| 69. | Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta. 2001;313:139-142. [PubMed] |
| 70. | Trejo-Becerril C, Pérez-Cárdenas E, Taja-Chayeb L, Anker P, Herrera-Goepfert R, Medina-Velázquez LA, Hidalgo-Miranda A, Pérez-Montiel D, Chávez-Blanco A, Cruz-Velázquez J. Cancer progression mediated by horizontal gene transfer in an in vivo model. PLoS One. 2012;7:e52754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | García-Olmo DC, Domínguez C, García-Arranz M, Anker P, Stroun M, García-Verdugo JM, García-Olmo D. Cell-free nucleic acids circulating in the plasma of colorectal cancer patients induce the oncogenic transformation of susceptible cultured cells. Cancer Res. 2010;70:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 72. | Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442-17452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1590] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 73. | Wang BG, Huang HY, Chen YC, Bristow RE, Kassauei K, Cheng CC, Roden R, Sokoll LJ, Chan DW, Shih IeM. Increased plasma DNA integrity in cancer patients. Cancer Res. 2003;63:3966-3968. [PubMed] |
| 74. | Suzuki N, Kamataki A, Yamaki J, Homma Y. Characterization of circulating DNA in healthy human plasma. Clin Chim Acta. 2008;387:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, Diaz LA, Goodman SN, David KA, Juhl H. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci USA. 2005;102:16368-16373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 944] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 76. | van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6904] [Cited by in RCA: 6356] [Article Influence: 276.3] [Reference Citation Analysis (0)] |
| 77. | Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 1720] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 78. | Chan KC, Jiang P, Zheng YW, Liao GJ, Sun H, Wong J, Siu SS, Chan WC, Chan SL, Chan AT. Cancer genome scanning in plasma: detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin Chem. 2013;59:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 79. | Castells A, Puig P, Móra J, Boadas J, Boix L, Urgell E, Solé M, Capellà G, Lluís F, Fernández-Cruz L. K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol. 1999;17:578-584. [PubMed] |
| 80. | Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1259] [Cited by in RCA: 1354] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 81. | Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1475] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 82. | Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, Antipova A, Lee C, McKernan K, De La Vega FM. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 83. | Sai S, Ichikawa D, Tomita H, Ikoma D, Tani N, Ikoma H, Kikuchi S, Fujiwara H, Ueda Y, Otsuji E. Quantification of plasma cell-free DNA in patients with gastric cancer. Anticancer Res. 2007;27:2747-2751. [PubMed] |
| 84. | Park JL, Kim HJ, Choi BY, Lee HC, Jang HR, Song KS, Noh SM, Kim SY, Han DS, Kim YS. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol Lett. 2012;3:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Lee TL, Leung WK, Chan MW, Ng EK, Tong JH, Lo KW, Chung SC, Sung JJ, To KF. Detection of gene promoter hypermethylation in the tumor and serum of patients with gastric carcinoma. Clin Cancer Res. 2002;8:1761-1766. [PubMed] |
| 86. | Zheng Y, Chen L, Li J, Yu B, Su L, Chen X, Yu Y, Yan M, Liu B, Zhu Z. Hypermethylated DNA as potential biomarkers for gastric cancer diagnosis. Clin Biochem. 2011;44:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Chen L, Su L, Li J, Zheng Y, Yu B, Yu Y, Yan M, Gu Q, Zhu Z, Liu B. Hypermethylated FAM5C and MYLK in serum as diagnosis and pre-warning markers for gastric cancer. Dis Markers. 2012;32:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 88. | Ling ZQ, Lv P, Lu XX, Yu JL, Han J, Ying LS, Zhu X, Zhu WY, Fang XH, Wang S. Circulating Methylated XAF1 DNA Indicates Poor Prognosis for Gastric Cancer. PLoS One. 2013;8:e67195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Gevensleben H, Garcia-Murillas I, Graeser MK, Schiavon G, Osin P, Parton M, Smith IE, Ashworth A, Turner NC. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin Cancer Res. 2013;19:3276-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 90. | Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2240] [Cited by in RCA: 2112] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 91. | Park KU, Lee HE, Park do J, Jung EJ, Song J, Kim HH, Choe G, Kim WH, Lee HS. MYC quantitation in cell-free plasma DNA by real-time PCR for gastric cancer diagnosis. Clin Chem Lab Med. 2009;47:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Lee HE, Park KU, Yoo SB, Nam SK, Park do J, Kim HH, Lee HS. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 93. | Reddi KK, Holland JF. Elevated serum ribonuclease in patients with pancreatic cancer. Proc Natl Acad Sci USA. 1976;73:2308-2310. [PubMed] |
| 94. | Maor D, Mardiney MR. Alteration of human serum ribonuclease activity in malignancy. CRC Crit Rev Clin Lab Sci. 1978;10:89-111. [PubMed] |
| 95. | Wieczorek AJ, Sitaramam V, Machleidt W, Rhyner K, Perruchoud AP, Block LH. Diagnostic and prognostic value of RNA-proteolipid in sera of patients with malignant disorders following therapy: first clinical evaluation of a novel tumor marker. Cancer Res. 1987;47:6407-6412. [PubMed] |
| 96. | Wieczorek AJ, Rhyner C, Block LH. Isolation and characterization of an RNA-proteolipid complex associated with the malignant state in humans. Proc Natl Acad Sci USA. 1985;82:3455-3459. [PubMed] |
| 97. | Kopreski MS, Benko FA, Kwak LW, Gocke CD. Detection of tumor messenger RNA in the serum of patients with malignant melanoma. Clin Cancer Res. 1999;5:1961-1965. [PubMed] |
| 98. | Lo KW, Lo YM, Leung SF, Tsang YS, Chan LY, Johnson PJ, Hjelm NM, Lee JC, Huang DP. Analysis of cell-free Epstein-Barr virus associated RNA in the plasma of patients with nasopharyngeal carcinoma. Clin Chem. 1999;45:1292-1294. [PubMed] |
| 99. | Dasí F, Martínez-Rodes P, March JA, Santamaría J, Martínez-Javaloyas JM, Gil M, Aliño SF. Real-time quantification of human telomerase reverse transcriptase mRNA in the plasma of patients with prostate cancer. Ann N Y Acad Sci. 2006;1075:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Chen XQ, Bonnefoi H, Pelte MF, Lyautey J, Lederrey C, Movarekhi S, Schaeffer P, Mulcahy HE, Meyer P, Stroun M. Telomerase RNA as a detection marker in the serum of breast cancer patients. Clin Cancer Res. 2000;6:3823-3826. [PubMed] |
| 101. | Silva JM, Rodriguez R, Garcia JM, Muñoz C, Silva J, Dominguez G, Provencio M, España P, Bonilla F. Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut. 2002;50:530-534. [PubMed] |
| 102. | Houseley J, LaCava J, Tollervey D. RNA-quality control by the exosome. Nat Rev Mol Cell Biol. 2006;7:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 103. | Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083-4099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 675] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 104. | Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-854. [PubMed] |
| 105. | Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3402] [Article Influence: 136.1] [Reference Citation Analysis (0)] |
| 106. | Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1730] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 107. | Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1960] [Cited by in RCA: 1739] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 108. | Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787-798. [PubMed] |
| 109. | Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38 Suppl:S8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 832] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 110. | Li C, Feng Y, Coukos G, Zhang L. Therapeutic microRNA strategies in human cancer. AAPS J. 2009;11:747-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 111. | Trang P, Weidhaas JB, Slack FJ. MicroRNAs as potential cancer therapeutics. Oncogene. 2008;27 Suppl 2:S52-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 112. | Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513-10518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5636] [Cited by in RCA: 6314] [Article Influence: 371.4] [Reference Citation Analysis (0)] |
| 113. | Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3218] [Cited by in RCA: 3554] [Article Influence: 209.1] [Reference Citation Analysis (0)] |
| 114. | Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1333] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 115. | Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2008;14:2588-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 602] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 116. | Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 117. | Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003-5008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2643] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 118. | Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2292] [Cited by in RCA: 2188] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 119. | Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223-7233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1525] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 120. | Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 1053] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 121. | Ichikawa D, Komatsu S, Konishi H, Otsuji E. Circulating microRNA in digestive tract cancers. Gastroenterology. 2012;142:1074-1078.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 122. | Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol Hematol. 2011;80:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 123. | Kang Y, Zhang J, Sun P, Shang J. Circulating cell-free human telomerase reverse transcriptase mRNA in plasma and its potential diagnostic and prognostic value for gastric cancer. Int J Clin Oncol. 2013;18:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 124. | Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 125. | Konishi H, Ichikawa D, Komatsu S, Shiozaki A, Tsujiura M, Takeshita H, Morimura R, Nagata H, Arita T, Kawaguchi T. Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br J Cancer. 2012;106:740-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 126. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [PubMed] |
| 127. | Ambros V. The functions of animal microRNAs. Nature. 2004;431:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7919] [Cited by in RCA: 8601] [Article Influence: 409.6] [Reference Citation Analysis (0)] |
| 128. | Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185-3193. [PubMed] |
| 129. | Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1483] [Cited by in RCA: 1452] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 130. | Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4442] [Article Influence: 277.6] [Reference Citation Analysis (0)] |
| 131. | Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, Fang G. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 376] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 132. | Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 133. | Funaki NO, Tanaka J, Kasamatsu T, Ohshio G, Hosotani R, Okino T, Imamura M. Identification of carcinoembryonic antigen mRNA in circulating peripheral blood of pancreatic carcinoma and gastric carcinoma patients. Life Sci. 1996;59:2187-2199. [PubMed] |
| 134. | Mori M, Mimori K, Ueo H, Karimine N, Barnard GF, Sugimachi K, Akiyoshi T. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer. 1996;68:739-743. [PubMed] |
| 135. | Aihara T, Noguchi S, Ishikawa O, Furukawa H, Hiratsuka M, Ohigashi H, Nakamori S, Monden M, Imaoka S. Detection of pancreatic and gastric cancer cells in peripheral and portal blood by amplification of keratin 19 mRNA with reverse transcriptase-polymerase chain reaction. Int J Cancer. 1997;72:408-411. [PubMed] |
| 136. | Soeth E, Vogel I, Röder C, Juhl H, Marxsen J, Krüger U, Henne-Bruns D, Kremer B, Kalthoff H. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res. 1997;57:3106-3110. [PubMed] |
| 137. | Yeh KH, Chen YC, Yeh SH, Chen CP, Lin JT, Cheng AL. Detection of circulating cancer cells by nested reverse transcription-polymerase chain reaction of cytokeratin-19 (K19)--possible clinical significance in advanced gastric cancer. Anticancer Res. 1998;18:1283-1286. [PubMed] |
| 138. | Noh YH, Im G, Ku JH, Lee YS, Ahn MJ. Detection of tumor cell contamination in peripheral blood by RT-PCR in gastrointestinal cancer patients. J Korean Med Sci. 1999;14:623-628. [PubMed] |
| 139. | Majima T, Ichikura T, Takayama E, Chochi K, Mochizuki H. Detecting circulating cancer cells using reverse transcriptase-polymerase chain reaction for cytokeratin mRNA in peripheral blood from patients with gastric cancer. Jpn J Clin Oncol. 2000;30:499-503. [PubMed] |
| 140. | Noh YH, Kim JA, Lim GR, Ro YT, Koo JH, Lee YS, Han DS, Park HK, Ahn MJ. Detection of circulating tumor cells in patients with gastrointestinal tract cancer using RT-PCR and its clinical implications. Exp Mol Med. 2001;33:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 141. | Sumikura S, Ishigami S, Natsugoe S, Miyazono F, Tokuda K, Nakajo A, Okumura H, Matsumoto M, Hokita S, Aikou T. Disseminated cancer cells in the blood and expression of sialylated antigen in gastric cancer. Cancer Lett. 2003;200:77-83. [PubMed] |
| 142. | Koike H, Ichikawa D, Ikoma H, Otsuji E, Kitamura K, Yamagishi H. Comparison of methylation-specific polymerase chain reaction (MSP) with reverse transcriptase-polymerase chain reaction (RT-PCR) in peripheral blood of gastric cancer patients. J Surg Oncol. 2004;87:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Friederichs J, Gertler R, Rosenberg R, Nahrig J, Führer K, Holzmann B, Dittler HJ, Dahm M, Thorban S, Nekarda H. Prognostic impact of CK-20-positive cells in peripheral venous blood of patients with gastrointestinal carcinoma. World J Surg. 2005;29:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 144. | Illert B, Fein M, Otto C, Cording F, Stehle D, Thiede A, Timmermann W. Disseminated tumor cells in the blood of patients with gastric cancer are an independent predictive marker of poor prognosis. Scand J Gastroenterol. 2005;40:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 145. | Seo JH, Choi CW, Kim BS, Shin SW, Kim YH, Kim JS, Lee SW, Choi JH, Park YT, Mok YJ. Follow-up study of peripheral blood carcinoembryonic antigen mRNA using reverse transcription-polymerase chain reaction as an early marker of clinical recurrence in patients with curatively resected gastric cancer. Am J Clin Oncol. 2005;28:24-29. [PubMed] |
| 146. | Kolodziejczyk P, Pituch-Noworolska A, Drabik G, Kulig J, Szczepanik A, Sierzega M, Gurda A, Popiela T, Zembala M. The effects of preoperative chemotherapy on isolated tumour cells in the blood and bone marrow of gastric cancer patients. Br J Cancer. 2007;97:589-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 147. | Tani N, Ichikawa D, Ikoma D, Tomita H, Sai S, Ikoma H, Okamoto K, Ochiai T, Ueda Y, Otsuji E. Circulating cell-free mRNA in plasma as a tumor marker for patients with primary and recurrent gastric cancer. Anticancer Res. 2007;27:1207-1212. [PubMed] |
| 148. | Koga T, Tokunaga E, Sumiyoshi Y, Oki E, Oda S, Takahashi I, Kakeji Y, Baba H, Maehara Y. Detection of circulating gastric cancer cells in peripheral blood using real time quantitative RT-PCR. Hepatogastroenterology. 2008;55:1131-1135. [PubMed] |
| 149. | Yie SM, Lou B, Ye SR, Cao M, He X, Li P, Hu K, Rao L, Wu SM, Xiao HB. Detection of survivin-expressing circulating cancer cells (CCCs) in peripheral blood of patients with gastric and colorectal cancer reveals high risks of relapse. Ann Surg Oncol. 2008;15:3073-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 150. | Bertazza L, Mocellin S, Marchet A, Pilati P, Gabrieli J, Scalerta R, Nitti D. Survivin gene levels in the peripheral blood of patients with gastric cancer independently predict survival. J Transl Med. 2009;7:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 151. | Kita Y, Fukagawa T, Mimori K, Kosaka Y, Ishikawa K, Aikou T, Natsugoe S, Sasako M, Mori M. Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer. 2009;100:153-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 152. | Qiu MZ, Li ZH, Zhou ZW, Li YH, Wang ZQ, Wang FH, Huang P, Aziz F, Wang DY, Xu RH. Detection of carcinoembryonic antigen messenger RNA in blood using quantitative real-time reverse transcriptase-polymerase chain reaction to predict recurrence of gastric adenocarcinoma. J Transl Med. 2010;8:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 153. | Saad AA, Awed NM, Abd Elkerim NN, El-Shennawy D, Alfons MA, Elserafy ME, Darwish YW, Barakat EM, Ezz-Elarab SS. Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Ann Surg Oncol. 2010;17:3059-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S. B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci. 2011;102:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 155. | Cao W, Yang W, Li H, Lou G, Jiang J, Geng M, Xi W, Ren R, Qu Q, Jin X. Using detection of survivin-expressing circulating tumor cells in peripheral blood to predict tumor recurrence following curative resection of gastric cancer. J Surg Oncol. 2011;103:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | Dardaei L, Shahsavani R, Ghavamzadeh A, Behmanesh M, Aslankoohi E, Alimoghaddam K, Ghaffari SH. The detection of disseminated tumor cells in bone marrow and peripheral blood of gastric cancer patients by multimarker (CEA, CK20, TFF1 and MUC2) quantitative real-time PCR. Clin Biochem. 2011;44:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 157. | Ren J, Chen Z, Juan SJ, Yong XY, Pan BR, Fan DM. Detection of circulating gastric carcinoma-associated antigen MG7-Ag in human sera using an established single determinant immuno-polymerase chain reaction technique. Cancer. 2000;88:280-285. [PubMed] |
| 158. | Lo YM, Chan WY, Ng EK, Chan LY, Lai PB, Tam JS, Chung SC. Circulating Epstein-Barr virus DNA in the serum of patients with gastric carcinoma. Clin Cancer Res. 2001;7:1856-1859. [PubMed] |
| 159. | Kanyama Y, Hibi K, Nakayama H, Kodera Y, Ito K, Akiyama S, Nakao A. Detection of p16 promoter hypermethylation in serum of gastric cancer patients. Cancer Sci. 2003;94:418-420. [PubMed] |
| 160. | Ichikawa D, Koike H, Ikoma H, Ikoma D, Tani N, Otsuji E, Kitamura K, Yamagishi H. Detection of aberrant methylation as a tumor marker in serum of patients with gastric cancer. Anticancer Res. 2004;24:2477-2481. [PubMed] |
| 161. | Koike H, Ichikawa D, Ikoma H, Tani N, Ikoma D, Otsuji E, Okamoto K, Ueda Y, Kitamura K, Yamagishi H. Comparison of serum aberrant methylation and conventional tumor markers in gastric cancer patients. Hepatogastroenterology. 2005;52:1293-1296. [PubMed] |
| 162. | Leung WK, To KF, Chu ES, Chan MW, Bai AH, Ng EK, Chan FK, Sung JJ. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer. 2005;92:2190-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 163. | Ikoma H, Ichikawa D, Daito I, Nobuyuki T, Koike H, Okamoto K, Ochiai T, Ueda Y, Yamagishi H, Otsuji E. Clinical application of methylation specific-polymerase chain reaction in serum of patients with gastric cancer. Hepatogastroenterology. 2007;54:946-950. [PubMed] |
| 164. | Tan SH, Ida H, Lau QC, Goh BC, Chieng WS, Loh M, Ito Y. Detection of promoter hypermethylation in serum samples of cancer patients by methylation-specific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncol Rep. 2007;18:1225-1230. [PubMed] |
| 165. | Abbaszadegan MR, Moaven O, Sima HR, Ghafarzadegan K, A’rabi A, Forghani MN, Raziee HR, Mashhadinejad A, Jafarzadeh M, Esmaili-Shandiz E. p16 promoter hypermethylation: a useful serum marker for early detection of gastric cancer. World J Gastroenterol. 2008;14:2055-2060. [PubMed] |
| 166. | Kolesnikova EV, Tamkovich SN, Bryzgunova OE, Shelestyuk PI, Permyakova VI, Vlassov VV, Tuzikov AS, Laktionov PP, Rykova EY. Circulating DNA in the blood of gastric cancer patients. Ann N Y Acad Sci. 2008;1137:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 167. | Wang YC, Yu ZH, Liu C, Xu LZ, Yu W, Lu J, Zhu RM, Li GL, Xia XY, Wei XW. Detection of RASSF1A promoter hypermethylation in serum from gastric and colorectal adenocarcinoma patients. World J Gastroenterol. 2008;14:3074-3080. [PubMed] |
| 168. | Chen Z, Fan JQ, Li J, Li QS, Yan Z, Jia XK, Liu WD, Wei LJ, Zhang FZ, Gao H. Promoter hypermethylation correlates with the Hsulf-1 silencing in human breast and gastric cancer. Int J Cancer. 2009;124:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 169. | Sakakura C, Hamada T, Miyagawa K, Nishio M, Miyashita A, Nagata H, Ida H, Yazumi S, Otsuji E, Chiba T. Quantitative analysis of tumor-derived methylated RUNX3 sequences in the serum of gastric cancer patients. Anticancer Res. 2009;29:2619-2625. [PubMed] |
| 170. | Zhang Y, Ye X, Geng J, Chen L. Epigenetic inactivation of deleted in lung and esophageal cancer 1 gene by promoter methylation in gastric and colorectal adenocarcinoma. Hepatogastroenterology. 2010;57:1614-1619. [PubMed] |
| 171. | Hibi K, Goto T, Shirahata A, Saito M, Kigawa G, Nemoto H, Sanada Y. Detection of TFPI2 methylation in the serum of gastric cancer patients. Anticancer Res. 2011;31:3835-3838. [PubMed] |
| 172. | Ng EK, Leung CP, Shin VY, Wong CL, Ma ES, Jin HC, Chu KM, Kwong A. Quantitative analysis and diagnostic significance of methylated SLC19A3 DNA in the plasma of breast and gastric cancer patients. PLoS One. 2011;6:e22233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 173. | Raja UM, Gopal G, Rajkumar T. Intragenic DNA methylation concomitant with repression of ATP4B and ATP4A gene expression in gastric cancer is a potential serum biomarker. Asian Pac J Cancer Prev. 2012;13:5563-5568. [PubMed] |
| 174. | Shirahata A, Sakuraba K, Kitamura Y, Yokomizo K, Gotou T, Saitou M, Kigawa G, Nemoto H, Sanada Y, Hibi K. Detection of vimentin methylation in the serum of patients with gastric cancer. Anticancer Res. 2012;32:791-794. [PubMed] |
| 175. | Balgkouranidou I, Karayiannakis A, Matthaios D, Bolanaki H, Tripsianis G, Tentes AA, Lianidou E, Chatzaki E, Fiska A, Lambropoulou M. Assessment of SOX17 DNA methylation in cell free DNA from patients with operable gastric cancer. Association with prognostic variables and survival. Clin Chem Lab Med. 2013;51:1505-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 176. | Xu W, Zhou H, Qian H, Bu X, Chen D, Gu H, Zhu W, Yan Y, Mao F. Combination of circulating CXCR4 and Bmi-1 mRNA in plasma: A potential novel tumor marker for gastric cancer. Mol Med Rep. 2009;2:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 177. | Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, Huang D, Chen X, Zhang H, Zhuang R. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 178. | Liu H, Zhu L, Liu B, Yang L, Meng X, Zhang W, Ma Y, Xiao H. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 179. | Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY, Yuasa Y, Kang D, Kim YS, You WC. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One. 2012;7:e33608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 180. | Tsai KW, Liao YL, Wu CW, Hu LY, Li SC, Chan WC, Ho MR, Lai CH, Kao HW, Fang WL. Aberrant expression of miR-196a in gastric cancers and correlation with recurrence. Genes Chromosomes Cancer. 2012;51:394-401. [PubMed] |
| 181. | Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol. 2012;138:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 182. | Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang L, Zhao C, Tao Y, Xu W. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Rep. 2012;5:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 183. | Zhang WH, Gui JH, Wang CZ, Chang Q, Xu SP, Cai CH, Li YN, Tian YP, Yan L, Wu B. The identification of miR-375 as a potential biomarker in distal gastric adenocarcinoma. Oncol Res. 2012;20:139-147. [PubMed] |
| 184. | Gorur A, Balci Fidanci S, Dogruer Unal N, Ayaz L, Akbayir S, Yildirim Yaroglu H, Dirlik M, Serin MS, Tamer L. Determination of plasma microRNA for early detection of gastric cancer. Mol Biol Rep. 2013;40:2091-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 185. | Kim SY, Jeon TY, Choi CI, Kim DH, Kim DH, Kim GH, Ryu DY, Lee BE, Kim HH. Validation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancer. J Mol Diagn. 2013;15:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 186. | Komatsu S, Ichikawa D, Tsujiura M, Konishi H, Takeshita H, Nagata H, Kawaguchi T, Hirajima S, Arita T, Shiozaki A. Prognostic impact of circulating miR-21 in the plasma of patients with gastric carcinoma. Anticancer Res. 2013;33:271-276. [PubMed] |
| 187. | Li C, Li JF, Cai Q, Qiu QQ, Yan M, Liu BY, Zhu ZG. MiRNA-199a-3p: A potential circulating diagnostic biomarker for early gastric cancer. J Surg Oncol. 2013;108:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |