Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.326
Revised: October 27, 2013
Accepted: November 12, 2013
Published online: January 7, 2014
Processing time: 174 Days and 5.9 Hours
Arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome is a rare genetic disorder and has not been described in China. We present a female infant with neonatal intrahepatic cholestasis from a Chinese family with ARC syndrome. All 23 coding exons and flanking introns of the VPS33B gene were amplified and sequenced using peripheral lymphocyte genomic DNA of the patient and her parents. Genetic testing revealed two novel mutations (c.1033delA and c.1567C>T) in the VPS33B gene. The patient is a compound heterozygote and her parents were heterozygous for each of the mutations.
Core tip: In our study, we present a female infant with neonatal intrahepatic cholestasis from a Chinese family, who was eventually diagnosed with arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome by genetic analysis. She will be the first patient with ARC syndrome reported in China. Genetic testing revealed two novel mutations (c.1033delA, p.I345LfsX8 and c.1567C>T, p.R523X) in VPS33B, which is the causative gene. The patient is a compound heterozygote and her parents were heterozygous for each mutation. Our paper will expand the worldwide distribution of ARC syndrome and the mutation spectrum of VPS33B.
-
Citation: Li LT, Zhao J, Chen R, Wang JS. Two novel
VPS33B mutations in a patient with arthrogryposis, renal dysfunction and cholestasis syndrome in mainland China. World J Gastroenterol 2014; 20(1): 326-329 - URL: https://www.wjgnet.com/1007-9327/full/v20/i1/326.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.326
Arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome (OMIM 208085) is a rare autosomal recessive multisystem disorder usually caused by germline mutations in the VPS33B gene[1,2]. Here we describe a Han ethnic patient from an unconsanguineous family with ARC syndrome, who presented with all three main symptoms (arthrogryposis, renal dysfunction and cholestasis) and ichthyosis at birth. She has two novel mutations in the VPS33B gene.
The female infant was born at the gestational age of 37 wk and 6 d by cesarean section because of breech presentation. Her birth weight was 2.46 kg and the parents were not consanguine. Physical examination showed mild ichthyosis (her skin was dry and scaly), low-set ear on the left side, and arthrogryposis in the form of bilateral dislocation of the hips, flexion contracture of the knee joints and rocker-bottom feet. Neonatal hearing screening revealed right-sided deafness. She developed jaundice at 3 d of age. At 27 d of age, she was transferred to the neonatal intensive care unit of our hospital for persistent hyperbilirubinemia, pneumonia and congenital deformities. Liver function tests showed markedly elevated total bilirubin, direct bilirubin and alkaline phosphatase and mild elevation of alanine aminotransferase and aspartate aminotransferase, but gamma glutamyl aminotransferase (γGT) was always normal (Table 1). Her white blood cell count was elevated remarkably, and urine protein and glucose were positive (Table 2). Serum creatinine and coagulation function were nearly normal, and blood gas analysis and thyroid function were normal. Screening for toxoplasma, rubella, cytomegalovirus, and herpes simplex was negative. Mass spectrometry analysis of serum amino acids and carnitine showed elevation of C5DC, C10:1 and C10 levels, while gas chromatography-mass spectrometry analysis of urinary organic acid levels revealed highly elevated 4-hydroxyphenylacetic acid and mild elevation of several other organic acids. An echocardiogram showed patent foramen ovale. Abdominal ultrasound showed moderate bilateral renal hyperechogenity and minimal hepatomegaly. A radionuclide scan of the hepato-biliary system revealed normal uptake of the tracer but no excretion into the bowel after 24 h. There was no abnormality on magnetic resonance imaging of the brain.
| Age (d) | TBIL (μmol/L) | DBIL (μmol/L) | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | GGT (IU/L) | TBA (μmol/L) | TP(g/L) | ALB (g/L) |
| Reference values | 5.1-17.1 | 0-6 | 0-40 | 0-40 | 42-383 | 7-50 | 0-10 | 60-83 | 35-55 |
| 26 | 154.6 | 128.5 | 34.0 | 44.0 | NA | NA | NA | NA | NA |
| 27 | 189.7 | 139.6 | 38.0 | 71.0 | 1088 | NA | NA | NA | NA |
| 33 | 183.1 | 146.4 | 49.0 | 51.0 | 940 | 33 | 115.0 | 50.6 | 33.1 |
| 41 | 162.4 | 135.5 | 34.0 | 50.0 | 1162 | 28 | 139.1 | 50.7 | 33.1 |
| Age (d) | WBC (× 109/L) | RBC (× 1012/L) | PLT (× 109/L) | HGB (g/L) | Urine pH | Urine protein | Urine glucose |
| Reference value | 4-10 | 3.5-5.5 | 100-300 | 110-160 | 6.0-8.0 | - | - |
| 29 | 22.5 | 3.03 | 508 | 98.0 | 6.0 | + | + |
| 34 | 23.0 | 2.91 | 688 | 96.0 | 7.0 | ++ | + |
| 41 | 19.2 | 2.28 | 662 | 75.2 | 5.0 | + | + |
She was treated with antibiotics, fat-soluble vitamins, including vitamin K and D, and a milk formula enriched with medium-chain triglycerides. The fever alleviated, but she failed to gain weight, weighing approximately 2.22 kg at the age of 1 mo, and died of liver failure at 6 mo of age. The diagnosis of ARC syndrome was made according to her main features, including arthrogryposis, renal dysfunction and cholestasis with low γGT.
With the approval of the ethics committee of the Children’s Hospital of Fudan University and after obtaining written informed consent from the parents, peripheral blood samples were obtained from the patient and her parents. Genomic DNA was extracted in a routine fashion. All 23 coding exons and flanking introns of the VPS33B gene were amplified and sequenced (primer sequences are available on request). A 25 μL reaction mixture used in this study contained 1.0 μL of genomic DNA, 2.5 μL of 10 × Ex Taq buffer, 2.0 μL of 2.5 mmol/L dNTP, 0.25 μL of 5u Ex Taq, 1.0 μL of each forward primer and reverse primer (diluted to 10 μmol/L), and 17.25 μL of ddH2O. Polymerase chain reaction (PCR) were performed under the following conditions: pre-denaturation at 95 °C for 5 min, followed by 35 thermal cycles, each composed of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °Cfor 40 s. The purified PCR products were sequenced directly on an ABI 3500 Genetic Analyzer and then analyzed using BioEdit software (North Carolina State University, Raleigh, United States). All of the sequences were blasted against GenBank to find the variations, and single nucleotide polymorphisms were excluded by using dbSNP and 1000 genomes database (website). NM_018668 was used as the VPS33B reference sequence.
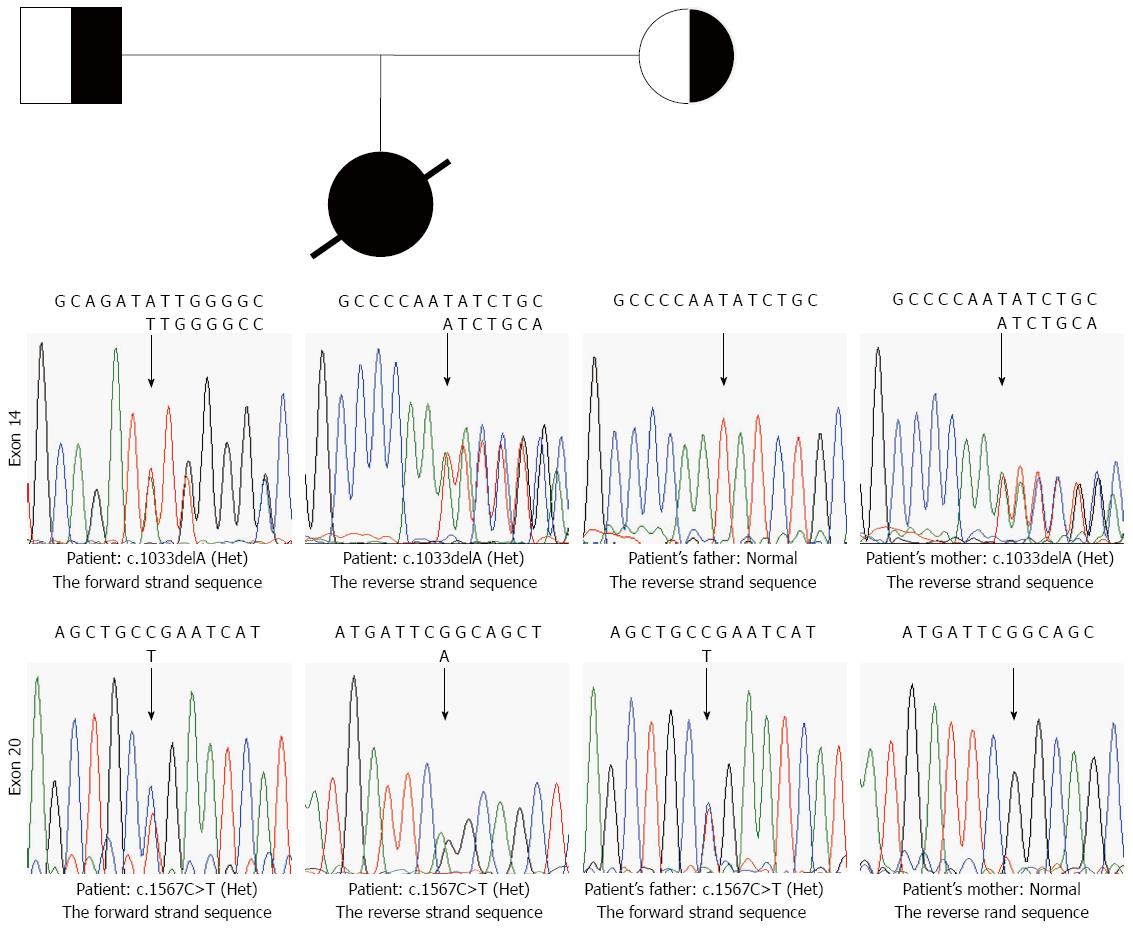
The patient was a compound heterozygote for c.1033delA and c.1567C>T. The deletion of one adenosine (A) between nucleotides 1032 and 1034 in exon 14 (Figure 1) modified the first nucleotide of codon 345, which codes for an isoleucine. The deletion of an A at this position did not only change the amino acid at codon 345, but a frame-shifting mutation was expected to result in the substitution of 6 abnormal amino acid residues (Gly346-Ser351), followed by a stop codon. This mutation was found heterozygous in the mother. The mutation c.1567C>T introduced a stop codon at codon 523 in exon 20 (Figure 1), resulting in an aberrant protein. c.1567C>T mutation was found heterozygous in the father. The two mutations had not been reported previously.
We report the first patient with ARC syndrome in China, and the patient carried two novel mutations in the VPS33B gene, which are the causative variations.
ARC syndrome is a rare, fatal cause of neonatal intrahepatic cholestasis without any known treatment. Gissen et al[1] mapped the disease to 15q26.1 and identified germline mutations in the VPS33B gene in 14 kindreds with ARC syndrome. VIPAR is another causative gene of ARC syndrome[3]. ARC syndrome presents with variable phenotypes in which the 3 main features are accompanied by many other systemic symptoms, including recurrent febrile illnesses, ichthyosis, abnormal platelets, bleeding tendency, and anomalies of the central nervous system[2,4-7]. Our patient presented with the 3 cardinal features plus ichthyosis, recurrent infection and failure to thrive. She died at the age of 6 mo.
The analysis of VPS33B indicated that our patient was a compound heterozygote. Her mother was heterozygous for a deletion mutation (c.1033delA, p.I345LfsX8) and her father was heterozygous for a nonsense mutation (c.1567C>T, p.R523X). The deletion mutation in exon 14 may alter the protein structure of VPS33B due to a reading frame shift with the formation of a stop codon in position 352. The resulting protein may be abnormal because it has 6 altered amino acids in addition to the premature termination of translation at codon 352. The nonsense mutation introduced a stop codon at codon 523 in exon 20, resulting in an aberrant protein. Over 30 different VPS33B mutations have been reported in ARC patients[2,8], but c.1033delA and c.1567C>T mutations were both novel.
In summary, we report a Chinese patient with ARC syndrome carrying two novel VPS33B mutations. Because there is no treatment for this syndrome, early recognition and genetic diagnosis are essential for the counseling of affected families and providing them with options, such as prenatal diagnosis in future pregnancies.
We thank Dr. Gissen for his kind advice on molecular tests of the VPS33B gene and Prof. Leung YK for the revision and editing of the manuscript. We also thank the patient and her parents for their kind cooperation.
P- Reviewer: Kramer H S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;36:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Gissen P, Tee L, Johnson CA, Genin E, Caliebe A, Chitayat D, Clericuzio C, Denecke J, Di Rocco M, Fischler B. Clinical and molecular genetic features of ARC syndrome. Hum Genet. 2006;120:396-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gürakan F, Utine E, Ozkan TB. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet. 2010;42:303-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Horslen SP, Quarrell OW, Tanner MS. Liver histology in the arthrogryposis multiplex congenita, renal dysfunction, and cholestasis (ARC) syndrome: report of three new cases and review. J Med Genet. 1994;31:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Di Rocco M, Callea F, Pollice B, Faraci M, Campiani F, Borrone C. Arthrogryposis, renal dysfunction and cholestasis syndrome: report of five patients from three Italian families. Eur J Pediatr. 1995;154:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Coleman RA, Van Hove JL, Morris CR, Rhoads JM, Summar ML. Cerebral defects and nephrogenic diabetes insipidus with the ARC syndrome: additional findings or a new syndrome (ARCC-NDI)? Am J Med Genet. 1997;72:335-338. [PubMed] |
| 7. | Eastham KM, McKiernan PJ, Milford DV, Ramani P, Wyllie J, van’t Hoff W, Lynch SA, Morris AA. ARC syndrome: an expanding range of phenotypes. Arch Dis Child. 2001;85:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Jang JY, Kim KM, Kim GH, Yu E, Lee JJ, Park YS, Yoo HW. Clinical characteristics and VPS33B mutations in patients with ARC syndrome. J Pediatr Gastroenterol Nutr. 2009;48:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |