Published online Mar 7, 2013. doi: 10.3748/wjg.v19.i9.1478
Revised: December 11, 2012
Accepted: December 22, 2012
Published online: March 7, 2013
Processing time: 147 Days and 9.9 Hours
Collagenous gastritis (CG) is characterized by patchy subepithelial collagen bands. Effective treatment and the clinical and histological outcome of CG in children are poorly defined. The aim of this study is to summarize the published literature on the clinical outcome and response to therapy of pediatric CG including two new cases. We performed a search in Pubmed, OVID for related terms; articles including management and clinical and/or endo-histologic follow up information were included and abstracted. Reported findings were pooled in a dedicated database including the corresponding data extracted from chart review in our patients with CG. Twenty-four patients were included (17 females) with a mean age of 11.7 years. The clinical presentation included iron deficiency anemia and dyspepsia. The reported duration of follow up (in 18 patients) ranged between 0.2-14 years. Despite most subjects presenting with anemia including one requiring blood transfusion, oral iron therapy was only documented in 12 patients. Other treatment modalities were antisecretory measures in 13 patients; proton pump inhibitors (12), or histamine-2 blockers (3), sucralfate (5), prednisolone (6), oral budesonide in 3 patients where one received it in fish oil and triple therapy (3). Three (13%) patients showed no clinical improvement despite therapy; conversely 19 out of 22 were reported with improved symptoms including 8 with complete symptom resolution. Spontaneous clinical resolution without antisecretory, anti-inflammatory or gastroprotective agents was noted in 5 patients (4 received only supplemental iron). Follow up endo-histopathologic data (17 patients) included persistent collagen band and stable Mononuclear cell infiltrate in 12 patients with histopathologic improvement in 5 patients. Neither collagen band thickness nor mononuclear cell infiltrate correlated with clinical course. Intestinal metaplasia and endocrine cell hyperplasia were reported (1) raising the concern of long term malignant transformation. In summary, CG in children is a chronic disease, typically with a variable clinical response and an indolent course that is distinct from the adult phenotype. Long term therapy usually inclused iron supplementation but cannot be standardized, given the chronicity of the disease, variability of response and potential for adverse events.
- Citation: Hijaz NM, Septer SS, Degaetano J, Attard TM. Clinical outcome of pediatric collagenous gastritis: Case series and review of literature. World J Gastroenterol 2013; 19(9): 1478-1484
- URL: https://www.wjgnet.com/1007-9327/full/v19/i9/1478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i9.1478
Collagenous gastritis (CG) is a rare idiopahic disorder characterized by the distinctive endoscopic-histopathologic finding of a thickened (> 10 μm) gastric sub-epithelial collagen layer in association with inflammatory cell infiltrate in the lamina propria[1]. Collagenous gastritis was first described in a 15 years old by Colletti et al[2] subsequent reports helped define the two distinct phenotypes of the disease in children and adult patients[1,3]. The classic pattern in children centers around upper gastrointestinal symptoms including abdominal pain and severe anemia[1,4-6]. In contrast to the adult phenotype, inflammatory changes and collagenous band deposition is usually limited to stomach and CG has a variable clinical and histopathological response to therapy[6,7]. Adult type CG is associated with several autoimmune processes and celiac disease[8] which is unusual in the childhood form. Concomitant collagenous colitis may be present and gastrointestinal symptoms include abdominal pain, malabsorptive symptoms and, protein loosing enteropathy[3,9]. Adult CG is characterized by the simultaneous occurrence of collageneous colitis and duodenitis, and absence of nodularity in the mucosa of the colon and duodenum. The histological appearance of adult onset CG is also characterized by extensive inflammation of the entire gastrointestinal tract.
The pathophysiology of collagenous gastritis is poorly understood; one hypothesis includes a primary vascular abnormality with increased vascular permeability resulting in deposition of extruded protein and collagen deposition. Alternatively a primary inflammatory process results in a secondary fibrotic scarring process in susceptible individuals. This is supported by the earlier observation of intraepithelial lymphocytic infiltrate and over expression of human leukocyte antigen DR and CD25[2] suggesting an immune mediated inflammatory process.
Effective treatment of CG in either age group remains elusive and poorly defined; treatment strategies have revolved around the etiopathologic observations suggesting an inflammatory process and the association with celiac disease. Both anti-inflammatory and anti-secretory measures as well as gluten free diet have been tried but there has been, to date, no comprehensive review of treatment strategies and outcomes in this population.
The purpose of this study is to synthesize the published experience for the clinical course and outcome following different treatment modalities in children with CG including two additional cases with one patient responsive to a combination of oral budesonide in fish oil.
We performed a comprehensive chart review of the two cases of pediatric Collagenous Gastritis presenting to our institution from 2009 to 2012, and accrued through query of our institutional dedicated pediatric endoscopic database. Relevant historic, clinical, endoscopic-histologic findings and treatment modalities were summarized and pooled with the cases reported in the literature.
We performed a literature search of Pubmed and Ovid for terms: collagenous gastritis, lymphocytic gastritis and Collagenous colitis. Accrued publications were filtered for inclusion of pediatric subjects. Related articles for relevant publications were also reviewed. Articles including pediatric subjects were accrued. We identified sixty peer reviewed publications from Pubmed and an additional 30 publications from Ovid. We excluded duplicate patient reports when identifiable and series where individual therapy and outcome pairs could not be determined.
We abstracted relevant demographic, clinical, endoscopic-histopathologic findings at presentation and upon follow up whenever available and all therapeutic interventions whenever reported. Reports defining therapeutic modalities and clinical or endoscopic-histopathologic outcome were included with our two cases above.
An 11 year old boy presented with profound, symptomatic iron deficiency anemia (hemoglobin 4.7 g/dL, mean corpuscular volume 52, serum iron 10 mg/dL, serum ferritin < 1.5 ng/dL), thrombocytosis (platelets were 540 000/μL). Celiac serology was negative. Upper endoscopy and colonoscopy with biopsy showed marked inflammatory changes and nodularity in the gastric body mucosa but otherwise normal findings. The corresponding gastric biopsies showed a mixed mucosal lymphocytic - neutrophilic infiltration and a prominent subepithelial collagen band (> 10 μm) that stained positive with Masson Trichrome. Wireless capsule endoscopy was also negative. The patient was treated with oral iron supplements, then standard dose omeprazole with continued periodic treatment with oral iron. Upon repeat esophago- gastroduodenoscopy at 16 years of age biopsies showed marked interval improvement in gastritis, the development of multiple gastric (fundic gland) polyps and resolution of the collagenous band. He is asymptomatic and has been weaned off omeprazole and is being monitored for iron deficiency.
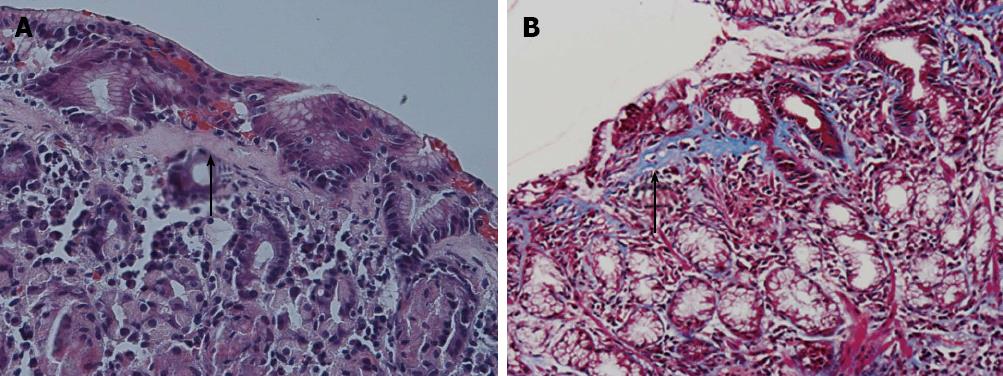
A 7 years old girl presented with chronic, mild, diffuse abdominal pain and pallor. Laboratory investigations confirmed iron deficiency anemia (hemoglobin 4.6 g/dL, mean cell volume 59.7, serum ferritin < 1.5 ng/dL) with platelets count of 435 000/μL) normal inflammatory indices (erythrocyte sedimentation rate and C-reactive protein), and negative celiac serology. Upon upper endoscopy and colonoscopy with biopsy moderate to severe hemorrhagic gastropathy was noted and the corresponding biopsies were reported showing a thick Masson Trichrome staining subepithelial hyaline band partly enveloping some of the superficial glands and moderate superficial chronic inflammatory infiltrate (Figure 1). The rest of the upper intestinal biopsies as well biopsies and endoscopic findings on complete simultaneous colonoscopy were normal.
The patient received standard dose esomeprazole approximately 1 mg/kg per day and oral iron supplementation (sodium feredetate) for 6 mo but upon follow up was reporting episodic, postprandial abdominal pain with corresponding epigastric tenderness on exam. Repeat endoscopy showed unchanged gross and histopathologic findings.
The treatment was escalated in view of refractory disease to include budesonide capsules (6 mg) crushed and suspended in 5 mL of fish oil given orally, daily and continuing esomeprazole and iron supplemention. Upon follow up, in 6 mo she was noted to have improved symptoms, mild constipation and was weaned off supplemental iron. Repeat endoscopy showed grossly improved gastritis (minimal diffuse antral nodularity), interval decreased inflammatory infiltrate but persistent collagen band on most biopsies. The patient was continued on the same medical treatment regimen and on follow up after a further 6 mo was noted to be asymptomatic, with stable hematologic indices.
Our search identified 16 publications and 22 pediatric cases with CG; their clinical presentation showed that anemia was predominantly observed in 17 cases (71%), Abdominal pain in 10 (41%), vomiting in 4 (17%), diarrhea in 4 (17%) and other presentations in 4 (17%) such as failure to thrive and hemoccult blood, hememesis and chest pain. their demographic data, therapeutic modalities duration of follow up and outcomes were pooled with our two cases and summarized in Table 1[1,2,4,6,9-20]. The majority of patients (17) were female, the mean age at presentation was 11.7 years (SD 4.5 years) and similar for both genders.
| Study | Age/gender | Treatment modalities | F/U | Histologic outcome | Clinical outcome |
| Colletti et al[2] | 15/F | Ranitidine sucralfate and furazolidane | N/R | No pathological improvement | No clinical improvement |
| Côté et al[1] | 9/F | Oral iron, sucralfate, omeprazole prednisolone | 2.5 | Unchanged subepithelial collagen bands Unchanged MN infiltrate Unchanged chronic active gastritis | Intermittent epigastric pain |
| Meunier et al[4] | 11/M | Oral iron | 6.25 | Chronic atrophic gastritis Unchanged subepithelial collagen bands Increasing severity MN infiltrate | N/R |
| 12/F | Oral iron | 0.6 | Unchanged histologic findings | Asymptomtic | |
| Winslow et al[13] | 14/F | Sucralfate, ranitidine Misoprostol, furazolidone Clarithromycin Metronidazole Omeprazole, prednisone | 12 | Progressive chronic active gastritis Increasing severity MN infiltrate Development of intestinal metaplasia Linear endocrine cell hyperplasia Stable subepithelial collagen bands | Intermittent abdominal pain |
| Mahadevan et al[19] | 15/F | Blood transfusion | 0.5 | Unchanged collagen band | N/R |
| Kori et al[6] | 12/F | Oral iron Omeprazole Clarithromycin-based triple therapy | 1 | Severe erosive gastritis histologically and macroscopically | Intermittent nausea and vomiting Normalized weight gain |
| 12/F | Oral, intravenous iron Omeprazole Clarithromycin-based triple therapy, predinsone | 6 | Unchanged subepithelial collagen bands Stable MN infiltrate | Marked clinical improvement, normalized weight gain, anemia Resolved, intermittent epigastric pain | |
| 12/F | Omeprazole | 0.2 | N/R | Improved abdominal pain and heartburn | |
| Kamimura et al[15] | 17/M | No therapy | 14 | Increased collagen band thickness, moderate MN cell infiltrate | Asymptomatic |
| Dray et al[14] | 15/F | Oral iron, triple therapy Prolong PPI, predinsone | 0.83 | N/R | Clinical remission |
| Ravikumara et al[20] | 9/F | Oral iron | 4.1 | Unchanged subepithelial collagen bands Stable MN infiltrate Decreased chronic gastritis | Asymptomatic |
| Leiby et al[17] | 0.16/M | Steroids, PPI mesalamine Bismuth subsalicylate | 6 | N/R | CG clinical improvement relapse after finishing steroids |
| Brain et al[9] | 16/F | Ranitidine Omeprazole Oral iron, exclusion diet | N/R | Unchanged histologic findings | Asymptomatic |
| Billiémaz et al[10] | 0.75/M | Prednisolone Budesonide Parenteral nutrition Gluten free diet | 14 | Showed a diffuse atrophic mucosa and an increase in the subcutaneous collagen in the gastrointestinal tract | Complete clinical improvement with TPN |
| Suskind et al[11] | 9/F | Oral iron | 0.33 | N/R | Asymptomatic |
| 15/M | Prednisone Lansoprazole Mesalamine | 1 | N/R | Asymptomatic | |
| Leung et al[12] | 15/F | Budesonide | 3.4 | Moderate gastric collagen deposition that decreased in thickness no IEL | Symptomatic improvement after therapy |
| 14/F | Pantoprazole Sucralfate | N/R | N/R | No improvement | |
| 19/M | Sucralfate | 0.25 | Moderate gastric collagen deposition in body/fundus, no IEL | Symptomatic improvement | |
| Wilson et al[16] | 12/F | Oral iron | N/R | N/R | Aymptomatic Normalized weight gain |
| Camarero Salces et al[18] | 9/F | Meslalazine | N/R | Unchanged | Unchanged |
| This series | 11/M | Oral iron Omeprazole | 5 | Decreased/resolved collagen bands Decreased chronic gastritis Decreased MN infiltrate (fundic gland polyps) | Improved abdominal pain |
| 7/F | Oral iron Esomeprazole Budesonide/fish oil | 1.5 | Unchanged subepithelial collagen band | Improved abdominal pain |
Gross endoscopic findings were abnormal in all reported cases, several showed gastric mucosal nodularity (11/24). Other reported finding were erythema, erosions, ulceration and hemorrhages in the gastric body, fundus and to a lesser extent in antrum.
Simultaneous colonoscopic findings were reported in 12 patients including 8 patients with gross and histological normal findings; in 3 patients there was collagenous colitis. In addition, an infantile case showed diffuse atrophic mucosa and increased subcutaneous collagen throughout gastrointestinal (GI) tract sections[10,11].
When reported (18 patients), the duration of follow up ranged (mean) between 0.2-14 (4) years. Duration of follow up did not significantly correlate with a consistent change in either outcome measure or disease phenotype. This is reflected by the reports by Winslow et al[13] and Kamimura et al[15], wherein follow up of 12 and 14 years respectively, and therefore into adulthood did not report features of adult onset type CG.
Despite most subjects presenting with anemia, with one patient requiring blood transfusion, oral iron therapy was only documented in 12 patients. Other treatment modalities were antisecretory measures in 13 patients; Proton pump inhibitors (12), or histamine-2 blockers (3). This includes 3 patients who received triple therapy for Helicobacter pylori (H. pylori) gastropathy in relation to the findings of CG. Sucralfate was used either alone or combined with other therapy in 5 patients.
Oral steroids were trialed in 9 patients either as systemic steroids (6) or oral budesonide (3) or were not specified (1). Steroids were used after other therapeutic modalities were ineffective. There were no cases reported in which steroids were able to eradicate the symptoms alone; in 6 cases intermittent response was noted; this included improved hematological indices or normalization of growth while recurrence of symptoms occurred in 3 cases after discontinuation of therapy[1,10,17]. The use of topical steroids produced variable success in 3 cases[10,12] and case 2. Our case used budosonide in fish oil and showed clinical and hematological improvement that led to eventual weaning of steroids.
In 6 individuals with CG treated with steroids and who underwent repeat endoscopy, histopathology was reported showing unchanged mononuclear (MN) cell infiltrate and collagen band in 3 patients increase in MN cells infiltrate in 2 patients and resolution of MN cells with decreased thickness of collagen in one case.
Additional measures included isolated patients treated with misoprostol, furazolidone, metronidazole and bismuth subsalicylate, hypoallergenic diet, gluten free diet and parenteral nutrition. Mesalamine was prescribed in 3 cases that were reported with concomitant collagenous colitis.
The clinical outcome was reported in 22 patients, repeat endoscopy and therefore histologic follow up was reported only in 17 patients. Three out of 22 patients showed no clinical improvement despite therapy; conversely, 19 out of 24 were reported with improved symptoms including 8 patients with complete symptom resolution. Spontaneous clinical resolution without antisecretory, anti-inflammatory or gastroprotective agents was noted in 5 patients (4 received only supplemental oral iron).
Complete resolution of symptoms was also reported after the use of oral prednisone in a patient with concomitant CC whereas another three patients with concomitant CC treated with mesalamine were reported with variable response.
In the patients with repeat endoscopy-histopathology reported; persistent histopathologic abnormalities were reported in 12 cases including persistent collagen band and stable mononuclear cell infiltrate. Histopathologic improvement was shown in 5 cases, 3 of them with decreased mononuclear cell infiltrate and decreased/resolved collagen bands in 2 patients on repeated endoscopy. In contrast, mononuclear cell infiltrate was increased in 2 patients[3,13]. Neither MN cell infiltration nor the collagen band changes correlated with the clinical course. Intestinal metaplasia and endocrine cell hyperplasia were reported in one case raising the concern of long term malignant transformation[13].
Collagenous gastritis is a rare diagnosis in children; its pathophysiology is unknown and is complicated by overlap with lymphocytic gastritis and celiac disease[8,12]. Pediatric CG is more common in girls and tends to present with severe anemia. In some cases pediatric CG coexists with collagenous sprue and collagenous colitis. The adult phenotype of collagenous gastritis is, in turn, associated with autoimmune disorders. The natural history, treatment modalities and long term outcome of pediatric CG are unknown. Herein, in addition to reporting two new cases of CG and a novel approach to treatment, we have reported on the most comprehensive review to date on the treatment modalities, response and outcome in children with CG described in the peer-reviewed literature.
The demographic outline and clinical presentation of our filtered dataset of treated children with CG and with described clinical and, or endoscopic-histopathologic outcomes reported appears representative of the whole population of pediatric cases reported in the literature. Our subset of cases includes a predominance of females, the mean age at diagnosis is 11.7 years and the duration of reported follow up (4 years) attests to the chronicity of the disease.
The presenting symptoms in our study; abdominal pain and iron deficiency anemia are identical to those reported elsewhere[9]. Although the pathophysiology of the iron deficiency remains unclear, most reports include the use of enteral iron supplementation and we have not come across any reference to parenteral iron supplementation alone, suggesting that blood loss rather than malabsorption, is thought to be the cause of iron deficiency.
Half the patients included in this series had undergone colonoscopy; four (16%) had collagenous colitis and three of them were treated with anti-inflammatory measures including mesalamine[11,17,18]. It was unclear whether lower gastrointestinal symptoms were present in the patients undergoing colonoscopy; it is likely that, as in our patients, this was mandated by suspicion of GI hemorrhage at the time of presentation. The collagen deposition of the entire gastrointestinal tract described in these cases was similar to those described in adult phenotype in their presentation. Although the reported subepithelial deposit in collagenous colitis tends to spread diffusely and continuously, collagen deposition and inflammatory infiltrate in collagenous gastritis tend to vary and are often irregular. The theory behind involvement of colon in some of these patients is that collagen bands arise from panenteric insult through the same pathological process in susceptible individuals, generally more severe in the colon in adulthood compared to children[9]. Anti-inflammatory drugs including corticosteroids have variable effect on collagenous colitis although relapses can occur, and diarrhea may resolve with or without treatment.
In our pooled analysis, coexistent collagenous colitis did not correlate with a worse prognosis. It is reasonable however to recommend colonoscopy in all patients with CG at the time of initial diagnosis in view of a potential response to therapy if coexistent CC is diagnosed.
A proven therapeutic paradigm does not exist for CG and our review failed to define a consistent response to the therapeutic strategies that were reported in the literature. It appears that the consensus approach to the treatment of isolated CG includes oral supplementation to address iron deficiency as discussed above; anti-secretory strategies, especially proton-pump inhibitors, and anti-inflammatory measures including systemic and, as in our case topically active steroids. In 3 of the patients, concomitant H. pylori infections were noted and warranted triple therapy[6,14]. Measures to detect H. pylori on endoscopy are therefore indicated when CG is diagnosed or suspected. This also raises interesting speculation of the possible association of H. pylori in the causation of CG.
A therapeutic strategy that includes long term anti-secretory and anti-inflammatory measures has its limitations, central to which, given the chronicity of the disease, is adverse events from long-term treatment. This is especially true given that our analysis is the first to suggest that pediatric CG is, in the most part an indolent, benign process. Adverse effects include systemic steroid side effects are exhaustively described elsewhere[21]. There are other potential adverse events, for example, the association of proton-pump inhibitor (PPI) use with neuroendocrine tumors as suggested by Winslow et al[13] in one patient in our pooled reports. PPI are also reported to interfere with iron absorption[22]. It therefore follows that, in addition to a clear explanation of our limited understanding of the efficacy of our treatment modalities, aside from oral iron supplementation, parents should also be offered treatments with the least adverse effects and for the shortest possible duration. Histamine-2-receptor Antagonists may be as suitable as PPI but entail less concern with long term use. Steroids may be considered a bridging therapy to achieve clinical and hematological response although there is a significant risk of recurrence of symptoms with discontinuation of therapy. Budesonide may be as efficacious as, and entail less potential for toxicity as systemic steroids.
There seems to be very limited evidence of restriction diet or gluten free diet improving CG. One patient was reported to respond to total parenteral nutrition but this seems an isolated case[10]. Several other treatment modalities (e.g., furazolidone) were also reported but with limited success.
This report highlights the disconnect between the endoscopic-histopathologic abnormalities that, in the large part, persisted in those patients with follow up endoscopy and the clinical outcome that was typically benign. This is especially true of the reported thickness of the pathognomonic supepithelial collagen band that remained unchanged in most of the patients. This observation contrasts with the conclusions drawn by Leung et al[12] who suggested that collagen thickness may correlate with severity of disease. In addition, mononuclear cell infiltrate did not correlate with collagen band thickness or clinical outcome.
In two reported cases, long term follow up over 12 years and into adulthood did not; however show a reversion to the adult-phenotype of the disease. This includes a 35-year-old woman presenting with a nodular pattern on Barium X-ray screening study 14 years after her initial presentation[15]. In this patient the endoscopic and histological findings of this disease were shown to progress gradually in absence of therapy. Endoscopically, the nodular appearance became more conspicuous, in addition to a thicker collagen band evident on biopsy in the context, however, of resolved clinical symptoms. In contrast, Winslow et al[13] reported on collagenous gastritis in a single patient who received multiple therapies including anti-inflammatory treatment during a 12-year period. The patient’s biopsy specimens showed a significant corpus endocrine cell hyperplasia, leading to speculation on an increased risk of endocrine neoplasia with gradual progression in disease severity, and unchanged collagen band over the 12-year period. In both cases no colonic involvement as described in adult phenotype reported after this long period. These reports suggest that the pediatric and adult disease phenotypes are different and that the pediatric form does not evolve in the adult form.
This study has several limitations; it is an attempt at a review of case reports and case series that were not, in the large part focused on therapy, response or long term outcome. There is potentially significant heterogeneity in the accuracy of reporting follow up duration, therapeutic modalities, histopathological and clinical response. Our sample size is a further significant limitation that impacts on the conclusions that can be drawn. Further studies with a more consistent method of qualifying endoscopic-histologic abnormalities are needed.
In conclusion, collagenous gastritis in children tends to be an isolated process that follows a generally benign course with limited long-term morbidity and no increased mortality reported to date. Initial investigation in children with CG needs to include investigation for H. pylori and colonoscopy to rule out collagenous colitis. Routine extensive investigation for autoimmune disorders appears unjustified. Response to medication is variable and needs to be individualized with the proviso that anecdotal evidence synthesized in this paper supports the use of oral iron supplementation and the judicious use of anti-secretory and anti-inflammatory agents for the shortest time feasible.
We would like to acknowledge Dr. Atif Ahmed Paediatric pathologist at Children Mercy Hospital for support in providing histological microphotographs.
P- Reviewer Kamimura K S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Côté JF, Hankard GF, Faure C, Mougenot JF, Holvoet L, Cézard JP, Navarro J, Peuchmaur M. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol. 1998;29:883-886. [PubMed] |
| 2. | Colletti RB, Trainer TD. Collagenous gastritis. Gastroenterology. 1989;97:1552-1555. [PubMed] |
| 3. | Lagorce-Pages C, Fabiani B, Bouvier R, Scoazec JY, Durand L, Flejou JF. Collagenous gastritis: a report of six cases. Am J Surg Pathol. 2001;25:1174-1179. [PubMed] |
| 4. | Meunier S, Villard F, Bouvier R, Lachaux A, Bertrand Y. Collagen gastritis, an unusual cause of anemia in children. Report of 2 cases. Arch Pediatr. 2001;8:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Park S, Kim DH, Choe YH, Suh YL. Collagenous gastritis in a Korean child: a case report. J Korean Med Sci. 2005;20:146-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Kori M, Cohen S, Levine A, Givony S, Sokolovskaia-Ziv N, Melzer E, Granot E. Collagenous gastritis: a rare cause of abdominal pain and iron-deficiency anemia. J Pediatr Gastroenterol Nutr. 2007;45:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Castellano VM, Muñoz MT, Colina F, Nevado M, Casis B, Solís-Herruzo JA. Collagenous gastrobulbitis and collagenous colitis. Case report and review of the literature. Scand J Gastroenterol. 1999;34:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Vesoulis Z, Lozanski G, Ravichandran P, Esber E. Collagenous gastritis: a case report, morphologic evaluation, and review. Mod Pathol. 2000;13:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Brain O, Rajaguru C, Warren B, Booth J, Travis S. Collagenous gastritis: reports and systematic review. Eur J Gastroenterol Hepatol. 2009;21:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Billiémaz K, Robles-Medranda C, Le Gall C, Gay C, Mory O, Clémenson A, Bouvier R, Teyssier G, Lachaux A. A first report of collagenous gastritis, sprue, and colitis in a 9-month-old infant: 14 years of clinical, endoscopic, and histologic follow-up. Endoscopy. 2009;41 Suppl 2:E233-E234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Suskind D, Wahbeh G, Murray K, Christie D, Kapur RP. Collagenous gastritis, a new spectrum of disease in pediatric patients: two case reports. Cases J. 2009;2:7511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Leung ST, Chandan VS, Murray JA, Wu TT. Collagenous gastritis: histopathologic features and association with other gastrointestinal diseases. Am J Surg Pathol. 2009;33:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Winslow JL, Trainer TD, Colletti RB. Collagenous gastritis: a long-term follow-up with the development of endocrine cell hyperplasia, intestinal metaplasia, and epithelial changes indeterminate for dysplasia. Am J Clin Pathol. 2001;116:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Dray X, Reignier S, Vahedi K, Lavergne-Slove A, Marteau P. Collagenous gastritis. Endoscopy. 2007;39 Suppl 1:E292-E293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Kamimura K, Kobayashi M, Narisawa R, Watanabe H, Sato Y, Honma T, Sekine A, Aoyagi Y. Collagenous gastritis: endoscopic and pathologic evaluation of the nodularity of gastric mucosa. Dig Dis Sci. 2007;52:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Wilson C, Thompson K, Hunter C. Nodular collagenous gastritis. J Pediatr Gastroenterol Nutr. 2009;49:157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Leiby A, Khan S, Corao D. Clinical challenges and images in GI. Collagenous gastroduodenocolitis. Gastroenterology. 2008;135:17, 327. [PubMed] |
| 18. | Camarero Salces C, Enes Romero P, Redondo C, Rizo Pascual JM, Roy Ariño G. Collagenous colitis and collagenous gastritis in a 9 year old girl: a case report and review of the literature. Acta Gastroenterol Belg. 2011;74:468-474. [PubMed] |
| 19. | Mahadevan S, Johnson J, Gould S, Baker C, Sullivan P. A rare case of Collagenous Gastritis. Arch Dis Child. 2002;86:A50-A51. |
| 20. | Ravikumara M, Ramani P, Spray CH. Collagenous gastritis: a case report and review. Eur J Pediatr. 2007;166:769-773. [PubMed] |
| 21. | Hoes JN, Jacobs JW, Verstappen SM, Bijlsma JW, Van der Heijden GJ. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis. 2009;68:1833-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |