Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.235
Revised: October 9, 2013
Accepted: October 19, 2013
Published online: January 7, 2014
Processing time: 205 Days and 23.2 Hours
AIM: To evaluate the clinical efficacy and safety of epirubicin, cisplatin, and 5-FU combination chemotherapy for the sorafenib-refractory metastatic hepatocellular carcinoma (HCC).
METHODS: From April 2009 to June 2012, 31 patients who were diagnosed with metastatic and progressive HCC after sorafenib treatment were retrospectively reviewed. Patients were treated with the combination of epirubicin (50 mg/m2 IV; day 1), cisplatin (60 mg/m2 IV; day 1), and 5-FU (1000 mg/m2 IV; day 1-3) [Epirubicin, cisplatin, 5-FU combination (ECF)], repeated every 4 wk.
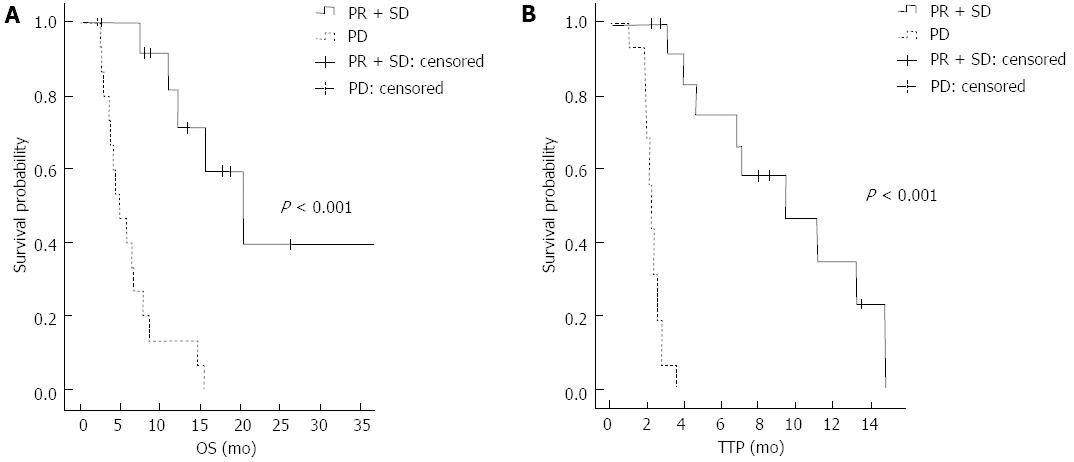
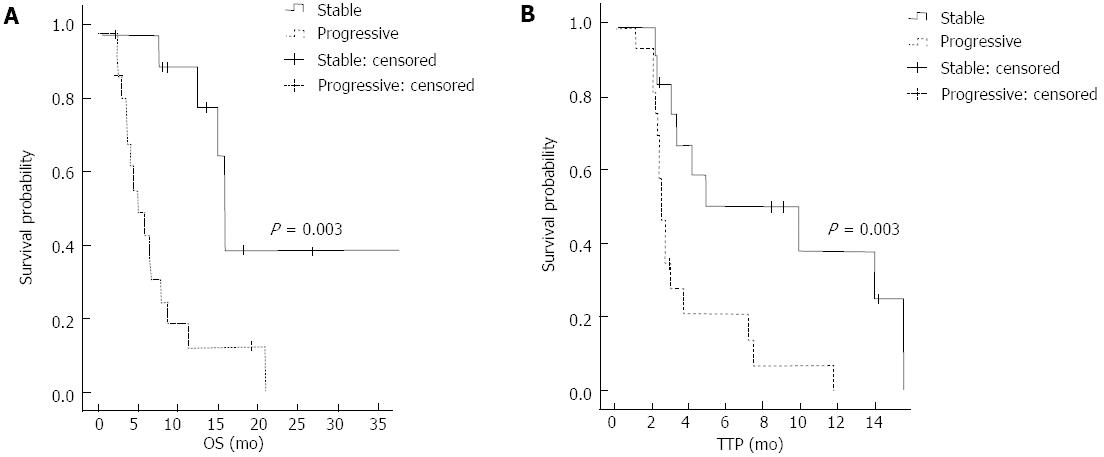
RESULTS: The overall response rate was 12.9%. Patients who responded to ECF chemotherapy showed a longer overall survival (OS) and time to progression (TTP) relative to those in the non-responder group (OS: 20.4 mo vs 4.9 mo, P < 0.001, TTP: 9.4 mo vs 2.2 mo, P < 0.001). Patients with a stable primary liver mass also exhibited a longer OS and TTP relative to those with progressive disease (OS: 13.4 mo vs 5.3 mo, P = 0.003; TTP: 9.4 mo vs 2.3 mo, P = 0.003). The most common hematologic toxicity was thrombocytopenia (87.2%), and the incidence of grade 3-4 neutropenia was 53.9%. Age older than 60, a stable primary mass, and a good response to chemotherapy were prognostic factors for OS and TTP.
CONCLUSION: This combination cytotoxic chemotherapy can serve as another treatment option after sorafenib failure for the subset of patients with advanced metastatic HCC.
Core tip: For advanced and metastatic hepatocellular carcinoma (HCC), sorafenib has been used as the standard systemic treatment. However, after failure to treat with sorafenib, no effective therapy is available. In the present study, we suggested that cytotoxic combination chemotherapy might be the another option for the treatment of progressive HCC. The patients with the age over 60 and a stable primary liver mass were benefit from the chemotherapy, leading to survival prolongation. Most clinical trials are currently focused on target agent because HCC is considered to be chemo-resistant cancer. Based on our data, new clinical trials using chemotherapy should be tried beyond sorafenib.
- Citation: Lee JE, Bae SH, Choi JY, Yoon SK, You YK, Lee MA. Epirubicin, Cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J Gastroenterol 2014; 20(1): 235-241
- URL: https://www.wjgnet.com/1007-9327/full/v20/i1/235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.235
Hepatocellular carcinoma (HCC) is the sixth most common cancer globally and the fifth in Korea[1,2]. The incidence of HCC is higher in Asia, and it is often associated with chronic liver disease or chronic alcoholic hepatitis[3]. Although several surveillance programs are actively ongoing in Asian countries including Korea, many patients visit the clinic when they have already reached the intermediate to advanced stage of HCC. Based on the Barcelona-Clinical Liver Cancer (BCLC) staging system, the intermediate stage group can benefit from chemo-embolization or radiofrequency ablation[4]. For advanced metastatic HCC, systemic therapy is needed, but an effective cytotoxic chemotherapy regimen has not yet been identified[5]. Recently, sorafenib has been reported as a standard therapy because it produced a significant survival benefit comparing to placebo in two randomized trials[4,6,7]. Furthermore, no more effective target agent is currently available to treat the progressive disease after sorafenib therapy, despite many clinical trials of new target agents[8].
For a long time, doxorubicin has served as the backbone of cytotoxic chemotherapy for HCC[5], yielding approximately a showing about 16% response rate[9]. Epirubicin is a doxorubicin derivative with a better therapeutic index[10], and a response rate of 17%[11]. As a single agent, cisplatin has also shown a 17% response rate for advanced HCC[12], and when this agent was combined with epirubicin and 5-FU, a higher response rate was achieved[13]. Although this combination chemotherapy was suggested a potential systemic therapy to treat for metastatic HCC in the past, only few trials for cytotoxic chemotherapy have been attempted due to the toxicity of the treatment and the underlying hepatic dysfunction of the patients.
In the present study, we retrospectively analyzed the clinical efficacy and safety of epirubicin, cisplatin, and 5-FU (ECF) combination chemotherapy in patients who presented progressive disease after sorafenib therapy to evaluate the potential benefit of these cytotoxic agents for advanced HCC.
From April 2009 to June 2012, the medical records of patients who presented advanced HCC in Seoul St. Mary’s Hospital were retrospectively reviewed. All patients had metastatic, progressive HCC after receiving treatment with sorafenib. The other eligible criteria included the following: (1) measurable lesions based on the response evaluation criteria in solid tumor (RECIST) criteria, ver. 1.0; (2) ECOG status 0 or 1; (3) Child-Pugh class A; (4) adequate bone marrow function, including platelet count ≥ 50000/mm3, absolute neutrophil count ≥ 1500/μL, and hemoglobin ≥ 8.0 g/dL; (5) adequate hepatic function, including aspartate aminotransferase and alanine aminotransferase ≤ 5 × upper normal limit and bilirubin ≤ 2.0 mg/dL; and (6) adequate renal function, serum creatinine ≤ 2 × upper normal limit. All procedures followed were in accordance with the ethical standards of the responsible committee on human experiments and the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for their inclusion in the study. This study was also approved by the Institutional Review Board of Seoul St. Mary’s Hospital, Catholic Medical Center.
Patients were treated with the combination of epirubicin (50 mg/m2 IV; day 1), cisplatin (60 mg/m2 IV; day 1), and 5-FU (1000 mg/m2 IV continuous; day 1-3) (ECF) every 4 wk. One liter of half saline was administered before and after the cisplatin. Echocardiography was performed in all patients to monitor their basic heart function before epirubicin treatment. Response evaluation was performed with CT scans every 2 cycles of chemotherapy using the RECIST criteria, ver. 1.0. Toxicity monitoring was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, ver. 3.0, during each cycle of chemotherapy. The treatment was continued until progressive disease or unacceptable toxicity was observed. If the patient failed to tolerate the chemotherapy, treatment was also stopped.
Overall survival (OS) was calculated from the start of the first day of ECF therapy to the death of the patient or the last follow-up date. Time to progression (TTP) was measured from the first day of the ECF chemotherapy to the date of disease progression, confirmed by CT scans. The disease control rate was defined as the partial response (PR) and the stable disease (SD), persisting at least 24 wk. OS and TTP were analyzed using Kaplan-Meier method. Cox regression models were used to analyze the statistical relationships between the prognostic factors and OS and TTP. All statistical analyses were carried out using SPSS, ver. 18.0.
A total 31 patients were retrospectively analyzed between April 1, 2009 and June 31, 2012. Baseline characteristics of the patients are described in Table 1. The median age was 53 years (range 36-71 years), and the majority of the patients were male (81%). Most of the patients (26 patients, 83.9%) presented as hepatitis B virus (HBV) positive and 4 patients presented with non-virus associated disease. A total of 13 patients (41.9%) presented with a stable primary liver mass at the start of ECF chemotherapy. Most of the patients presented with lung metastasis (90.3%), and other common metastatic sites included the lymph node (32.3%), bone (25.1%) and peritoneum (9.7%). All patients were previously treated with sorafenib and other local therapies.
| Numbers | |
| Age (range), yr | 531 (36-71) |
| Sex | |
| Male | 25 (81) |
| Female | 6 (19) |
| Etiology | |
| HBV | 26 (83.9) |
| HCV | 1 (3.2) |
| Others | 4 (12.9) |
| Status of primary liver mass | |
| Stable | 13 (41.9) |
| Progressive | 18 (58.1) |
| Metastatic sites | |
| Lung | 28 (90.3) |
| Lymph node | 10 (32.3) |
| Bone | 8 (25.1) |
| Peritoneum | 3 (9.7) |
| Previous treatment before sorafenib | |
| TACE | 29 (93.5) |
| Surgery | 17 (54.8) |
| Radiation | 12 (38.7) |
| Hepatic arterial infusion | 9 (29.0) |
| Radiofrequency ablation | 5 (16.1) |
A total of 102 cycles of ECF chemotherapy were administered to the 31 patients. The median number of cycles per patient was 2 cycles. PR was achieved in 4 patients, yielding a 12.9% response rate, and the disease control rate was 45.2% (Table 2). The median OS was 7.8 mo (range: 2.2-40.2 mo), and the median TTP was 2.7 mo (range: 10.0-14.8 mo).
| Response | n(%) |
| PR | 4 (12.9) |
| SD | 10 (32.3) |
| Disease control (PR + SD) | 14 (45.2) |
| Progressive disease | 17 (54.8) |
| Survival outcome | median (range) |
| Median TTP | 2.7 mo (1.0-14.8) |
| Median OS | 7.8 mo (2.2-40.2) |
In the survival analysis, patients who achieved PR or SD showed a better OS than the progressive disease (PD) group (median OS 20.4 mo vs 4.9 mo, 95%CI: 11.2-29.6, P < 0.001). The PR or SD group also showed a better TTP than the PD group (median TTP 9.4 mo vs 2.2 mo, 95%CI: 4.1-14.7, P < 0.001) (Figure 1). There was a significant association between the status of the primary liver mass at the start of treatment and the clinical outcome. Patients who presented a stable primary liver mass showed a better response than those who presented with a progressive primary liver mass (P = 0.004). The patients with a stable primary liver mass also showed a better OS and TTP than patients with a progressive primary liver mass (median OS 13.4 mo vs 5.3 mo, 95%CI: 14.4-16.8, P = 0.003; median TTP 9.4 mo vs 2.3 mo, 95%CI: 1.5-17.3, P = 0.003) (Figure 2).
In the univariate analysis, the presence of disease control was significantly associated with the OS and TTP (OS HR = 5.96, P < 0.001; TTP HR = 45.3, P < 0.001). There was also a correlation between presenting with a stable primary liver mass and OS and or TTP (OS HR = 4.22, P = 0.006; TTP HR = 3.56, P = 0.006). In addition, patients over 60 years of age achieved a better OS (HR = 0.09, P = 0.023) but not a better TTP (P = 0.143) (Table 3). These parameters also showed significant associations with OS and TTP in the multivariate analysis (Table 3).
| Characteristics | OS | TTP | ||
| HR | P value | HR | P value | |
| Univariate analysis | ||||
| Gender | ||||
| Male vs female | 0.870 | 0.804 | 0.87 | 0.793 |
| Age, yr | ||||
| < 60 vs≥ 60 | 0.090 | 0.023 | 0.46 | 0.143 |
| Primary liver mass control | ||||
| Yes vs no | 4.220 | 0.006 | 3.56 | 0.006 |
| Disease control | ||||
| PR + SD vs PD | 5.960 | < 0.001 | 45.3 | < 0.001 |
| Multivariate analysis | ||||
| Age, yr | ||||
| < 60 vs≥ 60 | 0.074 | 0.017 | 0.91 | 0.800 |
| Primary liver mass control | ||||
| Yes vs no | 3.660 | 0.026 | 1.38 | 0.540 |
| Disease control | ||||
| PR + SD vs PD | 4.310 | 0.011 | 36.69 | 0.001 |
The hematologic and non-hematologic toxicities are summarized in Table 4. There were no treatment-related deaths. Grade 3-4 neutropenia was the most common hematologic toxicity (53.9%); however, no febrile neutropenia developed. Among the non-hematologic toxicities, the most common toxicity was asthenia (26.4%). All of the toxicities were manageable.
| Grade 1/2 | Grade 3/4 | Total | |
| Hematologic | |||
| Anemia | 72 (70.6) | 10 (9.8) | 82 (80.4) |
| Neutropenia | 15 (14.7) | 55 (53.9) | 70 (68.6) |
| Thrombocytopenia | 61 (59.8) | 28 (27.4) | 89 (87.2) |
| Non-Hematologic | |||
| Nausea | 4 (3.9) | 0 | 4 (3.9) |
| Vomiting | 2 (1.9) | 0 | 2 (1.9) |
| Asthenia | 24 (23.5) | 3 (2.9) | 27 (26.4) |
| Anorexia | 2 (1.9) | 0 | 2 (1.9) |
| Microsites | 6 (5.8) | 0 | 6 (5.8) |
HCC is known to be a chemo-resistant cancer due to its high expression of the multidrug resistance (MDR) gene[14]. Based on the BCLC staging, patients presenting with early stage or intermediate stage disease can benefit from surgery, liver transplantation, or localized treatments such as TACE or radiofrequency ablation[4]. However, few treatment options are available for advanced HCC because most patients also suffer from chronic liver disease, such as chronic hepatitis and cirrhosis, leading to hepatic dysfunction. This underlying liver disease narrows the spectrum of the treatment options available for advanced HCC[14]. Although systemic therapy has played only a limited role over the past 30 years, newly developed novel agents that target signaling pathways have been attempted as treatments for advanced HCC[3]. Recently, sorafenib, an oral target agent, has been used as the standard treatment for advanced HCC because two large randomized phase III trials demonstrated that it was able to achieve a survival benefit compared with a placebo control[6,7]. However, sorafenib resulted in only 2-3 mo of benefit in the OS or TTP, so most patients eventually develop progressive disease after undergoing sorafenib treatment.
The Epirubicin, cisplatin, and 5-FU (ECF) combination regimen has shown some clinical benefits in advanced gastric cancer, biliary cancer and pancreatic cancer[15-17]. However, it had a poor response and survival outcome as first-line treatment for advanced HCC[18]. Another cytotoxic chemotherapy, the PIAF regimen (cisplatin, interferon alpha, doxorubicin, 5-fluorouracil) showed a better response, but it is too toxic to be tolerated by patients with advanced HCC[19]. In the present study, ECF chemotherapy showed modest activity in selected patients as second-line treatment. It achieved a promising clinical outcome in selected patients even if we treated the patients who had progressive disease after sorafenib treatment and other local therapy. In addition, all toxicities were manageable, and the patients tolerated the regimen well throughout the treatment cycles. In the trials of target agents, sorafenib prolonged the survival by only 2-3 mo[6,7], and the other target agents under investigation achieved only an additional 10% response rate[20]. Considering these data, our outcome was quite favorable, especially for patients with a PR or stable primary liver mass.
In the univariate and multivariate analyses of the survival outcome, age older than 60 years, a stable primary liver mass, and good disease control status were associated with a longer OS. Elderly patients over 60 years of age showed hazard ratios of 0.09 in the univariate analysis and 0.074 in the multivariate analysis, all of which indicated significant effects. These results are consistent with those of an Italian liver cancer group[21]. The status of the primary liver mass also could serve as a predictive and prognostic marker. Patients with a stable primary liver mass at the start of ECF chemotherapy had a better disease control rate, leading to a significantly longer OS and TTP, relative to patients with a progressive primary liver mass.
Although some selected patients showed a survival benefit in the present study, the overall survival increase was minimal across all patients. However, the change in the pain score according to 10-step numeric rating scale was 0.5 (range: -6 to 3, data not shown). Most patients had minimal pain that required a dosage increase in pain killers throughout the treatment period. We were unable to evaluate the quality of life improvement because this was a retrospective analysis. However, the minimal change of pain score suggested that ECF treatment might have a positive effect on pain control in advanced HCC.
Based on all these results, we suggest that ECF chemotherapy might offer another option a subset patient, i.e., those who have a stable primary liver lesion or are over 60 years of age. These patients might expect to achieve a survival benefit after failing to respond to sorafenib. There are some limitations in the present study. First, this is retrospective analysis with a small sample size, so the conclusions must be interpreted cautiously. Second, all patients enrolled in the study showed a good performance status with tolerable liver function (Child-Pugh score A). In practice, most of the patients who show progressive disease after sorafenib usually present with decreased liver function or poor performance status, so these patients could be cautiously selected to receive the second-line treatment.
After the sorafenib era, most clinical trials for advanced HCC have focused on new target agents compared to sorafenib being used to treat the control group[4,5]. The NCCN guideline recommends providing supportive care or a clinical trial as the treatment options after sorafenib failure[22]. However, patients who show good performance and liver function can be good candidates for active cytotoxic chemotherapy as a second-line treatment. It would be worthwhile to develop a clinical trial with less toxic cytotoxic combination chemotherapy. The inhibition of MDR1 has been reported to enhance the sensitivity of chemotherapy in vitro or in animal model systems[23-25]. These data suggest that combining a MDR1 inhibitor with chemotherapy can be another direction for the treatment of HCC, besides the new agent targeting signal pathway or tumor angiogenesis. Unfortunately, there have been few human clinical trials using this approach, so more preclinical or clinical trials are needed.
In conclusion, we have suggested that a cytotoxic combination regimen could offer an alternative treatment option after progression to sorafenib following careful patient selection. Patients who present with good ECOG, good liver function and a stable primary liver mass could be good candidates for systemic chemotherapy, and might expect to receive a survival benefit. To confirm our results, a prospective, randomized trial with a large sample size is warranted.
For advanced metastatic hepatocellular carcinoma (HCC), sorafenib has been used as standard systemic therapy. However, no effective systemic therapy is available yet for the progressive disease after treatment of sorafenib. New target agents are under investigation but fail to achieve survival prolongation. There were no recommendations about cytotoxic chemotherapeutic agents because HCC is known to be chemo-resistant cancer, and patients presented with poor hepatic dysfunction not to tolerate toxicity.
Epirubicin, 5-FU, and cisplatin have been tries in the treatment of HCC as single agent or embolic agents. This combination regimen has demonstrated some efficacy in several gastrointestinal malignancies. For progressive, advanced and metastatic HCC patients who are over 60 years or have a stable primary mass, combination cytotoxic chemotherapy showed good response, leading to survival prolongation. It might be another option for a subset patient with advanced HCC beyond progression of sorafenib.
In the previous trials, combination chemotherapy regimen showed poor response rate and was too toxic to tolerate for HCC patients with poor liver function. On the contrary, target agents are tolerable even for the patients with hepatic dysfunction, so new clinical trials for the treatment of advanced HCC are focused on target agents. However, these trials currently fail to show survival benefit. For the advanced patients with progressive disease after sorafenib therapy, more effective treatment is absolutely required. In the present study, authors tried with cytotoxic combination chemotherapy to treat patients with progressive, advanced, and metastatic HCC. In analysis of the clinical outcome, cytotoxic combination therapy demonstrated good response with survival benefit for some patients. Authors suggests that cytotoxic combination regimen should be worthwhile to try for the treatment of the progressive metastatic HCC patients
The study results suggest that the new clinical trials should be designed and performed using new, less toxic cytotoxic chemotherapy agents for the treatment of advanced, metastatic HCC
Progressive, advanced, metastatic HCC: In the treatment HCC with distant metastasis, sorafenib can be tried as first line treatment. However, drug resistance eventually develops during the sorafenib treatment. At this time, HCC is considered as “progressive and advanced HCC”. In this disease state, no effective treatment is available yet. In many clinical guidelines, observations with best supportive care or clinical trials are recommended as the treatment.
Lee et al report show combination chemotherapy using epirubicin, cisplatin and 5-FU can be used in selective HCC patients who are not responding to serafenib. In this study the authors tried chemotherapy using a combination of epirubicin, cisplatin and 5-FU in 31 HCC who were non-responders to serafenib. This combination chemotherapy show improved survival among patients who respond to treatment vs who are non-responders (20.4 mo vs 4.9 mo). The most common side effect seen among these patients were hematological toxicity including thrombocytopenia and neutropenia. Although the treatment success rate was not so high, the study deserve to be published due to the unique clinical study in humans.
P- Reviewers: Dash S, Wirth S S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Blivet-Van Eggelpoël MJ, Chettouh H, Fartoux L, Aoudjehane L, Barbu V, Rey C, Priam S, Housset C, Rosmorduc O, Desbois-Mouthon C. Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J Hepatol. 2012;57:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013;45:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Asghar U, Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol. 2012;56:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10238] [Article Influence: 602.2] [Reference Citation Analysis (2)] |
| 7. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4639] [Article Influence: 272.9] [Reference Citation Analysis (0)] |
| 8. | Siegel AB, Olsen SK, Magun A, Brown RS. Sorafenib: where do we go from here? Hepatology. 2010;52:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Nerenstone SR, Ihde DC, Friedman MA. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev. 1988;15:1-31. [PubMed] |
| 10. | Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790-800. [PubMed] |
| 11. | Hochster HS, Green MD, Speyer J, Fazzini E, Blum R, Muggia FM. 4’Epidoxorubicin (epirubicin): activity in hepatocellular carcinoma. J Clin Oncol. 1985;3:1535-1540. [PubMed] |
| 12. | Falkson G, Ryan LM, Johnson LA, Simson IW, Coetzer BJ, Carbone PP, Creech RH, Schutt AJ. A random phase II study of mitoxantrone and cisplatin in patients with hepatocellular carcinoma. An ECOG study. Cancer. 1987;60:2141-2145. [PubMed] |
| 13. | Ellis PA, Norman A, Hill A, O’Brien ME, Nicolson M, Hickish T, Cunningham D. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer. 1995;31A:1594-1598. [PubMed] |
| 14. | Rougier P, Mitry E, Barbare JC, Taieb J. Hepatocellular carcinoma (HCC): an update. Semin Oncol. 2007;34:S12-S20. [PubMed] |
| 15. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [PubMed] |
| 16. | Rao S, Cunningham D, Hawkins RE, Hill ME, Smith D, Daniel F, Ross PJ, Oates J, Norman AR. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer. 2005;92:1650-1654. [PubMed] |
| 17. | Evans TR, Lofts FJ, Mansi JL, Glees JP, Dalgleish AG, Knight MJ. A phase II study of continuous-infusion 5-fluorouracil with cisplatin and epirubicin in inoperable pancreatic cancer. Br J Cancer. 1996;73:1260-1264. [PubMed] |
| 18. | Boucher E, Corbinais S, Brissot P, Boudjema K, Raoul JL. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen). Cancer Chemother Pharmacol. 2002;50:305-308. [PubMed] |
| 19. | Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538. [PubMed] |
| 20. | Yau T, Wong H, Chan P, Yao TJ, Pang R, Cheung TT, Fan ST, Poon RT. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs. 2012;30:2384-2390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, Maraldi F, Di Nolfo MA, Del Poggio P, Benvegnù L. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | NCCN Clinical Practice Guidelines in Oncology, Hepatobiliary cancers ver. 2.0, 2012. Available from: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. |
| 23. | Sun Z, Zhao Z, Li G, Dong S, Huang Z, Ye L, Liang H, Qu J, Ai X, Zhang W. Relevance of two genes in the multidrug resistance of hepatocellular carcinoma: in vivo and clinical studies. Tumori. 2010;96:90-96. [PubMed] |
| 24. | Li G, Dong S, Qu J, Sun Z, Huang Z, Ye L, Liang H, Ai X, Zhang W, Chen X. Synergism of hydroxyapatite nanoparticles and recombinant mutant human tumour necrosis factor-alpha in chemotherapy of multidrug-resistant hepatocellular carcinoma. Liver Int. 2010;30:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wakamatsu T, Nakahashi Y, Hachimine D, Seki T, Okazaki K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int J Oncol. 2007;31:1465-1472. [PubMed] |