Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.310
Revised: October 11, 2013
Accepted: October 17, 2013
Published online: January 7, 2014
Processing time: 138 Days and 6 Hours
AIM: To assess the efficacy and safety of combination therapy based on S-1, a novel oral fluoropyrimidine, vs S-1 monotherapy in advanced gastric cancer (AGC).
METHODS: We searched PubMed, EMBASE and the Cochrane Library for eligible studies published before March 2013. Our analysis identified four randomized controlled trials involving 790 participants with AGC. The outcome measures were overall survival (OS), progression-free survival (PFS), overall response rate (ORR) and grade 3-4 adverse events.
RESULTS: Meta-analysis showed that S-1-based combination therapy significantly improved OS (HR = 0.77, 95%CI: 0.66-0.91, P = 0.002), PFS (HR = 0.58, 95%CI: 0.46-0.72, P = 0.000) and ORR (OR = 2.23, 95%CI: 1.54-3.21, P = 0.000). Sensitivity analysis further confirmed this association. Lower incidence of grade 3-4 leucopenia (OR = 4.06, 95%CI: 2.11-7.81), neutropenia (OR = 3.94, 95%CI: 2.1-7.81) and diarrhea (OR = 2.41, 95%CI: 1.31-4.44) was observed in patients with S-1 monotherapy.
CONCLUSION: S-1-based combination therapy is superior to S-1 monotherapy in terms of OS, PFS and ORR. S-1 monotherapy is associated with less toxicity.
Core tip: This is the first meta-analysis aimed to detect whether S-1-based combination therapy would be more effective and safer than S-1 monotherapy in patients with advanced gastric cancer (AGC). In the meta-analysis, the S-1-based combination therapy group shows great advantages of achieving better overall survival, progression-free survival and overall response rate for AGC compared with the S-1 monotherapy group. The grade 3-4 adverse events in the combination therapy group might be overcome with medical therapy. S-1-based combination therapy should be used as a standard chemotherapeutic regimen for AGC, at least in Asia.
-
Citation: Liu GF, Tang D, Li P, Wang S, Xu YX, Long AH, Zhou NL, Zhang LL, Chen J, Xiang XX. S-1-based combination therapy
vs S-1 monotherapy in advanced gastric cancer: A meta-analysis. World J Gastroenterol 2014; 20(1): 310-318 - URL: https://www.wjgnet.com/1007-9327/full/v20/i1/310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.310
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related deaths all over the world[1]. More than two-thirds of patients diagnosed with gastric cancer will have unresectable disease[2]. Even patients with an operable tumor have a high rate of recurrence, with a median survival of only 24 mo and a 5-year survival rate lower than 30%[3]. In the absence of curative treatment modalities, attempts have been made to control cancer-related symptoms and improve survival using surgery, chemotherapy and radiation. Chemotherapy in advanced gastric cancer (AGC) is important because most patients with gastric cancer develop metastases. Some of the combination chemotherapies have shown high overall response rate (ORR) and increased survival times.
S-1 is a novel oral fluoropyrimidine that has demonstrated antitumor activity against AGC when used either as a single agent or in combination with other chemotherapies. S-1 consists of the combination of a 5-FU prodrug called tegafur and the two enzyme inhibitors 5-chloro-2,4-dihydroxypyridine (CDHP) and oteracil potassium (Oxo), in a molar ratio of 1:0.4:1. Following oral ingestion, tegafur is converted to 5-FU in the liver through hydroxylation. CDHP inhibits the activity of dihydropyrimidine dehydrogenase, thereby allowing 5-FU to remain in high concentrations for a longer time in serum and tumor tissue. Oxo is distributed in the gastrointestinal tract at a high concentration following oral administration, and it prevents phosphorylation of 5-FU by inhibiting the effect of orotate phosphoribosyl transferase[4]. In East Asian countries such as Japan, S-1 monotherapy has been adopted as the standard chemotherapy regimen for inoperable and recurrent gastric cancer[5]. Several phase I/II studies have been performed to explore combinations of S-1 with other cytotoxic drugs such as CDDP[6], docetaxel[7], paclitaxel[8] and irinotecan[9]. All these combinations have been found to be promising, with response rates of 40% and higher and relatively favorable safety profiles.
A series of randomized controlled trials (RCTs) comparing these doublets with S-1 monotherapy were subsequently planned and conducted to seek optimal first-line treatments, but these have yielded findings that are not completely consistent, none of which have allowed definite conclusions about the efficacy and safety of these two therapies. Therefore, we conducted a meta-analysis to give an overview of the results of all eligible RCTs comparing S-1-based combination therapy with S-1 monotherapy as first-line chemotherapy of patients with gastric cancer.
All authors participated in the selection of trials for inclusion. The electronic searches were performed prior to March 2013 in The Cochrane Library, MEDLINE via PubMed, EMBASE, Science Citation Index Expanded, Chinese Biomedical Database and Chinese National Knowledge Infrastructure. The search strategy included the medical subject heading of “S-1”, “advanced gastric cancer”, and “randomized controlled trial”. The search was not limited by language or publication status. In addition, all abstracts and virtual meeting presentations from the American Society of Clinical Oncology conferences held between 2000 and 2013 were also searched for relevant RCTs. From these trials we were able to obtain numbers and characteristics of patients, treatment regimens and study outcomes including efficiency and toxicity. Two authors independently extracted and interpreted the data. Disagreements between the reviewers were resolved by discussion or by the third reviewer.
Studies that we identified had to meet the following criteria: (1) patients with AGC at baseline; (2) trials comparing S-1-based combination therapy with S-1 monotherapy; and (3) prospective phase II and III RCTs.
The following data were extracted: the first author’s name, publication year, the country where the study was performed, study duration, participants (number of patients, mean age), regimen, mean administration cycles, Eastern Cooperative Oncology Group performance status, overall survival (OS), progression-free survival (PFS), and grade 3 or 4 adverse events. The review team used a standardized form adapted from the Risk of Bias Criteria of the Cochrane Effective Practice and Organisation of Care (EPOC) Group to systematically identify study quality[10]. The instrument recorded 9 criteria, including whether studies used random and concealed allocation, documented similar baseline characteristics and outcomes between the intervention and control groups, and described a plan for missing data, as well as the likelihood of contamination between study groups, with a maximum score of 9. Two investigators independently conducted a literature search and extracted data. Any differences were resolved through discussion or by the third reviewer.
The end points used for this study were OS, PFS and ORR. Overall survival was defined as time from date of randomization to date of death from any cause, censoring patients who had not died at the date last known alive. Progression-free survival was defined as time from date of randomization to date of progressive disease or death from any cause. ORR was defined as the sum of partial and complete response rates, according to the Response Evaluation Criteria in Solid Tumors[11,12]. Toxicity was graded according to the United States National Cancer Institute’s common toxicity criteria (version 2.0, http://ctep.cancer.gov). Statistical analysis yielded the overall HR for OS and PFS, and the OR for ORR and adverse events. All the end points were analyzed by an intention-to-treat analysis, defined as all randomly assigned patients.
We assessed the heterogeneity between studies in meta-analysis by the Cochran Q test, and considered P values lower than 0.10 as an indicator of significant heterogeneity because of the low statistical power. We also calculated the inconsistency index I2 to quantify heterogeneity. I2 was documented for the percentage of the observed variation between studies that was caused by heterogeneity rather by chance[13]. The efficacy and safety of pooled estimates were calculated first using a fixed-effects model[12]. If any heterogeneity existed, then use of the fixed-effects model might have been invalid, so the following techniques were employed to explore it: (1) subgroup analysis; (2) sensitivity analysis performed by omitting one study at a time and investigating its influence on the overall meta-analysis estimate when necessary; and (3) if heterogeneity still existed, a random-effects model was applied to incorporate between-study heterogeneity in addition to sampling variation when calculating summary OR estimates and corresponding 95%CIs.
To investigate whether publication bias might affect the validity of the estimates, funnel plots were constructed. Funnel plot asymmetry was assessed using Begg’s test and Egger’s test[14,15]. All statistical tests were two-sided, and a P value < 0.05 was considered significant except where specifically noted. Software STATA version 12.0 (Stata Corporation, College Station, TX, United States) was used for all statistical analysis.
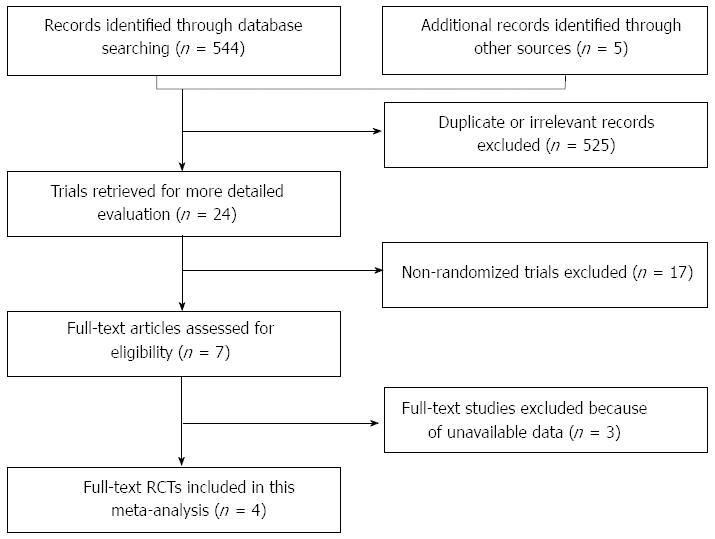
The initial searches led to the identification of 549 potentially eligible references. After screening the titles and abstracts, 525 studies were excluded because they were duplicate or clearly irrelevant (did not refer to clinical trials or did not assess the interventions specified in the protocol). The remaining 24 studies were selected for detailed evaluation. Twenty of these studies were excluded because they turned out not to be randomized and did not assess the interventions specified in the present protocol or were ongoing trials (no available data). Therefore, four RCTs were finally included in the present meta-analysis (Figure 1). A total of 790 participants were included in this meta-analysis, including 392 patients in the S-1-based combination group and 398 patients in the S-1 monotherapy group. More details of demographic and clinical characteristics of patients are listed in Tables 1 and 2.
| Ref. | Year | Country | Study design | Treatment groups | No. of patients | Regimen | Mediancycles | EPOCcriteria | Duration |
| Koizumi et al[16] | 2008 | Japan | Randomizedphase III study | Group A | 148 | S-1: 40-60 mg/m2, b.i.d days 1-21 plus cisplatin 60 mg/m2iv, on day 8, q.2.w | 4 | 7 | March 2001-Nov 2006 |
| Group B | 150 | S-1: 40-60 mg/m2, b.i.d days 1-28, q.2.w | 3 | ||||||
| Komatsu et al[17] | 2011 | Japan | Randomizedphase II study | Group A | 48 | S-1: 40-60 mg/m2,b.i.d days 1-14 plus irinotecan 75 mg/m2 iv, on days 1 and 15, q.4.w | 3 | 6 | Aug 2003-April 2007 |
| Group B | 47 | S-1: 40-60 mg/m2, b.i.d days 1-28, q.2.w | 2 | ||||||
| Narahara et al[18] | 2011 | Japan | Randomizedphase III study | Group A | 155 | S-1: 80 mg/m2, b.i.d days 1-21 plus irinotecan 80 mg/m2iv, on days 1 and 15, q.5.w | 4 | 6 | June 2004-April 2007 |
| Group B | 160 | S-1: 80 mg/m2, b.i.d days 1-28, q.6.w | 3 | ||||||
| Wang et al[19] | 2013 | China | Randomizedphase II study | Group A | 41 | S-1: 40-60 mg/m2, b.i.d days 1-14 plus paclitaxed 60 mg/m2iv, on days 1,8 and 15, q.4.w | 6 | 7 | Jan 2008-Dec 2011 |
| Group B | 41 | S-1: 40-60 mg/m2, b.i.d days 1-14, q.4.w | 5 |
| Wasaburo 2008 | Yoshito 2011 | Hiroyuki 2011 | Wang 2013 | |||||
| S-1-based | S-1 | S-1-based | S-1 | S-1-based | S-1 | S-1-based | S-1 | |
| Sex | ||||||||
| Men | 108 | 116 | 34 | 37 | 110 | 127 | 32 | 30 |
| Women | 40 | 34 | 14 | 10 | 45 | 33 | 9 | 11 |
| Age, yr | ||||||||
| Median | 62 | 62 | 70 | 63 | 63 | 63 | 63 | 61 |
| Rang | 33-74 | 28-74 | 47-78 | 24-76 | 33-75 | 27-75 | 35-74 | 31-73 |
| ECOG performances status | ||||||||
| 0 | 106 | 106 | 38 | 35 | 102 | 109 | 31 | 29 |
| 1 | 38 | 39 | 10 | 12 | 48 | 46 | 6 | 9 |
| 2 | 4 | 5 | NA | NA | 5 | 5 | 4 | 3 |
| Body surface area, m2 | ||||||||
| < 1.25 | 6 | 4 | 3 | 1 | NA | NA | 2 | 3 |
| 1.25-1.50 | 64 | 63 | 19 | 18 | NA | NA | 19 | 17 |
| > 1.50 | 79 | 83 | 26 | 28 | NA | NA | 20 | 21 |
| Disease status | ||||||||
| Unresectable | 118 | 119 | 33 | 33 | 129 | 133 | NA | NA |
| Recurrent | 30 | 31 | 15 | 14 | 26 | 27 | NA | NA |
| Histology | ||||||||
| Diffuse type | 103 | 89 | 25 | 25 | 93 | 88 | 28 | 30 |
| Intestinal type | 45 | 60 | 22 | 20 | 61 | 71 | 11 | 10 |
| Other not specified | 0 | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Primary tumor | ||||||||
| No | 53 | 58 | NA | NA | 62 | 67 | 15 | 17 |
| Yes | 95 | 92 | NA | NA | 93 | 93 | 26 | 24 |
Four RCTs were available for this meta-analysis. The included trials were conducted in Japan and China. All trials were published as full-text articles. We undertook detailed assessments of the relevant studies: treatment assignment was the typical method of “randomization” across trials in this meta-analysis. All four studies used proper methods for treatment allocation. None of the studies used double blinding. The quality of included studies was assessed by EPOC criteria, with the scores ranging from 6-7 (Table 1).
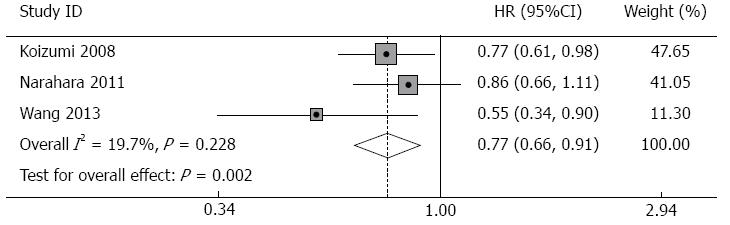
Overall survival: Survival data were available from three studies (Table 3). Patients receiving S-1 monotherapy had a median survival of 10.9 mo and a 1-year survival probability of 46.3%, while patients receiving the combination therapy had a median survival of 13.4 mo and a 1-year survival probability of 56.8%. The combined HR in the fixed-effects model for OS was 0.77 (95%CI: 0.66-0.91, P = 0.002), which indicated a favorable outcome in the combination therapy group for OS. There was no significant heterogeneity across the studies (I2 = 19.7%, P of heterogeneity = 0.288, Figure 2).
| Ref. | No. of patients | Median survival (mo) | 1-yr survival (%) | HR (95%CI) | P value |
| Koizumi et al[16] | 148 (Group A) | 11.0 | 46.7 | 0.77 (0.61-0.98) | < 0.0001 |
| 150 (Group B) | 13.0 | 54.1 | |||
| Narahara et al[18] | 47 (Group A) | 10.5 | 44.9 | 0.86 (0.66-1.11) | 0.233 |
| 48 (Group B) | 12.8 | 52.0 | |||
| Wang et al[19] | 160 (Group A) | 11.0 | 46.3 | 0.55 (0.34-0.90) | 0.020 |
| 155 (Group B) | 14.0 | 61.0 | |||
| Total | 355 (Group A) | 10.9 | 46.3 | 0.77 (0.66-0.91) | 0.000 |
| 353 (Group B) | 13.4 | 56.8 |
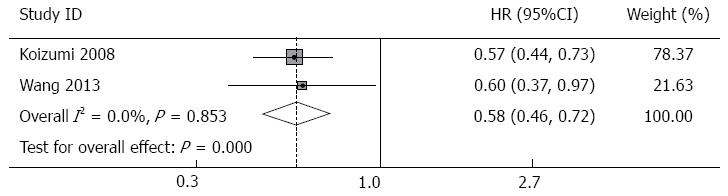
Progression-free survival: Data about progression-free survival were available for two studies (Table 4). Patients receiving S-1 monotherapy had a median progression-free survival of 4.0 mo, while patients receiving S-1-based combination therapy had a median progression-free survival of 6.0 mo. Pooled analysis of PFS in the combination group showed a significant difference compared with that in the monotherapy group, which indicated a favorable outcome in the combination therapy group for PFS (HR = 0.58, 95%CI: 0.46-0.72, P = 0.000). There was no evidence of inter-trial heterogeneity (I2 = 0.0%, P of heterogeneity = 0.853, Figure 3).
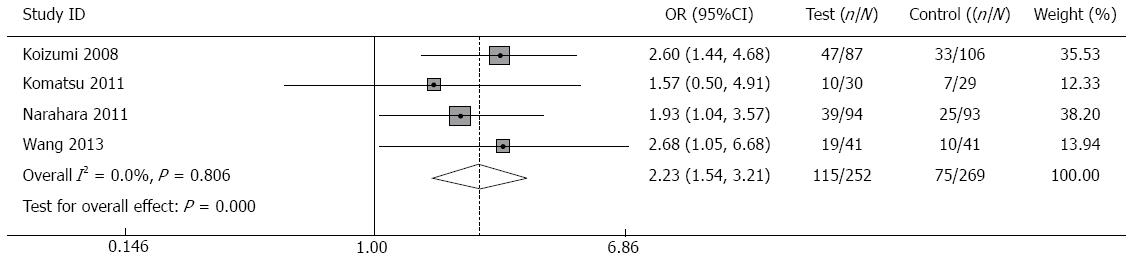
ORR: ORR data required for meta-analysis were available from four trials[16-19]. The pooled OR in the fixed-effects model for ORR was 2.23 (95%CI: 1.54-3.21, P = 0.000), which indicated a favorable outcome in the combination therapy group for ORR. There was no heterogeneity across studies (P of heterogeneity = 0.806, I2 = 0.0%, Figure 4).
Four studies[16-19] assessing 790 participants who were randomized to receive S-1-based combination therapy (n = 392) or S-1 monotherapy (n = 398) provided information on toxicity, analysis of which is shown in Table 5.
| Toxicity | S-1-based therapy | S-1 monotherapy | OR (95%CI) | Pvalue | Heterogeneity | |
| Grade 3-4 | n/N | n/N | Pvalue | I2 | ||
| Leucopenia | 44/392 | 12/398 | 4.06 (2.11-7.81) | < 0.01 | 0.728 | 0.00% |
| Neutropenia | 127/392 | 43/398 | 3.94 (2.70-5.77) | < 0.01 | 0.334 | 11.90% |
| Anaemia | 77/392 | 30/398 | 5.96 (3.03-11.73) | < 0.01 | 0.423 | 0.00% |
| Thrombocytopenia | 11/392 | 10/398 | 0.33 (0.10-1.12) | 0.076 | 0.900 | 0.00% |
| Anorexia | 91/392 | 47/398 | 2.40 (0.79-7.28) | 0.122 | 0.000 | 83.40% |
| Nausea | 41/392 | 16/398 | 2.80 (0.92-8.55) | 0.070 | 0.048 | 62.00% |
| Fatigue | 26/392 | 20/398 | 1.35 (0.74-2.46) | 0.336 | 0.506 | 0.00% |
| Vomiting | 15/392 | 9/398 | 1.71 (0.74-3.96) | 0.207 | 0.707 | 0.00% |
| Diarrhea | 35/392 | 16/398 | 2.41 (1.31-4.44) | < 0.01 | 0.614 | 0.00% |
| Stomatitis | 7/392 | 2/398 | 2.52 (0.71-8.79) | 0.151 | 0.994 | 0.00% |
Hematologic toxicity: Meta-analysis of four trials showed that grade 3-4 leucopenia was less likely to happen in patients receiving S-1 monotherapy, with no heterogeneity across studies (OR = 4.06, 95%CI: 2.11-7.81, P = 0.000; P of heterogeneity = 0.728, I2 = 0.0%).
The pooled OR showed that grade 3-4 neutropenia was significantly less prominent among participants receiving S-1 monotherapy relative to S-1-based combination therapy, and there was no heterogeneity across studies (OR = 3.94, 95%CI: 2.70-5.77, P = 0.000; P of heterogeneity = 0.334, I2 = 11.8%).
There was a significant difference in the pooled OR of anemia in the four trials (OR = 2.97, 95%CI: 1.90-4.64, P = 0.000), but heterogeneity across studies existed (P of heterogeneity = 0.012, I2 = 72.4%). Sensitivity analysis indicated that the trial reported by Narahara et al[18] was the main source of heterogeneity. When this study was omitted, the heterogeneity was eliminated (P of heterogeneity = 0.423, I2 = 0.0%), and the outcome of applying a fixed-effects model showed that grade 3-4 anemia was significantly less likely to happen in patients receiving S-1 monotherapy than S-1-based combination therapy (OR = 5.96, 95%CI: 3.03-11.7, P = 0.000).
Thrombocytopenia: The frequency of thrombocytopenia did not differ between two groups according to the pooled estimate for OR, and heterogeneity across studies did exist (P of heterogeneity = 0.058, I2 = 59.9%). Sensitivity analysis indicated that the trial reported by Koizumi et al[16] was the main source of heterogeneity. When this study was omitted, the heterogeneity was eliminated (P of heterogeneity = 0.90, I2 = 0.0%), but the pooled analysis did not show a significant difference between the two groups (OR = 0.33, 95%CI: 0.10-1.12, P = 0.076).
Non-hematologic toxicity: Meta-analysis showed that grade 3-4 anorexia was less likely to happen in patients receiving S-1 monotherapy (OR = 2.16, 95%CI: 1.47-3.16, P = 0.000), but heterogeneity across studies existed (P of heterogeneity = 0.000, I2 = 83.4%). Because heterogeneity could not be eliminated by sensitivity analysis, a random-effects model was applied (OR = 2.40, 95%CI: 0.79-7.28, P = 0.122; P of heterogeneity = 0.000, I2 = 83.4%).
The pooled OR of nausea showed heterogeneity across the four groups (P of heterogeneity = 0.048, I2 = 62%). Because heterogeneity could not be eliminated by sensitivity analysis, a random-effects model was performed, yielding an OR of 2.80 (95%CI: 0.92-8.55, P = 0.07).
Diarrhea: There was a significant difference in the pooled OR of diarrhea (OR = 2.41, 95%CI: 1.31-4.44, P = 0.005), with no heterogeneity across studies (P of heterogeneity = 0.614, I2 = 0.0%).
Meta-analysis of four trials showed that according to the pooled estimate for OR the frequency of these grade 3-4 adverse events did not differ between the two groups: fatigue (OR = 1.35, 95%CI: 0.74-2.46, P = 0.336; P of heterogeneity = 0.506, I2 = 0.0%), vomiting (OR = 1.71, 95%CI: 0.74-3.96, P = 0.207; P of heterogeneity = 0.707, I2 = 0.0%), and stomatitis (OR = 2.51, 95%CI: 0.71-8.79, P = 0.151; P of heterogeneity = 0.994, I2 = 0.0%).
Three trials[16,18,19]reported treatment-related deaths. Only one study[18] reported two patients in the S-1-based combination therapy died of potentially treatment-related conditions. There were no treatment-related deaths in either group in the other two studies[16,19].
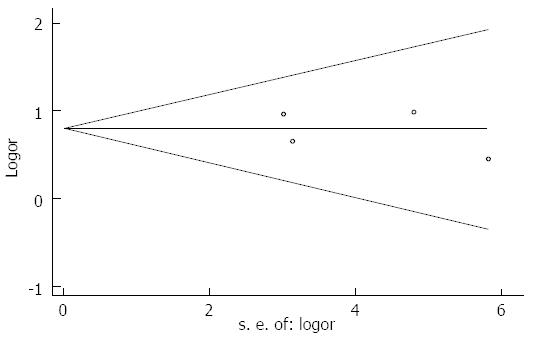
No publication bias was detected (Egger’s test: P = 0.827, Begg’s test: P = 0.734; Figure 5).
After years of disagreement about the utility of chemotherapy for AGC, several trials have demonstrated the efficacy of S-1 in both the adjuvant and primary settings, while combinations of S-1 with other cytotoxic therapies have been found promising, with higher response rates and relatively favorable safety profiles[20]. S-1 is convenient and offers an alternative to intravenous 5-FU. A recent meta-analysis reported that S-1-based combination therapy was associated with better OS and almost equivalent ORR and safety profile, compared with 5-FU-based therapy[21]. In addition, S-1 can be administered in the outpatient setting at lower costs, and its primary gastrointestinal side effects can be reasonably managed with antidiarrheal and antiemetic medications. The limited number of studies, with dissimilar criteria, methodologies, and evaluation standards, has likely resulted in inconsistent outcomes assessing S-1-based combination therapy vs S-1 monotherapy. Thus, this meta-analysis aimed to detect whether S-1-based combination therapy would be more effective and safer than S-1 monotherapy in patients with AGC.
Our study shows that OS (HR = 0.77, 95%CI: 0.66-0.91, P = 0.002) and PFS (HR = 0.58, 95%CI: 0.46-0.72, P = 0.000) were significantly increased in patients with ACG assigned to S-1-based combination therapy than in those assigned to S-1 monotherapy. With regard to the ORR (OR = 2.23, 95%CI: 1.54-3.21, P = 0.000), pooled analysis also showed that S-1-based combination therapy was superior to S-1 monotherapy. For hematologic toxicity, S-1-based combination therapy was associated with more grade 3-4 adverse events of leucopenia (11.2% vs 3.0%; P < 0.001), neutropenia (32.4% vs 10.8%; P < 0.001) and anemia (19.6% vs 7.5%; P < 0.001). For nonhematologic toxicity, incidence of grade 3-4 diarrhea (8.9% vs 4%; P = 0.005) was less prominent in the S-1 monotherapy group. With regard to grade 3-4 adverse events of thrombocytopenia, anorexia, nausea, fatigue, vomiting and stomatitis, there was no significant difference between two groups. Compared with S-1 monotherapy, S-1-based combination therapy was associated with longer OS and PFS, and higher ORR. The most common grade 3-4 adverse events associated with this regimen included leucopenia, neutropenia, anemia and diarrhea, all of these being more frequent than in patients receiving S-1 monotherapy. Overall results were confirmed when subjected to sensitivity analysis.
Overall survival, which requires prolonged follow-up, is the traditional endpoint for efficacy. The impact of first-line therapy on OS may be confounded by the effect of second- or third-line therapies. In the present meta-analysis, three RCTs reported OS. The present review suggests that OS was markedly increased in patients who received S-1-based combination therapy relative to S-1 monotherapy, without inter-study heterogeneity across the studies. As for leucopenia, neutropenia, anemia and diarrhea, these are more likely due to a byproduct of the higher cumulative effect of other chemotherapeutic agents, such as cisplatin. But all the toxicities were manageable, predictable and tolerable. S-1-based combination therapy was associated with more cases of grade 3 to 4 hematologic toxicities. To date, the availability of granulocyte colony-stimulating factors and erythropoietins could also improve the control of corresponding hematologic toxicities[22,23]. With regard to diarrhea, which was more frequent during S-1-based combination therapy, there seems to be a schedule-dependent toxicity, and it is likely that with the introduction of more effective antidiarrheal agents[24], the incidence of diarrhea can be further ameliorated.
Overall, quality of life could not be assessed in our study because none of studies included in the meta-analysis analyzed this endpoint.
As with any meta-analysis, the current study has possible limitations because evidence was combined from available studies. First, the quality of the trials affected the results: four of the studies included in this analysis were RCTs, but insufficient data might potentially limit detection of the effects of S-1-based combination therapy. Second, although four of the studies in the meta-analysis reported adequate randomization, absence of blinding might have resulted in an overestimate of the effects. Third, the second-line treatments were not reported, so it was not possible to consider their possible impact on survival. Although the role of second-line treatments has been a matter of debate, improved survival with the second-line administration of irinotecan over best supportive therapy has been reported by Thuss-Patience et al[25]. However, second-line treatments obviously do not alter PFS. Finally, the results from this meta-analysis need confirmation in the West because all four of the included trials were from Asia.
Recently introduced molecularly targeted therapies have been investigated in many solid malignancies, including gastric cancer. These include the tyrosine kinase inhibitors and monoclonal antibodies directed to critical tumor targets such as epidermal growth factor receptor 2 and vascular endothelial growth factor. Unfortunately, with the exception of trastuzumab, an HER-2 specific monoclonal antibody which was shown to improve survival in HER-2+ gastric and esophagogastric adenocarcinoma, none of these agents has been demonstrated to improve survival in comparison with chemotherapy[26]. As development of S-1-based combinations with other cytotoxic agents and biomarkers proceeds, the foreseeable combination of S-1 with targeted agents is an attractive option. In addition, the role of S-1 can be expanded in treating gastroesophageal cancers preoperatively as well as with radiation therapy.
This is the first meta-analysis in which we collected all the available trials that addressed this issue and all of them have been published as full-length articles. In conclusion, in the meta-analysis, the S-1-based combination therapy group shows great advantages of achieving better OS, PFS and ORR for AGC compared with the S-1 monotherapy group. The disadvantages such as more frequent grade 3-4 adverse events including leucopenia, neutropenia, anemia and diarrhea in the S-1-based combination therapy group might be overcome with medical therapy. Considering all the outcome benefits of S-1-based combination therapy over S-1 monotherapy in the current study, S-1-based combination therapy should be used as a standard chemotherapeutic regimen for AGC, at least in Asia. To further confirm these findings, additional large-scale randomized studies and Western studies are warranted.
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related deaths all over the world. S-1 is a novel oral fluoropyrimidine that has demonstrated antitumor activity against advanced gastric cancer (AGC) when used either as a single agent or in combination with other chemotherapies. However, whether S-1-based combination therapy or S-1 monotherapy are equally effective in the treatment of AGC is still contentious.
A series of randomized controlled trials comparing S-1-based combination therapy with S-1 monotherapy were planned and conducted to seek optimal first-line treatments, but these have yielded findings that are not completely consistent, none of which have allowed definite conclusions about the efficacy and safety of these two therapies.
This is the first meta-analysis to give an overview of the results of all eligible RCTs comparing S-1-based combination therapy with S-1 monotherapy with the aim of investigating whether S-1-based combination therapy was more effective than S-1 monotherapy in the treatment of patients with AGC as first-line chemotherapy. Several important conclusions might be used for future selection of S-1-based combination therapy or S-1 monotherapy for AGC patients’ treatments.
The study results suggest that S-1-based combination therapy is superior to S-1 monotherapy in terms of overall response rate, progression-free survival and overall survival without being associated with an increase in severe toxic effects.
S-1: A novel oral anticancer drug composed of tegafur, 5-chloro-2, 4-dihydroxypyridine (gimestat), and oteracil potassium in a molar ratio of 1:0.4:1, was developed in an effort to further enhance the therapeutic index of tegafur.
This is a well-performed meta-analysis aimed to detect whether S-1-based combination therapy would be more effective than S-1 monotherapy in patients with AGC, and its findings are interesting.
P- Reviewers: Imrie CW, Ishikawa T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 2. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 2762] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 3. | Ajani JA. Chemotherapy for gastric carcinoma: new and old options. Oncology (Williston Park). 1998;12:44-47. [PubMed] |
| 4. | Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 622] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Maehara Y. S-1 in gastric cancer: a comprehensive review. Gastric Cancer. 2003;6 Suppl 1:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207-2212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M, Sakamoto J. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Narahara H, Fujitani K, Takiuchi H, Sugimoto N, Inoue K, Uedo N, Tsukuma H, Tsujinaka T, Furukawa H, Taguchi T. Phase II study of a combination of S-1 and paclitaxel in patients with unresectable or metastatic gastric cancer. Oncology. 2008;74:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Uedo N, Narahara H, Ishihara R, Takiuchi H, Goto M, Fujitani K, Hirao M, Tsujinaka T, Imano M, Furukawa H. Phase II study of a combination of irinotecan and S-1 in patients with advanced gastric cancer (OGSG0002). Oncology. 2007;73:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Group CEPaOoC. EPOC resources for review authors, 2011. Available from: http://epoc.cochrane.org/epoc-resources. |
| 11. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13078] [Article Influence: 523.1] [Reference Citation Analysis (0)] |
| 12. | Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley 2000; 49-53. |
| 13. | Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40566] [Article Influence: 1448.8] [Reference Citation Analysis (2)] |
| 15. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10586] [Cited by in RCA: 12186] [Article Influence: 406.2] [Reference Citation Analysis (0)] |
| 16. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1422] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 17. | Komatsu Y, Takahashi Y, Kimura Y, Oda H, Tajima Y, Tamura S, Sakurai J, Wakasugi T, Tatebe S, Takahashi M. Randomized phase II trial of first-line treatment with tailored irinotecan and S-1 therapy versus S-1 monotherapy for advanced or recurrent gastric carcinoma (JFMC31-0301). Anticancer Drugs. 2011;22:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Narahara H, Iishi H, Imamura H, Tsuburaya A, Chin K, Imamoto H, Esaki T, Furukawa H, Hamada C, Sakata Y. Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer. 2011;14:72-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Wang X, Wang ML, Zhou LY, Lu XY, Yang JF, Yu HG. Randomized phase II study comparing paclitaxel with S-1 vs. S-1 as first-line treatment in patients with advanced gastric cancer. Clin Transl Oncol. 2013;15:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kubota T. The role of S-1 in the treatment of gastric cancer. Br J Cancer. 2008;98:1301-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Huang J, Cao Y, Wu L, Liao C, He Y, Gao F. S-1-based therapy versus 5-FU-based therapy in advanced gastric cancer: a meta-analysis. Med Oncol. 2011;28:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Myeloid growth factors-Version v1, 2011. Available from: http://www.nccn.org. |
| 23. | National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Cancer- and chemotherapy-induced anemia-Version v2, 2012. Available from: http://www.nccn.org. |
| 24. | Ippoliti C. Antidiarrheal agents for the management of treatment-related diarrhea in cancer patients. Am J Health Syst Pharm. 1998;55:1573-1580. [PubMed] |
| 25. | Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 436] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 26. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5328] [Article Influence: 355.2] [Reference Citation Analysis (3)] |