Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3223
Revised: January 24, 2014
Accepted: February 20, 2014
Published online: March 28, 2014
Processing time: 181 Days and 14 Hours
Inflammatory bowel disease (IBD), which comprises ulcerative colitis and Crohn’s disease, is characterized by inflammation of the gastrointestinal tract. The trefoil factors 1, 2, and 3 (TFF1-3) are a family of peptides that play important roles in the protection and repair of epithelial surfaces, including the gastrointestinal tract. TFFs may be involved in IBD pathogenesis and are a potential treatment option. In the present review, we describe the TFF family and their potential role in IBD by summarizing the current knowledge of their expression, possible function and pharmacological role in IBD.
Core tip: Ulcerative colitis and Crohn’s disease are characterized by mucosal inflammation. The trefoil factor (TFF) family consists of three peptides, TFF1, TFF2 and TFF3, and all are widely distributed in the mucous membranes of the gastrointestinal tract. The TFFs facilitate a significant role not only in mucosal repair but also in protecting mucous epithelia from a variety of insults. This review describes the trefoil factor family and the role of the peptide family in relation to inflammatory bowel disease (IBD), and we summarize the current knowledge of their expression, possible function and potential pharmacological role in IBD.
- Citation: Aamann L, Vestergaard EM, Grønbæk H. Trefoil factors in inflammatory bowel disease. World J Gastroenterol 2014; 20(12): 3223-3230
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3223.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3223
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two most common inflammatory bowel diseases (IBDs). The etiologies of both diseases are unknown but are considered to be multifactorial, involving the genetic composition of an individual, the commensal gut flora and the environment[1].
Studies of the mucosal barrier indicate that trefoil factors (TFF) facilitate a significant role not only in mucosal repair but also in protecting mucous epithelia from a variety of insults in the gastrointestinal tract[2]. In this respect, repair is essential for preventing inflammation and ulceration. In conjunction with other mechanisms, several products that are primarily secreted from the goblet cells, including TFF and mucins form the innate immune response and first line of defense in the mucus layer. How this fully occurs is still only partly understood[3].
In mammals, the trefoil factor family consists of three peptides: TFF1, TFF2 and TFF3; all three are widely distributed in the gastrointestinal tract and are present in virtually all mucous membranes. The importance of TFFs in the protection and repair of epithelial surfaces is well established[4].
TFF1 and TFF3 each have one trefoil domain, while TFF2 has two trefoil domains. The trefoil domain is characterized by a sequence of amino acid residues, in which 6 cysteines are linked by 3 disulphide bonds to form the “trefoil” disulphide loop structure or the clover-like shaped structure[5]. The resistance of the peptides to proteolytic digestion, acids and thermal degradation seems to be caused by the compact trefoil structure of the peptides[6,7]. TFF1 and TFF3 only contain one trefoil domain but have a seventh free cysteine, which is essential for the formation of dimers[6]. It is not clear whether the main part of naturally occurring TFF1 and TFF3 consists of monomers or dimers[2].
TFF2, formerly known as the Pancreatic Spasmolytic Polypeptide, was the first TFF to be isolated in the early 1980s from a side-fraction obtained during the purification of insulin from porcine pancreas[7]. The human homologue of TFF2 is produced primarily by mucus neck cells in the body and in antral glands of the stomach, while a small amount is expressed in Brunner’s gland in the duodenum[8,9]. The cloning of an estrogen-regulated gene from the MCF-7 human breast cancer cell line resulted in the identification of pS2, which is today known as TFF1[10]. TFF1 is also produced in the stomach by superficial gastric foveolar cells[11]. It was also discovered that these peptides share a new sequence motif, named the trefoil domain[5]. A third trefoil factor, TFF3, was identified in 1991 as a rat cDNA sequence[12] and a human cDNA sequence in 1993[13] that was initially known as the intestinal trefoil factor (ITF). TFF3 is expressed in the goblet cells of the small and large intestine[14] and is co-produced and secreted with mucin (MUC)2[15].
This review describes the TFF family and the role of this family as it relates to IBD and summarizes the current knowledge of their expression, possible function and pharmacological role in IBD.
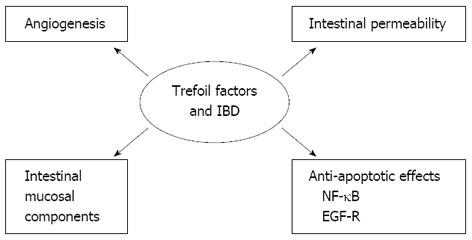
The physiologically relevant functions of TFFs are not clear, and the important question of how TFFs work remains unresolved. Do they work by cross-linking with mucins, via a receptor, or in a completely different way? Data suggest that TFFs may have multiple cellular functions that support their protective and repair functions[16,17]. Below, we describe the potential mechanisms involving anti-apoptotic properties, migration and invasion, angiogenesis, and interaction with mucins (Figure 1).
Anti-apoptotic properties are very important for epithelial restitution, where epithelial cells must migrate over the denuded area of the gut mucosa. In this process, the epithelial cells are vulnerable to apoptosis or anoikis, which is the form of apoptosis that is induced by anchorage-dependent cells detaching from the surrounding extracellular matrix. TFFs have been found to have anti-apoptotic effects in several cell lines[18,19], and this effect has been supported by the finding that TFF3-deficient mice have increased numbers of apoptotic cells in their colonic crypts[17]. Furthermore, TFF3 has anti-anoikic effects on intestinal epithelial cells via its activation of nuclear factor-kappa B (NF-κB)[20]. This effect of TFF was dependent on the activation of epidermal growth factor receptor (EGFR) and required TFF3 dimer[18].
An abnormal distribution or expression of tight junction proteins in gastrointestinal epithelial cells, which causes barrier dysfunction, is thought to be involved in IBD pathogenesis[21]. The effect of TFF3 on increased intestinal permeability and its association with tight junction proteins was evaluated in an in vitro intestinal epithelia barrier model in which colorectal epithelial cells were treated with platelet-activating factor (PAF). The analysis revealed that TFF3 suppressed the PAF-induced down-regulation of the tight junction proteins claudin-1 and zonula occludens-1. These proteins maintain the tight junction’s integrity and intestinal barrier function, and TFF3 thereby decreases mucosal permeability[22]. TFF3 induces the recovery of tight junction protein changes, which contributes to the TFF3-mediated stabilization and maintenance of intestinal epithelial barrier function. The findings may provide new insight into the protective functions of TFF3 in epithelial cells and demonstrate its potential for treatment of IBD[22,23].
The proliferative phase of wound healing is characterized by angiogenesis and pro-angiogenic properties, which are dependent on cyclooxygenase-2 and EGFR signalling and have been described for all TFFs[24].
Alterations in the intestinal mucous components may impair the barrier function of the mucin layer and may be a contributing factor to IBD[25]. In IBD, the mucin types and expression are affected by several factors. For example, the numbers of mucin-producing goblet cells are reduced in active disease and changes in the thickness and composition of the mucous gel layer may occur[26]. Although MUC2 is the major colonic mucin, alterations in the composition and concentrations of colonic mucins occur in IBD[26,27].
Several studies support the hypothesis that TFFs interact with mucins to enhance the mucosal barrier. TFFs and mucins are co-localized in the gut. When TTF3 and mucin were combined, they were more effective in protecting epithelial cells in an in vitro model of epithelial barrier, which indicates a joint effect in mucosal protection[28]. The TFFs may act differently when coupled with specific mucins, which is supported by the finding that each TFF co-localizes with its own unique mucin type in the ulcer-associated cell lineage (UACL) and normal gastrointestinal mucosa. For example TFF1 couples with MUC5AC, TFF2 couples with MUC6 and TFF3 couples with MUC2[29].
The combination of mucins and TFFs has been demonstrated to protect cell monolayers against injurious agents by increasing mucus viscosity and decreasing proton permeation[28,30]. TFF2 in particular has been shown to increase the viscosity and elasticity of porcine gastric mucus and may contribute to a more resilient protective barrier than TFF3. Conversely, TFF1 and TFF3 do not increase the viscosity of mucus but instead form small complexes with the mucins[31], which may be beneficial in the intestines. TFF1 binds to the von Willebrand Factor C domain of MUC2[32]. TFF3 was recently reported to form a disulfide-linked heteromer with IgG Fc binding protein, which could contribute to the stability of the mucin network in the mucus layer by interacting with MUC2 mucin[33].
In addition to the direct stabilization of the surface mucous layer, TFF-mucin interactions have also been shown to promote cellular effects such as cell migration and NO production[34,35].
In animal models of IBD induced by the intrarectal administration of 5% acetic acid, various and conflicting patterns of altered/increased TFF expression have been observed[36]. A possible explanation for the conflicting results observed in studies exploring the effects of TFFs in the treatment of gastrointestinal damage may be that different TFF forms, dosages and administration routes have been used in colitis models.
In general, the expression pattern of the TFFs in animal models differs from the pattern observed in humans, but the models have been very useful for investigating, for example, the temporospatial expression of TFFs after the induction of damage and the possible use of TFFs for pharmacologic intervention in IBD.
In vivo studies have clearly shown that TFFs have protective and healing effects when given exogenously following either enteral or parenteral administration. This finding suggests that TFFs might be useful in the IBD treatment. In this instance, rodent colitis models have been useful for examining the relationship between intestinal damage and the expression of the TFFs and thus examining the role of exogenously added TFFs in epithelial repair during instances of injured mucosa.
Mashimo et al[37] showed that mice lacking TFF3 had impaired mucosal healing, with poor epithelial regeneration after injury; those mice died from extensive colitis after oral administration of dextran sulfate sodium (DSS), an agent that causes mild epithelial injury in wild-type mice. The same was observed following chemotherapy and radiation-induced damage[38]. In addition, luminal treatment with recombinant TFF3 (rTFF3) restored the capacity for restitution in TFF3-knockout mice exposed to DSS and radiation-induced damage[37,38].
Although several animal studies have documented the effect of treatment with TFFs, the optimal administration route for treatment with TFFs remains unclear. In animal models of gastric ulceration, both oral and systemic treatments with TFFs are effective for protection, prevention and healing[39-43]. Subcutaneous infusions of recombinant TFF2 (rTFF2) or porcine TFF2 (pTFF2) decreased acute gastric ulceration damage by 50% without changing the gastric acid secretion[39,40]. In the gastric ulceration model, both orally and subcutaneously administered TFF2 had an effect; however, both treatments aggravated duodenal ulcerations. After oral administration, pTFF2 is bound to the mucus layer of the stomach and small intestine, but it does not reach the colonic mucosa[41]. The same dose of pTFF2, given subcutaneously, was superior to oral pTFF2 treatment. When administered orally as a prophylactic treatment, hTFF2 had a protective function in Non-steroidal anti-inflammatory drug (NSAID)-induced damage in rat gastric mucosa[42], whereas both rTFF2 and rTTF3 prevented ethanol- and indomethacin-induced gastric injury when given up to 2 h before injury. However, following intraperitoneal administration, the rTFF2 treatment had no effect[43].
The effects of TFFs have also been demonstrated in animal models of intestinal inflammation and damage. Here, the optimal route of administration is unclear, with some studies favoring oral treatment and others favoring systemic treatment[44-46]. In a DSS-induced experimental colitis model, pre-treatment with subcutaneously or intracolonically administered TFF2, ameliorated the clinical course of this chemically induced colitis, with the luminal route being superior to the parenteral route[44]. In another study, the distribution profile of subcutaneously and intraperitoneally administered TFF3 was very similar to the intravenous distribution, with a high uptake of tracer in the kidney and gastrointestinal organs. TFF3 availability was slightly faster following sc administration than following ip administration, and both administration routes would yield comparable pharmacological effects[45]. The molecular forms of TFFs also seem to play a role. In a DSS colitis model and in a model of colitis induced by the intraperitoneal injection of mitomycin luminal treatment with dimeric TFF3 was effective, whereas treatment with monomeric TFF3 had no effect. It is worth mentioning that a systemic TFF3 monomer treatment intensified the mucosal insults[47]. In a previous study, the subcutaneous administration of the hTFF1 dimer was proven to be more compelling than the TFF1 monomer[48].
The DSS model is considered to be suitable for studying acute epithelial damage, but the model lacks the chronic inflammation characteristics of IBD. In colitis models that are more representative of IBD, such as the dinitrobenzene sulphonic acid model, hTFF2 has shown enhancing effects on colonic epithelial repair and a decrease in local inflammation after luminal application. In addition, endogenous concentrations of TFF2 and TFF3 were increased in the active phase of colitis and reduced to basal levels after hTFF2 treatment[49].
The chronic production of NO via inducible nitric oxide synthase (iNOS) leads to tissue damage and inflammation. In a study involving local intracolonic hTFF2 treatment, the in vitro inhibition of NO and iNOS in monocytes was observed, along with a reduction in the levels of the damaging reactive oxygen species and a decrease in colitis. These findings further indicate that TFFs exert a positive effect on mucosal protection[50].
In combination therapy, TFF3 and EGF act in a synergistic manner to stimulate cell migration in vitro and can potentially provide a more effective and safe approach for treating intestinal ulcerations[51]. This may reduce the degree of colonic injury and may prove to be useful when treating colitis in patients with a disease that is beyond the reach of enema therapy[46].
Poulsen et al[52] showed that orally given TFF did not reach the colon. Systematically administered TFF2 and TFF3 bind to the gastric mucosal surface and are transported to the lumen. Whether this occurs in the colon is uncertain, but it seems that less of the systemically administered TFF is present in the colon than in the stomach[52,53]. The intragastric administration of the TFF1-secreting Lactococcus lactis (L. lactis) in DSS-induced colitis was followed by the active delivery of TFF at the colonic mucosa. A significant protective effect was observed, which may represent a new therapeutic approach that involves the in situ secretion of TFF by orally administered L. lactis[54].
In another recent attempt to improve the application of TFFs, a recombinant adenoviral vector containing the human ITF (hITF) gene was constructed and shown both to promote cellular migration in an in vitro intestinal wound model and to improve the healing of intestinal mucosal injury[55].
Several studies have shown that the cytokines and transcription factors that are related to the immune system and important in IBD can regulate the expression of TFFs and vice versa.
The tumor necrosis factor alpha (TNF-α) triggering of NF-κB activation is known to be a proinflammatory factor in the pathogenesis of IBD and may contribute to the development of ulcerations. Toll-like receptor-4 (TLR4)/NF-κB expression is essential for the activation of human intestinal epithelial cells and the subsequent expression of cytokines. Using cell culture studies, it was shown that both TNF-α and NF-κB induced the down-regulation of TFF3 by repressing transcription and in experimental colitis, the increase in the epithelial expression of NF-κB coincided with reduced TFF3 expression during the acute phase[56].
In a more recent study, the intraperitoneal application of rTFF3 promoted a protective effect against colitis (trinitrobenzene sulphonic acid-induced) and was accompanied by a reduction in TNF-α expression in the colonic endothelium. The protective effect was also paralleled by a reduction in TLR4/NF-κB expression, indicating that hTFF3 may have therapeutic potential through the inhibition of the TLR4/NF-κB signaling pathways[57].
Podolsky et al[58] investigated the possible linkage between TLR2 that plays a key role in the innate immune system as well as in the digestive system. This possible linkage was studied for TFF3 in a DSS-induced colitis model and for TLR2 and TFF3 in knockout mice models. The oral administration of a TLR2 agonist in TFF3 and TLR2 knockout mice causes anti-apoptotic protection of the TFF3 stress-induces inflammatory intestinal mucosa. Recombinant TFF3 administration decreased morbidity and mortality during acute colonic injury in a TLR2-deficient mice model. These findings imply that TLR2 exerts diverse mucosa-protective properties in different epithelial cell types, critically suppressing mucosal apoptosis and the associated leukocyte influx during acute colitis by regulating TFF3 in goblet cells.
Several studies have indicated that TFF expression may be regulated via the EGFR; TFFs and EGF are co-expressed in the UACL cell line[59]. The transcription of TFF1 is enhanced by EGF[60], and EGFR activation is required for the auto- and cross-induction of TFFs and for the anti-apoptotic effect of TFF3[18]. Additionally all TFFs have been shown to cause transient phosphorylation of the EGFR[61]. However, no binding to the EGFR has been demonstrated, and the mechanisms remain unclear.
In a recent study, it was shown that dietary supplementation with conjugated linoleic acid, which may have anti-inflammatory effects, protected against DSS-induced colitis in a process involving the induction of TFF3[62].
Overall, multiple studies have investigated, with conflicting results, the relation between TFFs and cytokines as well as the transcription factors related to the immune system. More studies are clearly needed to describe the regulation of TFFs in IBD and thus pave the way for drug development.
Although multiple in vitro and animal studies since the discovery of TFF 30 years ago have documented the crucial role of TFFs in the epithelial restitution of the gut, few studies have been performed in IBD patients to investigate the clinical potential of TFF in IBD.
TFF are expressed in several tissues that contain mucus-secreting cells, but they are most markedly expressed in the gastrointestinal tract. At this site, each TFF is co-localized with its unique mucin type. For example, TFF1 is co-localized with MUC5AC, TFF2 is co-localized with MUC6, and TFF3 is co-localized with MUC2[63,64]. TFF1 and TFF2 are primarily located in the stomach[8,11], whereas TFF3 is predominantly present in the mucous cells of the small and large intestine[14]. Several studies have documented the supportive and protective functions of TFFs in the human gastrointestinal tract. Those studies have shown the up-regulated expression of all three TFFs at the site of mucosal damage in IBD[65,66], peptic ulcer[67] and the neoexpression of TFF1 in UC with histologically severe disease[68].
The UACL, which occurs at sites of chronic gastrointestinal ulceration including IBD expresses a number of peptides that have been implicated in the repair of damaged mucosa, such as the TFFs[13,69]. In small intestinal Crohn’s disease, TFF2 mRNA is expressed in the acinar and proximal duct cells, while TFF1 mRNA and peptide are found in the distal duct cells and in the surface cells[65]. As in normal gastrointestinal mucosa, the co-localization of specific TFFs and mucins is observed in IBD[29,68].
The co-localization of TFF3 with DMBT1 in IBD, which has been proposed to have a role in cell differentiation and growth, indicates that DMBT1-TFF3 interactions may play a role in IBD[70].
Quantitative measurements of TFFs have been important tools for elucidating the biological functions of the peptides and exploring their role as biomarkers for IBD. Larger than normal serum concentrations of TFF2 and TFF3, i.e., 2000 to 10000 and 140 to 500 times higher, respectively, have been measured in bowel discharges[71]. All three TFFs are present in sera from healthy individuals[72]; in line with immunohistochemical studies showing increased expression in IBD, the sera concentrations of the peptides were also elevated in IBD patients[73-75]. The TTF3 concentrations were significantly higher in UC patients and their levels correlated with the clinical and biochemical parameters of disease activity.
Because of large biological variations, measurements of TFFs are not useful as clinical biomarkers for disease activity in CD and UC[72,75]. However, quantitative measurements may still be important and should be included in continued research in the area.
In clinical studies TFF peptides are considered promising for the treatment of inflammatory conditions of mucous membranes. In IBD the effect of TFF3 enemas, given in combination with oral mesalazine in patients with mild-to-moderate left-sided UC, have been tested. UC patients were given a total daily dose of 750 mg of dimeric rTFF3 in 75 mL enemas (dosage concentration of 10 mg/mL), similar to the luminally administered doze used in animal models of gut injury that has proven effective. The TFF3 enema was well tolerated, but in this first human study no additional benefit of TFF3 treatment was detected compared to the effect of 5-aminosalicylic acid treatment alone[76]. One possible explanation may be the rapid decay of TFFs observed in the colon[71]. In future trials, the systemic route should be explored.
Since the discovery of the TFFs, a number of animal studies and studies on UC and CD patients have shown that the peptides are linked to inflammatory conditions in the gut. Although a significant role in mucosal protection and repair has been established for the TFFs, the full knowledge of their biological functions in IBD remains elusive. The quantitative measurements of the peptides in patients with IBD have been less promising, due to large inter-individual variations, and their future use as biomarkers in IBD is uncertain. Future studies are needed to show whether the peptides have a potential as a novel therapeutic in IBD. Additionally further identification of the regulatory mechanisms that can affect TFF expression may aid in the development of new drugs for treating IBD.
P- Reviewers: Gassler N, Marina IG, M’Koma AE, Pellicano R S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 2. | Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 468] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Podolsky DK. Mechanisms of regulatory peptide action in the gastrointestinal tract: trefoil peptides. J Gastroenterol. 2000;35 Suppl 12:69-74. [PubMed] |
| 4. | Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Thim L. A new family of growth factor-like peptides. ‘Trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989;250:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Thim L, Wöldike HF, Nielsen PF, Christensen M, Lynch-Devaney K, Podolsky DK. Characterization of human and rat intestinal trefoil factor produced in yeast. Biochemistry. 1995;34:4757-4764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Jørgensen KH, Thim L, Jacobsen HE. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept. 1982;3:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Tomasetto C, Rio MC, Gautier C, Wolf C, Hareuveni M, Chambon P, Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990;9:407-414. [PubMed] |
| 9. | Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110-1116. [PubMed] |
| 10. | Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895-7903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 569] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, Chenard MP, Batzenschlager A, Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 259] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA. 1991;88:11017-11021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 223] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Hauser F, Poulsom R, Chinery R, Rogers LA, Hanby AM, Wright NA, Hoffmann W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci USA. 1993;90:6961-6965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Podolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, DeBeaumont M, Sands BE, Mahida YR. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694-6702. [PubMed] |
| 15. | Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem. 2007;55:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Efstathiou JA, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright NA, Bodmer WF, Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3122-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci USA. 2000;97:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Kinoshita K, Taupin DR, Itoh H, Podolsky DK. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: structure-function analysis of human intestinal trefoil factor. Mol Cell Biol. 2000;20:4680-4690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Bossenmeyer-Pourié C, Kannan R, Ribieras S, Wendling C, Stoll I, Thim L, Tomasetto C, Rio MC. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J Cell Biol. 2002;157:761-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Chen YH, Lu Y, De Plaen IG, Wang LY, Tan XD. Transcription factor NF-kappaB signals antianoikic function of trefoil factor 3 on intestinal epithelial cells. Biochem Biophys Res Commun. 2000;274:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Xu LF, Teng X, Guo J, Sun M. Protective effect of intestinal trefoil factor on injury of intestinal epithelial tight junction induced by platelet activating factor. Inflammation. 2012;35:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Meyer zum Büschenfelde D, Tauber R, Huber O. TFF3-peptide increases transepithelial resistance in epithelial cells by modulating claudin-1 and -2 expression. Peptides. 2006;27:3383-3390. [PubMed] |
| 24. | Rodrigues S, Van Aken E, Van Bocxlaer S, Attoub S, Nguyen QD, Bruyneel E, Westley BR, May FE, Thim L, Mareel M. Trefoil peptides as proangiogenic factors in vivo and in vitro: implication of cyclooxygenase-2 and EGF receptor signaling. FASEB J. 2003;17:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Podolsky DK. Lessons from genetic models of inflammatory bowel disease. Acta Gastroenterol Belg. 1997;60:163-165. [PubMed] |
| 26. | Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 338] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Tytgat KM, Opdam FJ, Einerhand AW, Büller HA, Dekker J. MUC2 is the prominent colonic mucin expressed in ulcerative colitis. Gut. 1996;38:554-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 237] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, Wright NA. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Tanaka S, Podolsky DK, Engel E, Guth PH, Kaunitz JD. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997;272:G1473-G1480. [PubMed] |
| 31. | Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest. 2002;32:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Tomasetto C, Masson R, Linares JL, Wendling C, Lefebvre O, Chenard MP, Rio MC. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000;118:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Albert TK, Laubinger W, Müller S, Hanisch FG, Kalinski T, Meyer F, Hoffmann W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res. 2010;9:3108-3117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 322] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 35. | Tan XD, Liu QP, Hsueh W, Chen YH, Chang H, Gonzalez-Crussi F. Intestinal trefoil factor binds to intestinal epithelial cells and induces nitric oxide production: priming and enhancing effects of mucin. Biochem J. 1999;338:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Itoh H, Tomita M, Uchino H, Kobayashi T, Kataoka H, Sekiya R, Nawa Y. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996;318:939-944. [PubMed] |
| 37. | Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 522] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 38. | Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology. 2004;126:796-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Playford RJ, Marchbank T, Chinery R, Evison R, Pignatelli M, Boulton RA, Thim L, Hanby AM. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | McKenzie C, Marchbank T, Playford RJ, Otto W, Thim L, Parsons ME. Pancreatic spasmolytic polypeptide protects the gastric mucosa but does not inhibit acid secretion or motility. Am J Physiol. 1997;273:G112-G117. [PubMed] |
| 41. | Poulsen SS, Thulesen J, Christensen L, Nexo E, Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999;45:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Cook GA, Thim L, Yeomans ND, Giraud AS. Oral human spasmolytic polypeptide protects against aspirin-induced gastric injury in rats. J Gastroenterol Hepatol. 1998;13:363-370. [PubMed] |
| 43. | Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 188] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Soriano-Izquierdo A, Gironella M, Massaguer A, May FE, Salas A, Sans M, Poulsom R, Thim L, Piqué JM, Panés J. Trefoil peptide TFF2 treatment reduces VCAM-1 expression and leukocyte recruitment in experimental intestinal inflammation. J Leukoc Biol. 2004;75:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Kjellev S, Thim L, Pyke C, Poulsen SS. Cellular localization, binding sites, and pharmacologic effects of TFF3 in experimental colitis in mice. Dig Dis Sci. 2007;52:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | FitzGerald AJ, Pu M, Marchbank T, Westley BR, May FE, Boyle J, Yadollahi-Farsani M, Ghosh S, Playford RJ. Synergistic effects of systemic trefoil factor family 1 (TFF1) peptide and epidermal growth factor in a rat model of colitis. Peptides. 2004;25:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Poulsen SS, Kissow H, Hare K, Hartmann B, Thim L. Luminal and parenteral TFF2 and TFF3 dimer and monomer in two models of experimental colitis in the rat. Regul Pept. 2005;126:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Marchbank T, Westley BR, May FE, Calnan DP, Playford RJ. Dimerization of human pS2 (TFF1) plays a key role in its protective/healing effects. J Pathol. 1998;185:153-158. [PubMed] |
| 49. | Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Giraud AS, Pereira PM, Thim L, Parker LM, Judd LM. TFF-2 inhibits iNOS/NO in monocytes, and nitrated protein in healing colon after colitis. Peptides. 2004;25:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Chinery R, Playford RJ. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond). 1995;88:401-403. [PubMed] |
| 52. | Poulsen SS, Thulesen J, Nexø E, Thim L. Distribution and metabolism of intravenously administered trefoil factor 2/porcine spasmolytic polypeptide in the rat. Gut. 1998;43:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Kjellev S, Nexø E, Thim L, Poulsen SS. Systemically administered trefoil factors are secreted into the gastric lumen and increase the viscosity of gastric contents. Br J Pharmacol. 2006;149:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Sun Y, Zhu Y, Wang L, Mao X, Peng X, Peng Y. Recombinant adenovirus-mediated intestinal trefoil factor gene therapy for burn-induced intestinal mucosal injury. PLoS One. 2013;8:e62429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Loncar MB, Al-azzeh ED, Sommer PS, Marinovic M, Schmehl K, Kruschewski M, Blin N, Stohwasser R, Gött P, Kayademir T. Tumour necrosis factor alpha and nuclear factor kappaB inhibit transcription of human TFF3 encoding a gastrointestinal healing peptide. Gut. 2003;52:1297-1303. [PubMed] |
| 57. | Teng X, Xu LF, Zhou P, Sun HW, Sun M. Effects of trefoil peptide 3 on expression of TNF-alpha, TLR4, and NF-kappaB in trinitrobenzene sulphonic acid induced colitis mice. Inflammation. 2009;32:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 59. | Wright NA, Poulsom R, Stamp GW, Hall PA, Jeffery RE, Longcroft JM, Rio MC, Tomasetto C, Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990;162:279-284. [PubMed] |
| 60. | Nunez AM, Berry M, Imler JL, Chambon P. The 5’ flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989;8:823-829. [PubMed] |
| 61. | Liu D, el-Hariry I, Karayiannakis AJ, Wilding J, Chinery R, Kmiot W, McCrea PD, Gullick WJ, Pignatelli M. Phosphorylation of beta-catenin and epidermal growth factor receptor by intestinal trefoil factor. Lab Invest. 1997;77:557-563. [PubMed] |
| 62. | Borniquel S, Jädert C, Lundberg JO. Dietary conjugated linoleic acid activates PPARγ and the intestinal trefoil factor in SW480 cells and mice with dextran sulfate sodium-induced colitis. J Nutr. 2012;142:2135-2140. [PubMed] |
| 63. | Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16:319-334. [PubMed] |
| 64. | Matsuoka Y, Pascall JC, Brown KD. Quantitative analysis reveals differential expression of mucin (MUC2) and intestinal trefoil factor mRNAs along the longitudinal axis of rat intestine. Biochim Biophys Acta. 1999;1489:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Wright NA, Poulsom R, Stamp G, Van Norden S, Sarraf C, Elia G, Ahnen D, Jeffery R, Longcroft J, Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1992;193:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Shaoul R, Okada Y, Cutz E, Marcon MA. Colonic expression of MUC2, MUC5AC, and TFF1 in inflammatory bowel disease in children. J Pediatr Gastroenterol Nutr. 2004;38:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Rio MC, Chenard MP, Wolf C, Marcellin L, Tomasetto C, Lathe R, Bellocq JP, Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991;100:375-379. [PubMed] |
| 68. | Longman RJ, Poulsom R, Corfield AP, Warren BF, Wright NA, Thomas MG. Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. J Histochem Cytochem. 2006;54:1335-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 320] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 70. | Madsen J, Sorensen GL, Nielsen O, Tornøe I, Thim L, Fenger C, Mollenhauer J, Holmskov U. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS One. 2013;8:e64441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Kjellev S, Vestergaard EM, Nexø E, Thygesen P, Eghøj MS, Jeppesen PB, Thim L, Pedersen NB, Poulsen SS. Pharmacokinetics of trefoil peptides and their stability in gastrointestinal contents. Peptides. 2007;28:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Samson MH. Quantitative measurements of trefoil factor family peptides: possibilities and pitfalls. Scand J Clin Lab Invest. 2013;73:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Vestergaard EM, Poulsen SS, Grønbaek H, Larsen R, Nielsen AM, Ejskjaer K, Clausen JT, Thim L, Nexø E. Development and evaluation of an ELISA for human trefoil factor 3. Clin Chem. 2002;48:1689-1695. [PubMed] |
| 74. | Vestergaard EM, Brynskov J, Ejskjaer K, Clausen JT, Thim L, Nexø E, Poulsen SS. Immunoassays of human trefoil factors 1 and 2: measured on serum from patients with inflammatory bowel disease. Scand J Clin Lab Invest. 2004;64:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Grønbaek H, Vestergaard EM, Hey H, Nielsen JN, Nexø E. Serum trefoil factors in patients with inflammatory bowel disease. Digestion. 2006;74:33-39. [PubMed] |
| 76. | Mahmood A, Melley L, Fitzgerald AJ, Ghosh S, Playford RJ. Trial of trefoil factor 3 enemas, in combination with oral 5-aminosalicylic acid, for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2005;21:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |