Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2499
Revised: October 16, 2013
Accepted: January 2, 2014
Published online: March 14, 2014
Processing time: 180 Days and 23.2 Hours
Irritable bowel syndrome (IBS) is common in the society. Among the putative pathogeneses, gut dysmotility results in pain and disturbed defecation. The latter is probably caused by the effect of abnormal gut water secretion. The interaction between abnormal gas accumulation, abdominal pain and bloating remains controversial. Visceral hypersensitivity and its modification along with the central transmission are the characteristics of IBS patients. The identification of biologic markers based on genetic polymorphisms is undetermined. Imbalanced gut microbiota may alter epithelial permeability to activate nociceptive sensory pathways which in turn lead to IBS. Certain food constituents may exacerbate bowel symptoms. The impact of adult and childhood abuses on IBS is underestimated. Using the concept of biopsychosocial dysfunction can integrate multidimensional pathogeneses. Antispasmodics plus stool consistency modifiers to treat the major symptoms and defecation are the first-line drug treatment. New drugs targeting receptors governing bowel motility, sensation and secretion can be considered, but clinicians must be aware of their potential serious side effects. Psychiatric drugs and modalities may be the final options for treating intractable subjects. Probiotics of multi-species preparations are safe and worth to be considered for the treatment. Antibiotics are promising but their long-term safety and effectiveness are unknown. Diet therapy including exclusion of certain food constituents is an economic measure. Using relatively safe complementary and alternative medicines (CAMs) may be optional to those patients who failed classical treatment. In conclusion, IBS is a heterogeneous disorder with multidimensional pathogeneses. Personalized medicines with multidisciplinary approaches using different classes of drugs, psychiatric measures, probiotics and antibiotics, dietary therapy, and finally CAMs, can be considered.
Core tip: Irritable bowel syndrome (IBS) is common in the society. Patients with this disorder have a poor quality of life with severe impact on their social and economic burdens. Its pathogenesis remains evolutional, involving biological, psychiatric and social factors. Therefore, the biopsychosocial dysfunctional model has attempted to integrate all the above mentioned mechanisms in order to understand how IBS can develop under such complex interaction. Since the etiology of IBS is heterogeneous, the currently recommended treatments are multidisciplinary and also individualized, e.g., using different classes of drugs, psychiatric measures, probiotics and antibiotics, dietary therapy, and finally complementary and alternative medicines.
- Citation: Chang FY. Irritable bowel syndrome: The evolution of multi-dimensional looking and multidisciplinary treatments. World J Gastroenterol 2014; 20(10): 2499-2514
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2499
Irritable bowel syndrome (IBS) is an essential member of the functional gastrointestinal disorder (FGID) family. According to the globally accepted Rome III definition, it is characterized by chronic and recurrent abdominal pain/discomfort associated with disturbed defecation[1,2]. As a functional disorder, IBS definition remains evolutional over recent decades. For example, the Manning criteria released in 1978 are just to identify IBS. The later released Rome I-III criteria are broadly to diagnose all FGIDs including IBS. Now the Rome IV criteria are undergoing preparation but not formally announced[3]. Regarding various criteria, a review indicated that the Manning criteria are the most valid and accurate, whereas the Rome III criteria are not valid and are poorly adopted, especially for clinical trials[4]. It is also controversial whether abdomen pain is virtually required to diagnose IBS. For example, constipation-predominant IBS (IBS-C) and functional constipation are two exactly distinct FGIDs because the latter lacks obvious abdominal pain, but a study indicated that their discrepancy was not easily to detect since marked overlapping was observed between the two conditions[1,5]. Accordingly, an expert meeting recommended that current criteria to diagnose IBS need further revision, particularly the significance of abdominal bloating should be included and the pain component is best to de-emphasize[6]. Overall, IBS is common in the society with worldwide prevalence ranging from 5% to 15%[3,7-10]. The reported IBS prevalence is determined by a number of factors including subject gender, used criteria, questionnaires, study methods, locations, geographical characters, cultural and social backgrounds, and ethnicity[3,8,9,11]. Clinically, IBS is not only confined to the colon but may also extend to other organs and systems since IBS individuals usually have multiple comorbidities such as dyspepsia, gastro-esophageal reflux disease, interstitial cystitis, fibromyalgia, chronic fatigue, insomnia, headache/migraine and psychiatric disturbances[12-18]. Owing to the commonly associated somatic comorbidities and high level of psychiatric disturbances, IBS subjects often have absenteeism, reduced quality of life (QoL) and multiple healthcare seeking behaviors, which lead to great social and economic burdens[13,16,19-21]. Because IBS is a functional disorder with multi-dimensional looking, current IBS management is towards multidisciplinary approaches[1,7,22-24]. The purpose of this review attempts to introduce what are the updated pathogeneses and managements of IBS based on the multi-dimensional looking and multidisciplinary approaches.
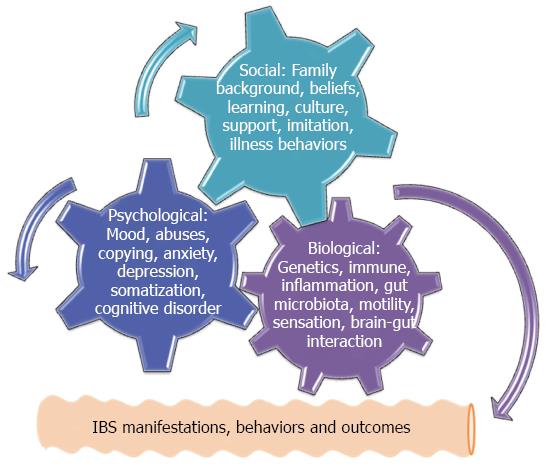
Current mechanisms to address IBS pathogenesis consist of the defects involving biological, chemical, physical, environmental, economic, cultural, moral, and spiritual events, particularly these defects may interact with each other and lead to IBS. Overall, these mechanisms can be simply categorized into three major issues in terms of biological defects, psychological disturbances and social impacts[1,25]. In order to illustrate and understand why a disease or disorder will develop under the complex interaction involving many mechanisms, the framework of a biopsychosocial model has been introduced to unify biological, psychological and social issues together to indicate their final interaction[25]. Figure 1 briefly depicts that the original existence of any defects among three categories during the early life and adolescent period may initiate biopsychosocial interaction and the following IBS symptoms.
Alternatively, genetics- or environment-determined biological defects at any level of neural control and modulation of gut motility, digestion, sensation, endocrine, secretion and immune functions may result in IBS symptoms, while the psychological disturbances which are closely related to a number of social impacts such as early life abuses, stresses, social learning, and copying patterns are able to trigger neuroimmune reactions via the brain-gut axis and lead to exacerbated IBS symptoms[25]. Most importantly, the biopsychosocial model is characterized by bi-directional causality and feedback. Accordingly, any adolescent modification coming from the biological, psychological and social impacts manifests different levels of symptoms, behaviors and outcomes of IBS in adulthood, while their symptomatic manifestations are in turn to modify the existent psychological and social events[1,25-27]. This is why many associated comorbidities are reported among IBS subjects. Interestingly, the biopsychosocial model is not only confined to the IBS but also adopted in many pain related disorders such as migraine, tension headache, chronic fatigue syndrome, and fibromyalgia[28,29]. Thereafter, a concept of central sensitivity syndromes is proposed to unite these comorbidities that apparently share the same biopsychosocial dysfunction[30,31]. Although the unified biopsychosocial model can help easily to understand how IBS will develop under this interaction, the individual pathogeneses are worthy of being introduced before their final integration.
Based on the predominant defecation pattern, various IBS subtypes are traditionally defined[1]. Accordingly, it is reasonable to speculate that bowel dysmotility may result in IBS, particularly the disordered defecation. For instance, abnormal small intestinal motility was indicated to lead to IBS in some subjects[32]. In addition, rapid small intestinal transit among the diarrhea-predominant IBS (IBS-D) subjects and delayed transit in IBS-C subjects were reported[33,34]. Using ingested radiopaque markers to count scattering index representing small intestinal transit, another study pointed out the same transit among three categories in terms of IBS-C, IBS-D and control subjects[35]. Accordingly, observed small intestinal dymotility is likely to exist in certain IBS subjects, but the intra- and inter-individual variations in motility measurements limit their interpretation of small intestinal dysmotility in clinical usefulness[36].
Defecation is a complex event involving the coordination of colon transit, high amplitude propagated contractions (HAPC) and pelvic floor synergia while the integrated central (CNS), autonomic and enteric nervous systems (ENS) are virtually required to mediate their correct process[37-39]. Abnormal colon motilities have been observed among IBS subjects. For example, the total colonic transit time in IBS-D patients measured by ingested radiopaque markers was prolonged after pinaverlum bromide treatment. The effectiveness of this agent to treat IBS-D appears via correction of abnormal colon transit[40]. Similarly, a radiopaque study confirmed again that Japanese IBS-D subjects had accelerated colon transit compared to controls, whereas those in IBS-C subjects and controls were the same[35]. Left colonic segmentation pressure waves and HAPC were altered among some non-IBS-C patients[41]. Besides, certain IBS-C patients had delayed total colon and right segmental transit[42]. Like small intestinal transit, it is concluded that abnormal colon transit probably exists in some, but not all IBS subjects, because IBS is heterogeneous in its pathophysiology.
Regarding the autonomic nervous activity, IBS-D subjects manifested an enhanced adrenergic sympathetic dominance compared with controls and IBS-C subjects, while this dominance was likely the effect of vagal withdrawal rather than true enhancement[43]. As one of mechanisms leading to functional constipation, pelvic floor dyssynergia was also observed in some non-IBS-D patients[34,44]. Since pelvic floor dyssynergia obviously overlaps with the spectrum of functional anorectal disorder defined by the Rome III criteria[25], it is debatable what is the demarcation between IBS and functional anorectal disorder. Overall, colonic dysmotility probably exists in certain, but not all IBS patients. Using various colon motility measurements to diagnose IBS may be unreliable.
Gut water component has been a main factor to determine hard or loose stool. IBS subtypes are traditionally classified by the predominant stool pattern. Alternatively, it means that the gut water secretion in IBS subjects may be different. Unlike other mechanisms that are extensively evaluated, only a few studies have addressed this issue. For example, a rat IBS model study pointed out that the fecal water content was lower in IBS-C rats, whereas an excessive secretion existed in the IBS-D group[45]. The densities of some peptides mediating gut motility, secretion and sensation, e.g., serotonin, peptide YY, pancreatic polypeptide, enteroglucagon, somatostatin, etc. were obviously reduced in human IBS colon. It looks to mean that the abnormal gut water secretion is one of components leading to IBS[46]. In addition, using lubiprostone with the ability to increase gut water secretion in softening stool for IBS-C subjects appears to support the role of gut water secretion in IBS[47]. Overall, the abnormal gut water secretion should not be forgotten as a candidate of IBS pathogeneses.
Both abdominal bloating and fullness are common among IBS subjects. Therefore, abnormal bowel gas accumulation may account for these annoying symptoms[6]. Unfortunately, bowel gas studies report conflicting results. An earlier study did not find abnormal bowel gas accumulation among the very limited IBS like subjects[48]. In contrast, IBS patients had impaired transit and tolerance to the loading of intestinal gas[49]. A Japanese study pointed out the excessive bowel gas volume among IBS subjects. However, neither symptoms nor subtypes correlated well with abnormal bowel gas accumulation[50]. This means that other factors apart from bowel gas may be responsible for the bloating symptom. Alternatively, bloating symptom is additionally associated with visceral hypersensitivity and delayed transit, and the impaired gas handling may be observed in some, but not all IBS subjects[51].
Abdominal pain has been a key component of IBS. It is expected that visceral hypersensitivity may account for IBS. Studies using rectal balloon distension repeatedly confirmed that IBS subjects have diminished threshold and exaggerated painful severity to balloon distension[41]. Accordingly, visceral hypersensitivity appears a candidate of biological hallmark to diagnose IBS[52]. In fact, hypersensitivity among IBS subjects is not only confined to the colon but also extends upward to CNS[53-55]. For example, abnormal activation of certain brain regions following painful rectal stimulation confirmed the altered processing of afferent signals along the brain-gut axis[54]. Visceral hypersensitivity is additionally modified by the gender, peptide, immune and emotional factors[14,25,56,57]. The central projection and modulation of visceral pain are complex, and many transmitted tracts have not been clearly revealed. It is believed that prefrontal lobe may modulate the neural activities coming from limbic and paralimbic regions, anterior cingulate cortex, and hypothalamus, which in turn down modifies the activities of descending inhibitory and facilitatory pathways through the periaqueductal gray and pontomedullary nuclei. The neuronal activities among these cortico-limbic pontine networks can coordinate the final perception of cognitional and emotional impacts on the visceral pain and discomfort in IBS subjects[56,58].
Based on the neuroimage technique, IBS subjects were observed to have long-term micro-structural brain changes, particularly the regions integrating sensory information and cortico-thalamic modulation[59]. These observed brain structural changes among IBS patients appear to challenge the concept of IBS as a functional disorder without existing structural abnormality. The altered functional connectivity between brainstem pain-modulating circuits and cortical-limbic centers suggests a bi-directional interaction between pain and mood. Interestingly, this dysfunctional pain network not only exists in IBS but also is observed among other comorbidities, e.g., migraine, fibromyalgia, anxiety disorders, etc.[60]. Allodynia is a pain condition originated from a stimulus, which does not normally provoke pain. Alternatively, it is a central hypersensitivity phenomenon with diminished threshold to triggers[61]. Apart from visceral hypersensitivity, IBS subjects also had cutaneous allodynia following a number of repetitive nociceptive thermal stimuli[62,63]. Overall, the broadly existing somatic, visceral and central hypersensitivities support why IBS patients always have multiple somatic and psychiatric comorbidities.
Twins are an ideal model to resolve whether genetics or environmental factor is essential to determine IBS in a family. Unfortunately, the results of twin studies are conflicting. Concordance for IBS was significant among monozygotic (17.2%) twins compared to dizygotic (8.4%) twins[64,65]. In contrast, similar prevalences were reported between monozygotic (17%) and dizygotic (16%) twins[66]. A meta-analysis based on twin studies further indicated that heritability is more significant among migraineurs (50%) compared to IBS subjects (25%)[67]. It means that both environmental factors and learning behaviors, rather than the heredity only, are the necessary determinants leading to IBS. This viewpoint confirms again that IBS is most likely the final result of biopsychosocial dysfunction involving the interaction of genetically determined biological and psychological factors and exposed environmental factors coming from biological, psychological and social events. Of mitochondrial dysfunctions and associated DNA sequence variants of maternal inheritance, 60% were related to bowel dysfunction, whereas 16% were probable non-maternal inheritance. This suggests that defective mitochondrial energy metabolism among matrilineal relatives probably leads to FGIDs including IBS[68]. Overall, genetics may be a factor leading to IBS, but environmental and learning factors are also involved.
There are numerous peptides/substances and their corresponding receptors that are involved in IBS pathogenesis. Their roles are mainly to mediate gut motility, sensation, permeability, secretion and immune response. The most frequently addressed peptides include 5-hydroxytyptamine (5-HT), cholecystokinin, glucagon-like peptide, somatostatin, neuropeptide Y, endocannabinoid, vasoactive intestinal polypeptide, corticotropin releasing hormone (CRH), etc.[1,7,23,41,46,69-72]. For example, the fact that 5-HT related agonists and antagonists have been developed effectively to treat either IBS-C or IBS-D patients strongly suggests that certain peptide dysfunction is one of important mechanisms leading to IBS[1,7,23]. Second, human IBS colon was observed to have low densities of gut peptides including serotonin, peptide YY, pancreatic polypeptide, enteroglucagon, somatostatin, etc.[46]. Third, CRH has been a main mediator of stress response in the brain-gut axis, while IBS is believed a dysfunctional brain-gut link which can be exaggerated via CRH related stress[71].
Peptide abnormalities among IBS subjects are sometimes genetically determined. Accordingly, variation of genotypes or polymorphisms among those genes governing peptide synthesis and metabolism, mucosal ion channel functions, reuptake of neurotransmitters and their optimal functioning in receptors, and inflammation susceptibility may account for the IBS phenotypes and symptomatic severity[73]. Some genetic polymorphisms have been identified in relation to IBS even with impacts on the therapeutic response, e.g., CRH-R1 gene polymorphism of TT genotype of rs7209436 and rs242924 among Japanese IBS patients, SS genotype of serotonin reuptake transporter polymorphism among Indian C-IBS subjects, mitochondrial adenosine triphosphate 6 and 8 polymorphisms among Chinese IBS-D patients, and serotonin transporter promoter genetic polymorphisms influencing response to alosetron therapy among American IBS-D patients[74-77]. Current IBS candidate genes consist of serotonin transporter (SLC6A4), norepinephrine transporter (NET), alpha-2A-adrenergic receptors (ADRA2A), interleukin-10 (IL-10), G protein β3 subunit (GNβ3), sodium channel (SCN5A), etc.[78]. Regarding genes controlling inflammation, a meta-analysis indicated that high producer IL-10 (-1082 G/G) polymorphism diminishes the IBS risk in the European IBS population, whereas tumor necrotic factor (TNF) (-308 G/G) polymorphism increases IBS susceptibility and TNF (-308 G/A) polymorphism decreases IBS susceptibility in the Asian IBS population[79]. Overall, IBS genetic polymorphism studies are criticized with drawbacks of very limited case number, inconsistent results, lack of reproducibility, heterogeneous nature of IBS, etc., while no single gene is globally confirmed to be responsible to IBS[80]. Nevertheless, genetic polymorphisms or pharmacogenetics open a door using an optimal substance to treat appropriate subjects via proper genetic mapping in the future.
Human fetus is initially sterile before birth and begins to be infected by many microorganisms since birth through the contact with external environment, while the human immune system is gradually maturing to adapt and tolerate the challenge of exposed microorganisms. Among organs with microorganism residence, the colon owns the most number of resided microorganisms[81]. In fact, the colon microbiota provides numerous physiologic events, namely, supplying energy, nutrient accessibility including short-chain fatty acids, enhancing immune and normal homeostasis, influencing organ development such as morphogenesis of the bone and visceral organs, and even the host metabolism[81,82]. Regarding their clinical impact, inflammatory bowel disease has been the consequence of uncontrolled and imbalanced gut microbiota with altered defense system, permeability and immune response[83]. Similarly, dysfunctional gut microbiota may activate mucosal innate immune responses, which in turn increase epithelial permeability, activate nociceptive sensory pathways, dysregulate the ENS, and finally lead to various FGIDs including IBS. For example, a 16S rRNA-based microbiota profiling study demonstrated both quantitative and qualitative changes of mucosal and fecal gut microbiota among IBS subjects[84]. Second, Japanese IBS subjects had much higher counts of Veillonella and Lactobacillus than controls, while the products of microbiota such as acetic acid, propionic acid and total organic acids were also significantly higher among these subjects[85]. Third, the methanogenic flora in North Indian IBS patients measured via lactose hydrogen breath test was lower compared to controls and this observation was suggested to be the nature of flatulence among them[86].
Apart from the suggested alteration in brain gut axis functions, colonic immunological changes such as chronic and low-grade immune activation are reported among IBS patients. The mediators released by these immune responses may have an impact on the functions of gut mucosal permeability and nerves, leading to the further closed interaction between the immune system and the brain gut axis and finally the observed IBS symptoms[87,88]. For example, post-infectious IBS is to address a phenomenon that previous enteritis may be followed with IBS symptoms, particularly the IBS-D seen months later[89]. Briefly, these patients have excessive numbers and increased activation of mucosal immune cells including mast cells and lymphocytes. In addition, releasing factors such as proteases, histamine, and prostanoids attenuate permeability and activate abnormal neural response, leading to abdominal pain and changed bowel habits, which correlate well with IBS symptoms[88,89]. In addition, psychological stress and activation of Toll-like receptors are also involved in the neuroimmune response among these subjects[56,57]. Besides, antibiotic therapy reduced stress induced visceral hypersensitivity, enhanced bacterial wall adherence and increased luminal s-IgA levels in dysbiotic mice[90]. Considered together, emotional stress, gut microbiota and host immune system interact with each other to respond with altered bowel motor, sensory and secretory functions observed among IBS subjects.
The experience of certain food ingestion and its following abdominal symptoms are common among the population. For example, acute chili ingestion aggravated abdominal pain and burning symptoms of FGID subjects[91]. Regarding the self-reported food elicited bowel symptoms of IBS subjects, most of them believed that certain diets such as beans, apple, flour, and plum could trigger bowel symptoms, particularly those foods rich in carbohydrates, fat, and biogenic amines such as milk, wine and pork, while women reported more intolerable food items than men[92]. On the other hand, an objective study indicated that IBS patients did not consume different food calories and constituents, but they usually tried to avoid diets rich in fermentable oligo-, di-, monosacharides, and polyols (FODMAP), and their diets often contained low contents of calcium, magnesium, phosphorus, vitamin B2 and vitamin A[93]. Regarding the relationship between ingested food and gut microbiota composition, a recent study observed that IBS subjects consuming a restriction diet with a lower content of fermentable short-chain carbohydrates for 4 wk had adequate relief of bowel symptoms, while the concentration and proportion of luminal bifidobacteria were diminished together[94]. In summary, food owing to its certain components seems to be a factor leading to IBS, but the food intolerance of IBS subjects does not mean food allergy.
Childhood abuses including sexual issue are the significant worldwide health burden. For example, abuse has been a main risk factor leading to health problems including shaken baby syndrome and behavioral regression during the developmental period, while its long-term risks consist of mental health disorders, substance use disorders and chronic physical complains in the later adult life[95]. Unfortunately, both physical and sexual abuses are common and underestimated among IBS patients[96]. In addition, these victims often manifest severe pain perception, psychological distress, and poorer health outcome[97]. Their perceptive pattern was already centrally confirmed via advanced neuroimage to show an enhanced nociception[98].
Early life trauma is able to increase future visceral pain perception. Accordingly, maternally separated neonatal rodents are used to create a model to study the relationship between early life stress, visceral sensation and depression related disorders including IBS. It was indicated that water avoidance stress increased pain perception and activated somatosensory cortex, periaquaductal gray and hippocampus in the maternally separated rats[99]. In addition, maternally separated rats had significantly increased 5-HT content after colorectal distension[100]. This model also pointed out that the colon of maternally separated rats had elevated circulating levels of interleukin-6 in addition to gut dysfunction[101]. Considered together, neonatal maternal separation appears a stress in rats with exacerbated neurochemical, inflammatory responses, and visceral hyperalgesia in the colon and CNS comparable to IBS subjects. It is of interest whether the neonatal separation story does truly happen in the society leading to IBS. A study to explore the childhood events among IBS adults confirmed that loss and separation during childhood, in the current family and conflicted or dependent maternal relationships were common among some IBS patients[102]. In summary, avoidance of any kind of childhood abuses is necessary to demolish future adult onset of IBS, FGIDs and psychiatric events.
With regard to IBS treatment, patient-centered approach with a strong and effective communication between patients and clinicians has been emphasized to increase the treatment satisfaction and diminish utilization of health care sources[23,103]. In fact, the development of active drugs to exhibit an efficacy greater than placebo in treating heterogeneous IBS is not easily to achieve, because IBS subjects often experience an excellent efficiency up to 40%-50% to placebo treatment[23,104]. Psychologically, placebo effect is believed the total response of treating expectancy, repetitive administration named conditioning and a non-specific psychological effect supported from givers. Now the placebo effect could be well confirmed in the brain via functional neuroimage[54]. Table 1 summarizes the multidisciplinary approaches that are optional to treat IBS.
| Category | Functions | Examples |
| Antispasmodics | Antagonists of muscarinic receptors and calcium channels of smooth muscle | Cimetropium bromide, dicyclomine, hyoscine butylbromide, mebeverine, otilonium bromide, peppermint oil, pinaverium bromide, trimebutine maleate |
| Antidiarrheals | Agonists of μ-opioid receptors | Loperamide |
| Laxatives | Osmotic, stimulant | Bisacodyl, lactulose, magnesium citrate, magnesium sulfate, polyethylene glycol |
| Bulking agents | Water binding to increase stool bulk | Methylcellulose, psyllium, wheat bran |
| Receptor targeted new drugs | Agonists and antagonists of 5-HT | Alosetron, cilansetron, naronapride, prucalopride, ramosetron, tegaserod |
| Chloride channel activators | Lubiprostone | |
| Agonists of GC-C | Linaclotide | |
| Antagonists of NK1 receptors | Ezlopitant, TAK 637 | |
| Agonists of κ-opioid receptors | Asimadoline | |
| Agonists of α2 adrenergic receptors | AGN-203818, clonidine, solabegron | |
| Antagonists of CCK1 receptors | Loxiglumide | |
| Agonists of somatostatin receptors | Octreotide | |
| Psychiatrics | Tricyclic antidepressants | Amitriptyline, desipramine, doxepin, imipramine, trimipramine |
| SSRIs | Citalopram, fluoxetine, paroxetine, venlafaxine | |
| Psychotherapy | Biofeedback, cognitive behavioral therapy, dynamic psychotherapy, hypnotherapy, relaxation training | |
| Probiotics | To balance gut microbiota | VSL-3, lactobacilli, bifidobacteriae |
| Fecal transplantation | Living microbiota supplement | Through nasogastric tube, enema or colonoscopy |
| Anti-inflammation | Mast cell stabilizers, PAR-2 blockers TRPV receptor type 1 and 4 blockers | Capsazepine, GB88, ketotifen, RN1734 |
| Antibiotics | To inhibit gut microorganisms | Neomycin, rifaximin |
| Miscellaneous | Antinociceptive substance | Melatonin |
| Bile acid sequestrant | Cholestyramine | |
| To diminish inflammation? To absorb bacteria and enterotoxins? | Diosmectite | |
| Food | To enhance immunity? | Kiwifruit |
| Complementary and alternative medicine | Mysterious | Acupuncture, aromatic therapy, ginger, herb drugs, holistic medicine, homeopathy, massage, reflexology |
Antispasmodics that can block muscarinic receptors and calcium channels of gut smooth muscle cells have been the oldest drugs to treat IBS for decades because of disturbed bowel motility and its effect on abdominal pain are commonly observed among these patients[1,23,34,105,106]. Unfortunately, their effectiveness and recommended evidence are not fair owing to the trial drawbacks including different IBS definitions, limited case number, inappropriate end-points, evaluation methods, dosing, duration, side effect recording, etc.[3,23,106]. Apart from hyoscine butylbromide, the only available antispasmodic in United States, other marketed antispasmodics include dicyclomine, mebeverine, pinaverium, otilonium bromide, peppermint oil, trimebutine maleate, etc.[1,2,23,39,106-108]. Overall, a meta-analysis indicated that antispasmodics are beneficial for IBS patients when abdominal pain is the predominant symptom of subjects attempted to treat[109]. Based on their long-term marketing, antispasmodics remain the first-line drugs to treat IBS but their probable anticholinergic side effects are best to warn before the prescription.
Disordered defecation has been another concern of IBS subjects and normalization of defecation via various approaches such as antidiarrheals for IBS-D and laxatives or bulking agents for IBS-C is recommended[1,23,106]. Regarding the IBS-D treatment using loperamide, it is a synthetic opiate derivative with an agonistic effect on μ-opioid receptors but scant opioid CNS effects. Its antidiarrheal effect comes from directly simulating gut water absorption and is further augmented by an antisecretory activity mediated by calmodulin antagonism, a property not shared by other opioids[110]. Loperamide appears the only antidiarrheal recommended to treat IBS-D during the acute or chronic diarrhea[1,7,106,110,111]. Earlier trials already supported its efficacy over placebo in treating stool consistency, urgency, borborygmi and abdominal pain[112,113]. However, a meta-analysis pointed out that it seems to reduce diarrhea but does not relieve abdominal pain among IBS subjects[109].
Laxatives have long been recommended to treat the constipation concern of IBS-C subjects[1,23,106]. Surprisingly, laxatives are not well evaluated whether they do have effectiveness in treating IBS-C, because most clinical experiences are adopted from those of functional constipation treatments. Only a small-scaled study pointed out that polyethylene glycol vs placebo improved stool frequency but not ameliorated abdominal pain among IBS-C subjects[114]. Until now, the evidence to recommend laxatives in treating IBS-C remains controversial[23,106].
Bulking agents including natural and artificial fibers are also recommended to treat constipated subjects including IBS patients. Basically, unabsorbed soluble agents such as psyllium and polycarbophil are dissolved and fermented in colon water to form a gel in turn to shorten colon transit time and to stimulate defecation, whereas insoluble agents such as corn fiber and wheat bran have limited change in gut, but they increase fecal mass to help defecation[115]. Reported trials indicated a limited benefit for constipation and no effect to attenuate other IBS symptoms[116]. Furthermore, a meta-analysis did not support its efficacy in treating IBS symptoms including stool frequency, abdominal pain and bloating[109]. According to the types of bulking agents, another meta-analysis pointed out that soluble fibers improve global symptoms, whereas insoluble fibers even exacerbate the clinical outcome[23,115]. As fermentable substances, the commonly reported side effects of bulking agents such as bloating, abdominal distension and flatulence are best to inform before the prescription[23].
Since the end of last century, new drugs targeting receptors known to have pharmacological effects on IBS have been emerging. Of them, 5-HT related drugs including agonists and antagonists are most promising because their efficacies over placebo were critically evaluated based on the high quality controlled trials and finally approved by the authorities[23,106]. For example, IBS-D can be treated using alosetron and cilansetron which have antagonistic activity on 5-HT3 receptors to delay bowel transit, reduce colonic tone and HAPC, blunt gastrocolic reflex and decrease visceral sensation, particularly with obvious therapeutic effect among female patients[7,23,117,118]. Nevertheless, this group should be used with very caution because of the possibility of serious side effects including severe constipation and ischemic colitis. Now they are only restricted to female IBS-D patients when conventional therapies have failed[7,23,106]. Ramosetron is another potent and selective 5-HT3 receptor antagonist that can attenuate abnormal colonic function and abdominal pain in experimental animals. Clinical studies conducted in East Asia confirmed its benefits on abdominal pain/discomfort and bowel habits in both male and female IBS-D patients, but it also had side effect of hard stool. Until now, no ischemic colitis was reported based on a small number of cases exposed to it[23,119].
Regarding IBS-C treatment, tegarserod and prucalopride showed an agonistic activity on 5-HT4 receptor-mediated release of 5-HT from mucosal enterochromaffin cells, which promotes ascending excitatory contraction and descending inhibitory relaxation to enhance bowel motility through a series of chain reactions. Apart from attenuating visceral hypersensitivity, these agonists owing to different affinities with 5-HT4 receptors may account for variable prokinetic potentials and side effects[120-123]. Clinically, 5-HT4 agonists diminish bloating and abdominal pain/discomfort with the improved satisfaction to defecation concerns such as stool consistency and straining[23,106,124]. Unfortunately, tegaserod was withdrawn due to serious cardiovascular adverse events. It is indicated that nonselective 5-HT4 agonists such as cisapride and tegaserod may interact with human ether-à-go-go related cardiac potassium channels to have the chance of causing heart arrhythmia, whereas selective 5-HT4 agonists such as prucalopride and naronapride are believed to have cardiovascular safety[123]. Tegaserod was reintroduced in United States in 2007 under a limited and restricted using for women younger than 55 years and not at risk for cardiovascular events[23,123]. It remains uncertain whether prucalopride can effectively treat IBS-C as tegaserod, although its efficacy was confirmed among functional constipation subjects[106]. Renzapride is a substance to own both activities of 5-HT4 agonist and 5-HT3 antagonist, and its development for IBS-C patients was halted because of the disappointing limited effects in a phase III trial[23].
Lubiprostone is a newly approved drug available in United States, United Kingdom and Japan to treat constipated subjects including IBS patients. It is a synthetic bicyclic fatty acid derivative of prostaglandin E1 with the ability to stimulate cystic fibrosis transmembrane conductance regulator (CFTR) dependent chloride channels of enterocytes to increase small intestinal secretion of fluid, mucin and electrolytes and finally to improve bowel functions including defecation[23,46,125]. Lubiprostone is safe and effective to treat constipated subjects, but it has some side effects, with nausea being the most common, followed by diarrhea, abdominal pain, bloating, and even the very rare events of dyspnea and ischemic colitis[23,126,127].
Similarly, linaclotide was marketed in United States and Europe to treat severe constipated patients including IBS patients in 2012[128]. It is a synthetic 14-amino-acid peptide of guanylate cyclase C (GC-C) agonist mainly to increase intestinal fluid secretion and gut transit. Unlike lubiprostone, linaclotide first activates GC-C receptors on the luminal surface of enterocytes to enhance intra- and extracellular levels of cyclic guanosine monophosphate and in turn promote CFTR to secrete chloride and bicarbonate into gut lumen to improve defecation. Interestingly, the activation of GC-C receptors also diminishes visceral pain[23,128,129]. Clinically, linaclotide improves abdominal pain/discomfort, bloating and the defecated symptoms of straining, incomplete defecation and stool consistency of IBS-C patients. Meta-analyses confirmed its superior efficacy over placebo to treat IBS-C and functional constipation[128,130,131]. The most common side effect of linaclotide has been severe diarrhea (20%), thus subjects with a tendency to water and electrolyte imbalance are not indicated. Until now, its long-term safety has not been established yet[128,129,131].
Currently, many new drugs targeting the specific receptors responsible for motility, visceral sensation, gut secretion, neuroimmune and brain-gut axis are being developed to treat IBS. Basically, the key factors in terms of clear mechanisms involving whole pathophysiology, good oral bioavailablity, no CYP dependent metabolism, best once daily, least interaction with food and other drugs, no unwanted metabolites, long-term maintenance ability, good safety records and so forth may determine whether these new drugs can be accepted to treat IBS[132]. Because too many new drugs are under development, only a few examples are briefly introduced here. First, TAK 637 is a selective antagonist of smooth muscle neurokinin 1 receptors that activate intestinal muscle contraction. This agent reduced rabbit abdominal contractions induced by colorectal distension via inhibition of neurokinin 1 receptors, mainly located in the spinal cord, and it also reduced colonic transit and defecation in a Mongolian gerbil IBS model. Unfortunately, its development was halted because of serious side events that occurred in two animal species[133]. Second, opioid kappa receptors are located on the cholinergic terminals of ENS with the ability to inhibit cholinergic transmission and gut motility. Asimadoline, an agonist of these receptors, reduces gut wall neurotransmitter releasing to exhibit both analgesic and anti-diarrheal effects[7,132,134]. A recent phase III trial on IBS-D patients observed excellent results to treat pain and defecation related concerns such as frequency, urgency and bloating[134]. Third, clonidine initially used to treat hypertension with the commonly reported constipation side effect is a α2 adrenergic receptor agonist. It increased colonic and rectal compliance, and reduced tone, pain, gas sensation and rectal urgency of healthy subjects[135]. A trial also indicated its effect on IBS-D patients with reduced abdominal pain, satisfactory relief of global IBS symptoms and improved disturbed defecation in spite of side effects of drowsiness, dizziness and dry mouth[132,136]. Owing to the obvious CNS effects, clonidine is apparently unable to treat IBS. Other adrenergic agonists such as AGN-203818 and solabegron with the purpose to treat IBS are undergoing evaluation[23].
Severe and intractable IBS patients who fail conventional therapy may consider the psychiatric approaches such as anxiolytic agents, antidepressants, cognitive behavioral therapy, dynamic psychotherapy and even hypnotherapy[1,23,106,137]. According to the recommendations, antidepressants are only indicated when abdominal pain is the main concern. Its benefits are likely the central antinociceptive effect plus bowel effect[23,106]. When treating IBS patients using either tricyclic antidepressants (TCAs) or selective 5-hydroxytryptamine re-uptake inhibitors (SSRIs), their symptomatic subtypes should be considered. For example, SSRIs such as paroxetine decrease orocecal and whole gut transit times in IBS-C patients. In contrast, TCAs such as imipramine prolong orocecal and whole gut transit times in IBS-D patients[138]. Meta-analyses indicated that IBS global symptoms are improved using both TCAs and SSRIs no matter its subtypes while SSRIs are more tolerable than TCAs owing to their obvious prokinetic effect, but their long-term safety remains unknown[23,106]. Other psychiatric measures are also recommended to treat intractable IBS. Overall, the drawbacks of these non-drug approaches include expert dependence, being unable to have blinding studies, methodological deviation and scant clinical experiences among most gastroenterologists. Nevertheless, experts recommended its good global symptom improvement and less adverse events[1,23]. It may be employed to severe and intractable subjects when all available and conventional treatments have failed.
Since an abnormal composition of gut microbiota exists among IBS patients, modification of gut microbiota components through exogenous supplement or inhibition of them using antibiotics appears promising to treat IBS patients[81,139]. Probiotics prepared as empiric base of “immune-boosting and health-enhancing” for century are live microbial supplements in attempt to improve gut microbial balance[81,140]. Pharmacologically, the benefits of probiotics consisting of anti-pathogenic ability via secretion of bacteriocins, acidification of the colon by fermentation, anti-inflammation to protect gut mucosa, alteration of mucosal response to stress, barrier enhancement, immune-modulating effects, and inhibition of visceral hypersensitivity justify their use to treat IBS[141,142]. Unfortunately, the worldwide probiotic preparations are not standardized. The most commonly used strains and species include Streptococcus thermophilus, Lactobacillus rhamnosus Lc705, Bifidobacteria, Lactobacillus rhamnosus GG, L., Bifidobacterium animalis ssp., Lactis Bb12, and non-pathogenic yeasts such as Saccharomyces boulardii. However, no two preparations are the same and the extrapolation of therapeutic responses from one to another may be problematic[23,142,143]. It was indicated that probiotic cocktail had potent anti-inflammatory properties of suppressing mucosal inflammation and restoring cytokine balance[143]. Overall, probiotics are safe without serious side effects but the benefit magnitude and the most effective species or strains are undetermined. Multi-species preparations are probable the best to treat IBS[23,84,143-145].
Live fecal microbiota transplantation is an incredible approach to treat various bowel diseases including inflammatory bowel disease, Clostridium difficile infection and even IBS. The fecal content can be administered via nasogastric tube, enema and colonoscopy[146]. Limited data indicated that constipated patients treated with colonoscopically delivered fecal microbiota had immediately improved defecation, bloating and abdominal pain[147]. It is unknown whether it is applicable to IBS-C subjects. Apart from microorganism supplement, new drugs targeting colonic low-grade inflammation are being developed, e.g., mast cell stabilizer, transient receptor potential vanilloid receptor type 1 and 4 blockers, protease-activated receptor 2 blockers, etc. It appears too early to predict their chance of success[7,132].
Antibiotics provide another route to treat imbalanced gut microbiota. For example, rifaximin has been proved in several non-diarrhea IBS controlled trials to improve global symptoms, abdominal pain, dysfunctional defecation and bloating[23,148,149]. Regarding IBS-C patients, neomycin treatment improved global symptoms and constipation. The success of this treatment depended upon the presence and post-treatment elimination of methane[150]. Owing to the chronic and recurrent nature of IBS, the effectiveness and safety of long-term or repeated use of antibiotics to treat IBS remain controversial.
Food restricted approaches such as avoidance of FODMAP items and individual evaluation of the effects of protein-, fat- and carbohydrate-rich/poor diets are recommended to reduce some IBS symptoms[84,93,94]. Likewise, a fermentable short-chain carbohydrates restricted diet significantly improved IBS symptoms of United Kingdom patients[94]. In contrast, another study indicated that dietary manipulation of poorly absorbed short-chain carbohydrates increased total amount of gut gas including hydrogen production to exaggerate the bowel symptoms of Australia IBS patients, thus avoidance of this food constituent is recommended[151]. Food elimination towards IgG antibodies in certain IBS patients effectively reduced bowel symptoms[152,153]. Interestingly, kiwifruit is a natural remedy to own laxative ability, particularly among the elderly population[154]. A study found that 4-wk kiwifruit consumption diminished colon transit time, increased defecation frequency, and finally improved the bowel function of IBS-C subjects[155]. Since kiwifruit may support the immune function to reduce the occurrence and severity of flu-like illness, it is unknown whether its efficacy to treat IBS is relevant to enhanced gut immunity[156]. Overall, the restriction of certain diets may be recommended to all IBS patients, but the routine use of food restriction or supplement without an appropriate drug therapy may not be perfect.
The intensity of pain perception is usually lower during the night dark hours when blood melatonin level is higher. Consequently, melatonin is considered an antinociceptive substance with the mechanisms broadly involving opioid, benzodiazepine, α(1)- and α(2)-adrenergic, serotonergic, cholinergic and melatonergic (1) and (2) receptors[157]. A short-term oral melatonin treatment improved abdominal pain, distension and abnormal defecation sensation in female IBS patients, whereas the defecation frequency and stool consistency were not affected[158]. Bile acid malabsorption is common among chronic diarrhea subjects and even IBS-D patients. A meta-analysis indicated that this event might be underestimated since about a third of IBS-D patients had moderate to severe bile acid malabsorption[159]. This may explain why cholestyramine is recommended to treat IBS-D patients[1,160]. Mesalazine was observed to reduce the number of mast cells and the subsequent release of mediators and diminish gut permeability and sensitivity in IBS patients, thus a large-scale mesalazine trial is undergoing in an attempt to know whether it can treat IBS-D patients. The final results are expected toward the end of 2013[161]. Diosmectite is inorganic aluminomagnesium silicate clay with a strong adsorbent ability. It is used to treat acute watery diarrhea based on the suggested mechanisms to diminish inflammation and mucolysis, to modify mucus rheologic and to adsorb bacteria, enterotoxins, viruses and other potentially diarrheogenic substances[162]. With regard to IBS treatment, diosmectite diminished abdominal pain and bloating intensity in IBS-D patients, but its effect on the disturbed defecation was not observed[163].
Traditionally, complementary and alternative medicines (CAM) is a medical practice not belonging to the current conventional medicine with therapeutic effects determined by the cultural, ethnic, social, religion, education and economic backgrounds. The CAM theories are markedly deviated from the conventional medicine in terms of heterogeneity, disease mechanisms, diagnostic approaches, therapeutic measures, judging efficacies, etc.[164,165]. Now herb drugs based on Chinese, Indian, Ayurvedic, and Tibetan preparations, acupuncture, aloes, aromatic therapy, ginger, homeopathy, probiotics, peppermint oil, reflexology, massage, colon irrigation, holistic medicine, aromatherapy, Qi gong, bioelectromagnetic field therapy, etc. are categorized as CAM[166]. Interestingly, certain CAM members have been acknowledged by the conventional medicine to treat IBS, e.g., probiotics and peppermint oil.
Clinically, many IBS patients do seek CAM before they encounter clinicians[167]. Herb drugs are the most often used but their effects are conflicting. In fact, the therapeutic effects of herb drugs are very hard to evaluate and compare each other since they are criticized with the drawbacks of mixture of variable botanical components, neither purified nor quality control, lack of preclinical animal study, unique preparation as family secret, publication bias, no reported adverse events, absent negative reports, etc.[23,168]. For instance, a trial conducted on Australian Caucasians with IBS indicated the very promising effect over placebo in relieving bowel symptoms even after discontinuation[168]. In contrast, herb mixture to treat Chinese IBS patients residing in Hong Kong did not reveal any benefits judged by global symptom and individual bowel symptoms[169]. It is unknown whether certain herb drugs claimed effective to treat IBS have the true pharmacological effect or just enhanced placebo response.
Acupuncture is a well-known old Chinese traditional medicine. Basically, it exhibits the physiological impacts on neural, humoral, opioid and serotonegic pathways with the effects of normalized motility, inhibited acid output, antinociceptive effect, reduced rectal hypersensitivity and altered 5-HT functions[170-172]. Acupuncture looks promising to treat FGIDs including IBS. Apart from Chinese studies, the effects of acupuncture to treat IBS among Western people are conflicting. For example, 10 weekly acupuncture sessions compared to placebo procedure for United Kingdom IBS patients reduced their symptomatic severity and its efficacy even persisted over a 1-year period[173]. Another study using 3-wk true acupuncture and cross-over with another 3-wk sham procedure conducted on United States IBS patients did not support its superiority over sham procedure to treat the global symptom and symptomatic intensities[174]. Overall, meta-analyses repeatedly indicated that acupuncture has no effect to the general wellbeing, individual bowel symptoms and QoL of IBS patients[175-177]. Finally, NICE guidance also does not recommend using acupuncture to treat IBS[178].
Homeopathy is popular among the CAM. Unlike conventional medicine, it means that “a substance is capable of inducing a series of symptoms in a healthy living system, and low doses of the same substance can cure these symptoms under certain circumstances”[179]. Homeopathy is claimed effectively to treat IBS. Now a three-arm trial based on 5 sessions of true homeopathic treatment plus usual care vs placebo-homeopathy plus usual care vs usual care alone is undergoing in United Kingdom and the final result is expected to resolve whether homeopathy is truly effective to treat IBS[180]. Regarding IBS patients who failed all conventional treatments, CAM may be considered as a supplement or alternative with expected efficacy equal to enhanced placebo effect if they do not have any intolerable or serious side effects.
Current Rome III criteria-based diagnosis of IBS remains to have limitations, particularly the differentiation from constipation. It probably needs the resolution of coming new criteria. Since IBS is heterogeneous based on the multidimensional pathogeneses, using biopsychosocial dysfunction is effectively to integrate all old and emerging IBS pathogeneses in terms of gut dysmotility, abnormal gut water secretion and gas accumulation, visceral hypersensitivity, impaired mucosal permeability, dysfunctional brain-gut axis, genetic abnormalities, disturbed gut microbiota and immune system, psychological disturbances, impacts from food and various abuses, etc. Now multidisciplinary approaches using drugs with different mechanisms of action, imposing psychiatric measures, giving probiotics and antibiotics, possessing diet therapy, and CAM treatment, can be considered individually to treat the major clinical symptoms and other associated concerns.
P- Reviewers: Bashashati M, Kanazawa M, Kitazawa H, Quigley E, Ukena SN S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] |
| 2. | Chang JY, Talley NJ. An update on irritable bowel syndrome: from diagnosis to emerging therapies. Curr Opin Gastroenterol. 2011;27:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Ballou SK, Keefer L. Multicultural considerations in the diagnosis and management of irritable bowel syndrome: a selective summary. Eur J Gastroenterol Hepatol. 2013;25:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 4. | Dang J, Ardila-Hani A, Amichai MM, Chua K, Pimentel M. Systematic review of diagnostic criteria for IBS demonstrates poor validity and utilization of Rome III. Neurogastroenterol Motil. 2012;24:853-e397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Wong RK, Palsson OS, Turner MJ, Levy RL, Feld AD, von Korff M, Whitehead WE. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol. 2010;105:2228-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Pimentel M, Talley NJ, Quigley EM, Hani A, Sharara A, Mahachai V. Report from the multinational irritable bowel syndrome initiative 2012. Gastroenterology. 2013;144:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2013;14:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Chang FY, Lu CL, Chen TS. The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil. 2010;16:389-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 10. | Liu J, Hou X. A review of the irritable bowel syndrome investigation on epidemiology, pathogenesis and pathophysiology in China. J Gastroenterol Hepatol. 2011;26 Suppl 3:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Quigley EM, Abdel-Hamid H, Barbara G, Bhatia SJ, Boeckxstaens G, De Giorgio R, Delvaux M, Drossman DA, Foxx-Orenstein AE, Guarner F. A global perspective on irritable bowel syndrome: a consensus statement of the World Gastroenterology Organisation Summit Task Force on irritable bowel syndrome. J Clin Gastroenterol. 2012;46:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Lovell RM, Ford AC. Prevalence of gastro-esophageal reflux-type symptoms in individuals with irritable bowel syndrome in the community: a meta-analysis. Am J Gastroenterol. 2012;107:1793-1801; quiz 1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Vandvik PO, Wilhelmsen I, Ihlebaek C, Farup PG. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther. 2004;20:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 14. | Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Whitehead WE, Palsson OS, Levy RR, Feld AD, Turner M, Von Korff M. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102:2767-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Lu CL, Chang FY, Lang HC, Chen CY, Luo JC, Lee SD. Gender difference on the symptoms, health-seeking behaviour, social impact and sleep quality in irritable bowel syndrome: a Rome II-based survey in an apparent healthy adult Chinese population in Taiwan. Aliment Pharmacol Ther. 2005;21:1497-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Chang FY, Lu CL. Irritable bowel syndrome and migraine: bystanders or partners? J Neurogastroenterol Motil. 2013;19:301-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser AS, Klapp BF, Mönnikes H. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | MacLean EW, Palsson OS, Turner MJ, Whitehead WE. Development and validation of new disease-specific measures of somatization and comorbidity in IBS. J Psychosom Res. 2012;73:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Brun-Strang C, Dapoigny M, Lafuma A, Wainsten JP, Fagnani F. Irritable bowel syndrome in France: quality of life, medical management, and costs: the Encoli study. Eur J Gastroenterol Hepatol. 2007;19:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Camilleri M. Evolving concepts of the pathogenesis of irritable bowel syndrome: to treat the brain or the gut? J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S46-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 24. | Gwee KA, Bak YT, Ghoshal UC, Gonlachanvit S, Lee OY, Fock KM, Chua AS, Lu CL, Goh KL, Kositchaiwat C. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [PubMed] |
| 26. | Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131-139. [PubMed] |
| 28. | Kröner-Herwig B, Gassmann J. Headache disorders in children and adolescents: their association with psychological, behavioral, and socio-environmental factors. Headache. 2012;52:1387-1401. [PubMed] |
| 29. | Van Houdenhove B, Egle UT. Fibromyalgia: a stress disorder? Piecing the biopsychosocial puzzle together. Psychother Psychosom. 2004;73:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339-356. [PubMed] |
| 31. | Smith HS, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. 2011;14:E217-E245. [PubMed] |
| 32. | Thompson DG, Laidlow JM, Wingate DL. Abnormal small-bowel motility demonstrated by radiotelemetry in a patient with irritable colon. Lancet. 1979;2:1321-1323. [PubMed] |
| 33. | Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: an Oriental study. Clin Sci (Lond). 1998;95:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Horikawa Y, Mieno H, Inoue M, Kajiyama G. Gastrointestinal motility in patients with irritable bowel syndrome studied by using radiopaque markers. Scand J Gastroenterol. 1999;34:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Posserud I, Ersryd A, Simrén M. Functional findings in irritable bowel syndrome. World J Gastroenterol. 2006;12:2830-2838. [PubMed] |
| 37. | Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil. 2012;24:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Gras B, Magge S, Bloom A, Lembo A. Motility disorders of the colon and rectum. Curr Opin Gastroenterol. 2013;29:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Lu CL, Chen CY, Chang FY, Chang SS, Kang LJ, Lu RH, Lee SD. Effect of a calcium channel blocker and antispasmodic in diarrhoea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2000;15:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Clemens CH, Samsom M, Van Berge Henegouwen GP, Smout AJ. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Shekhar C, Monaghan PJ, Morris J, Issa B, Whorwell PJ, Keevil B, Houghton LA. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology. 2013;145:749-757; quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol. 2001;96:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Prott G, Shim L, Hansen R, Kellow J, Malcolm A. Relationships between pelvic floor symptoms and function in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Chen Y, Li Z, Yang Y, Lin L, Zhang H. Role of glucagon-like peptide-1 in the pathogenesis of experimental irritable bowel syndrome rat models. Int J Mol Med. 2013;31:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Schey R, Rao SS. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig Dis Sci. 2011;56:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Levitt MD. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N Engl J Med. 1971;284:1394-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 104] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 50. | Koide A, Yamaguchi T, Odaka T, Koyama H, Tsuyuguchi T, Kitahara H, Ohto M, Saisho H. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Agrawal A, Whorwell PJ. Review article: abdominal bloating and distension in functional gastrointestinal disorders--epidemiology and exploration of possible mechanisms. Aliment Pharmacol Ther. 2008;27:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141-G154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 53. | Kellow JE, Azpiroz F, Delvaux M, Gebhart GF, Mertz HR, Quigley EM, Smout AJ. Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology. 2006;130:1412-1420. [PubMed] |
| 54. | Lee HF, Hsieh JC, Lu CL, Yeh TC, Tu CH, Cheng CM, Niddam DM, Lin HC, Lee FY, Chang FY. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Cady RK, Farmer K, Dexter JK, Hall J. The bowel and migraine: update on celiac disease and irritable bowel syndrome. Curr Pain Headache Rep. 2012;16:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 57. | Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K. Pathogenesis of irritable bowel syndrome--review regarding associated infection and immune activation. Digestion. 2013;87:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Camilleri M, Di Lorenzo C. Brain-gut axis: from basic understanding to treatment of IBS and related disorders. J Pediatr Gastroenterol Nutr. 2012;54:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Maizels M, Aurora S, Heinricher M. Beyond neurovascular: migraine as a dysfunctional neurolimbic pain network. Headache. 2012;52:1553-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Giamberardino MA, Costantini R, Affaitati G, Fabrizio A, Lapenna D, Tafuri E, Mezzetti A. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain. 2010;151:307-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 62. | Piché M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Stabell N, Stubhaug A, Flægstad T, Nielsen CS. Increased pain sensitivity among adults reporting irritable bowel syndrome symptoms in a large population-based study. Pain. 2013;154:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799-804. [PubMed] |
| 65. | Hotoleanu C, Popp R, Trifa AP, Nedelcu L, Dumitrascu DL. Genetic determination of irritable bowel syndrome. World J Gastroenterol. 2008;14:6636-6640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol. 2005;100:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Nielsen CS, Knudsen GP, Steingrímsdóttir ÓA. Twin studies of pain. Clin Genet. 2012;82:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Burnett BB, Gardner A, Boles RG. Mitochondrial inheritance in depression, dysmotility and migraine? J Affect Disord. 2005;88:109-116. [PubMed] |
| 69. | van der Schaar PJ, van Hoboken E, Ludidi S, Masclee AA. Effect of cholecystokinin on rectal motor and sensory function in patients with irritable bowel syndrome and healthy controls. Colorectal Dis. 2013;15:e29-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion. 2008;78:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42 Suppl 17:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 72. | Storr MA, Yüce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil. 2008;20:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Camilleri M. Genetics of human gastrointestinal sensation. Neurogastroenterol Motil. 2013;25:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Sato N, Suzuki N, Sasaki A, Aizawa E, Obayashi T, Kanazawa M, Mizuno T, Kano M, Aoki M, Fukudo S. Corticotropin-releasing hormone receptor 1 gene variants in irritable bowel syndrome. PLoS One. 2012;7:e42450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Sikander A, Rana SV, Sinha SK, Prasad KK, Arora SK, Sharma SK, Singh K. Serotonin transporter promoter variant: Analysis in Indian IBS patients and control population. J Clin Gastroenterol. 2009;43:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Wang WF, Li X, Guo MZ, Chen JD, Yang YS, Peng LH, Wang YH, Zhang CY, Li HH. Mitochondrial ATP 6 and 8 polymorphisms in irritable bowel syndrome with diarrhea. World J Gastroenterol. 2013;19:3847-3853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425-432. [PubMed] |
| 78. | Camilleri M, Katzka DA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Genetic epidemiology and pharmacogenetics in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1075-G1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Bashashati M, Rezaei N, Bashashati H, Shafieyoun A, Daryani NE, Sharkey KA, Storr M. Cytokine gene polymorphisms are associated with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2012;24:1102-e566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Camilleri M. Genetics and irritable bowel syndrome: from genomics to intermediate phenotype and pharmacogenetics. Dig Dis Sci. 2009;54:2318-2324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 405] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 82. | Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2392] [Article Influence: 199.3] [Reference Citation Analysis (0)] |
| 83. | Nagalingam NA, Lynch SV. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:968-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 84. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 648] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 85. | Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512-59, 512-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 86. | Sunderman FW. Therapeutic properties of sodium diethyldithiocarbamate: its role as an inhibitor in the progression of AIDS. Ann Clin Lab Sci. 1991;21:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 88. | Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 89. | Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150-153. [PubMed] |
| 90. | Aguilera M, Vergara P, Martínez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol Motil. 2013;25:e515-e529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 91. | Gonlachanvit S. Are rice and spicy diet good for functional gastrointestinal disorders? J Neurogastroenterol Motil. 2010;16:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 92. | Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 465] [Article Influence: 38.8] [Reference Citation Analysis (1)] |
| 93. | El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review). Int J Mol Med. 2012;29:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 95. | Buckingham ET, Daniolos P. Longitudinal outcomes for victims of child abuse. Curr Psychiatry Rep. 2013;15:342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Beesley H, Rhodes J, Salmon P. Anger and childhood sexual abuse are independently associated with irritable bowel syndrome. Br J Health Psychol. 2010;15:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396-404. [PubMed] |
| 99. | Wouters MM, Van Wanrooy S, Casteels C, Nemethova A, de Vries A, Van Oudenhove L, Van den Wijngaard RM, Van Laere K, Boeckxstaens G. Altered brain activation to colorectal distention in visceral hypersensitive maternal-separated rats. Neurogastroenterol Motil. 2012;24:678-85, e297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2007;292:G849-G856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | O’Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2011;300:G241-G252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Lowman BC, Drossman DA, Cramer EM, McKee DC. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 1987;9:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Di Palma JA, Herrera JL. The role of effective clinician-patient communication in the management of irritable bowel syndrome and chronic constipation. J Clin Gastroenterol. 2012;46:748-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Pitz M, Cheang M, Bernstein CN. Defining the predictors of the placebo response in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:237-247. [PubMed] |
| 105. | Camilleri M, Andresen V. Current and novel therapeutic options for irritable bowel syndrome management. Dig Liver Dis. 2009;41:854-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome--a European perspective. Aliment Pharmacol Ther. 2006;24:183-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 107. | Chang FY, Lu CL, Luo JC, Chen TS, Chen MJ, Chang HJ. The evaluation of otilonium bromide treatment in asian patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 256] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 110. | Regnard C, Twycross R, Mihalyo M, Wilcock A. Loperamide. J Pain Symptom Manage. 2011;42:319-323. [PubMed] |
| 111. | Wald A. Irritable bowel syndrome--diarrhoea. Best Pract Res Clin Gastroenterol. 2012;26:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci. 1984;29:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 113. | Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol. 1996;31:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Khoshoo V, Armstead C, Landry L. Effect of a laxative with and without tegaserod in adolescents with constipation predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 116. | Snook J, Shepherd HA. Bran supplementation in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1-8. [PubMed] |
| 118. | Chey WD, Cash BD. Cilansetron: a new serotonergic agent for the irritable bowel syndrome with diarrhoea. Expert Opin Investig Drugs. 2005;14:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1202-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 120. | Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844-854.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 121. | De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 122. | Tack J, Corsetti M. Prucalopride: evaluation of the pharmacokinetics, pharmacodynamics, efficacy and safety in the treatment of chronic constipation. Expert Opin Drug Metab Toxicol. 2012;8:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35:745-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 124. | Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev. 2007;CD003960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | De Lisle RC. Lubiprostone stimulates small intestinal mucin release. BMC Gastroenterol. 2012;12:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Chamberlain SM, Rao SS. Safety evaluation of lubiprostone in the treatment of constipation and irritable bowel syndrome. Expert Opin Drug Saf. 2012;11:841-850. [PubMed] |
| 127. | Sherid M, Sifuentes H, Samo S, Deepak P, Sridhar S. Lubiprostone induced ischemic colitis. World J Gastroenterol. 2013;19:299-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | McWilliams V, Whiteside G, McKeage K. Linaclotide: first global approval. Drugs. 2012;72:2167-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Berntgen M, Enzmann H, Schabel E, Prieto Yerro C, Gómez-Outes A, Salmonson T, Musaus J. Linaclotide for treatment of irritable bowel syndrome--the view of European regulators. Dig Liver Dis. 2013;45:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 130. | Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714-1724; quiz p.1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 131. | Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1084-1092.e3; quiz e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 132. | De Ponti F. Drug development for the irritable bowel syndrome: current challenges and future perspectives. Front Pharmacol. 2013;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Camilleri M. Treating irritable bowel syndrome: overview, perspective and future therapies. Br J Pharmacol. 2004;141:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 134. | Mangel AW, Hicks GA. Asimadoline and its potential for the treatment of diarrhea-predominant irritable bowel syndrome: a review. Clin Exp Gastroenterol. 2012;5:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 135. | Malcolm A, Camilleri M, Kost L, Burton DD, Fett SL, Zinsmeister AR. Towards identifying optimal doses for alpha-2 adrenergic modulation of colonic and rectal motor and sensory function. Aliment Pharmacol Ther. 2000;14:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Camilleri M, Kim DY, McKinzie S, Kim HJ, Thomforde GM, Burton DD, Low PA, Zinsmeister AR. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1:111-121. [PubMed] |
| 137. | Dekel R, Drossman DA, Sperber AD. The use of psychotropic drugs in irritable bowel syndrome. Expert Opin Investig Drugs. 2013;22:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 138. | Gorard DA, Libby GW, Farthing MJ. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 146] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 139. | Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 140. | Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil. 2007;19:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 141. | Verdú EF, Bercík P, Bergonzelli GE, Huang XX, Blennerhasset P, Rochat F, Fiaux M, Mansourian R, Corthésy-Theulaz I, Collins SM. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127:826-837. [PubMed] |
| 142. | Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 143. | Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 144. | Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 376] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 145. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 146. | Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 147. | Tummuru MK, Blaser MJ. Characterization of the Campylobacter fetus sapA promoter: evidence that the sapA promoter is deleted in spontaneous mutant strains. J Bacteriol. 1992;174:5916-5922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 148. | Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28-35; quiz 36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 149. | Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 150. | Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 151. | Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 152. | Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 153. | Aydinlar EI, Dikmen PY, Tiftikci A, Saruc M, Aksu M, Gunsoy HG, Tozun N. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache. 2013;53:514-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 154. | Drummond L, Gearry RB. Kiwifruit modulation of gastrointestinal motility. Adv Food Nutr Res. 2013;68:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 155. | Chang CC, Lin YT, Lu YT, Liu YS, Liu JF. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac J Clin Nutr. 2010;19:451-457. [PubMed] |
| 156. | Stonehouse W, Gammon CS, Beck KL, Conlon CA, von Hurst PR, Kruger R. Kiwifruit: our daily prescription for health. Can J Physiol Pharmacol. 2013;91:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 157. | Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A. Melatonin in antinociception: its therapeutic applications. Curr Neuropharmacol. 2012;10:167-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 158. | Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 159. | Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 160. | Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 161. | Leighton MP, Lam C, Mehta S, Spiller RC. Efficacy and mode of action of mesalazine in the treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D): study protocol for a randomised controlled trial. Trials. 2013;14:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 162. | Dupont C, Vernisse B. Anti-diarrheal effects of diosmectite in the treatment of acute diarrhea in children: a review. Paediatr Drugs. 2009;11:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Chang FY, Lu CL, Chen CY, Luo JC. Efficacy of dioctahedral smectite in treating patients of diarrhea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2007;22:2266-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 164. | Hussain Z, Quigley EM. Systematic review: Complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 165. | Koop CE. The future of medicine. Science. 2002;295:233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 166. | Chang FY, Lu CL. Treatment of irritable bowel syndrome using complementary and alternative medicine. J Chin Med Assoc. 2009;72:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 167. | Kong SC, Hurlstone DP, Pocock CY, Walkington LA, Farquharson NR, Bramble MG, McAlindon ME, Sanders DS. The Incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. 2005;39:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 168. | Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 169. | Leung WK, Wu JC, Liang SM, Chan LS, Chan FK, Xie H, Fung SS, Hui AJ, Wong VW, Che CT. Treatment of diarrhea-predominant irritable bowel syndrome with traditional Chinese herbal medicine: a randomized placebo-controlled trial. Am J Gastroenterol. 2006;101:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 170. | Takahashi T. Acupuncture for functional gastrointestinal disorders. J Gastroenterol. 2006;41:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 171. | Yin J, Chen JD. Gastrointestinal motility disorders and acupuncture. Auton Neurosci. 2010;157:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 172. | Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:306-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 173. | MacPherson H, Tilbrook H, Bland JM, Bloor K, Brabyn S, Cox H, Kang’ombe AR, Man MS, Stuardi T, Torgerson D. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterol. 2012;12:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 174. | Lembo AJ, Conboy L, Kelley JM, Schnyer RS, McManus CA, Quilty MT, Kerr CE, Drossman D, Jacobson EE, Davis RB. A treatment trial of acupuncture in IBS patients. Am J Gastroenterol. 2009;104:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 175. | Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006;CD005111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 176. | Manheimer E, Cheng K, Wieland LS, Min LS, Shen X, Berman BM, Lao L. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 177. | Manheimer E, Wieland LS, Cheng K, Li SM, Shen X, Berman BM, Lao L. Acupuncture for irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:835-847; quiz 848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 178. | Dalrymple J, Bullock I. Diagnosis and management of irritable bowel syndrome in adults in primary care: summary of NICE guidance. BMJ. 2008;336:556-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 179. | Bellavite P, Conforti A, Piasere V, Ortolani R. Immunology and homeopathy. 1. Historical background. Evid Based Complement Alternat Med. 2005;2:441-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 180. | Peckham EJ, Relton C, Raw J, Walters C, Thomas K, Smith C. A protocol for a trial of homeopathic treatment for irritable bowel syndrome. BMC Complement Altern Med. 2012;12:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |