Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1327
Revised: January 21, 2013
Accepted: January 23, 2013
Published online: February 28, 2013
Processing time: 155 Days and 17.5 Hours
A 57-year-old man presented with intermittent dull abdominal pain after a period of 1 year. Abdominal computed tomography (CT) was performed. Except for the endoscopy, the work-up for possible medical causes remained inconclusive. An open-abdomen, partial surgical excision of the stomach was performed after the unsuccessful endoscopic resection. The pathology report revealed a glomus tumor of the stomach. Importantly, glomus tumors of the stomach are rare and are almost always benign. Therefore, the most important current role of imaging associated with the diagnostic approach and therapeutic plan for a glomus tumor is to differentiate it from other gastric submucosal tumors (SMTs). We report this case with representative radiologic findings, including CT and endoscopic ultrasound (EUS) reports, and also correlate them with clinical and pathologic presentations that can help in the early detection and differentiation of gastric SMTs from other SMTs. As such, the purpose of this report is to provide a better understanding of relevant CT and EUS features. Alternative treatments should be considered carefully according to the imaging results.
- Citation: Tang M, Hou J, Wu D, Han XY, Zeng MS, Yao XZ. Glomus tumor in the stomach: Computed tomography and endoscopic ultrasound findings. World J Gastroenterol 2013; 19(8): 1327-1329
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1327
Glomus tumors of the stomach are rare; predominantly benign; and commonly described as solitary, well-defined gastric submucosal tumors (SMTs) in the antrum with non-specific clinical manifestations[1-4]. Gastrointestinal stromal tumors (GISTs), undoubtedly the most common malignant SMTs of the stomach, have the capacity to become malignant and later metastasize. Therefore, the most important and plausible current role of imaging in the diagnostic approach and therapeutic plan for a glomus tumor is to differentiate it from other SMTs[1,4]. We report this case with representative radiologic findings, including computed tomography (CT) and endoscopic ultrasound (EUS) reports, and also correlate them with clinical pathologic presentations that can help in the early detection and differentiation of GISTs from other SMTs. The diagnosis was surgically confirmed. Contrast-enhanced CT showed peripheral nodular or homogeneous strong enhancement in the arterial phase and prolonged enhancement in the delayed phase[1-6].
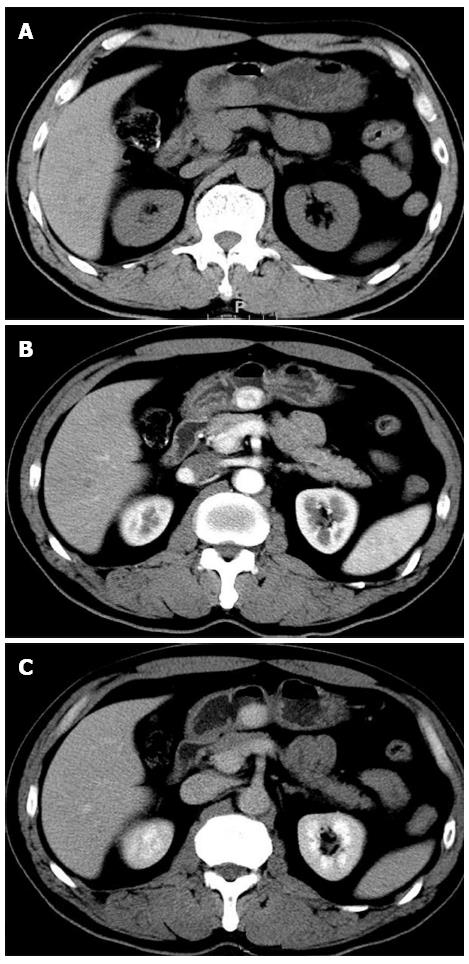
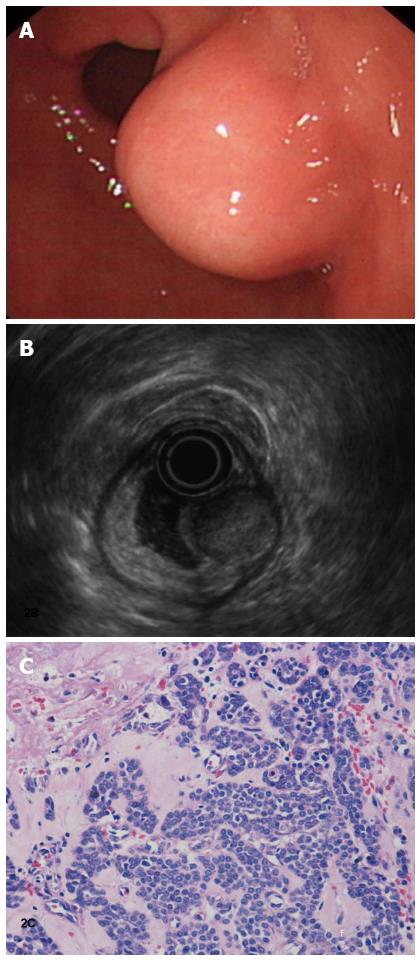
One year ago, a 57-year-old man presented to the local primary hospital with intermittent dull abdominal pain, especially when hungry. At that time, an upper gastrointestinal endoscope revealed mild diffuse gastritis. The patient underwent conservative medical management for 8 mo, during which the abdominal pain disappeared. Over a recent 3-wk period, however, the pain reappeared at levels higher than observed previously. The patient was transferred to our hospital and subjected to further investigation. A plain CT scan of the tumor revealed a clear regular contour and an isodensity mass (Figure 1A). The mass showed dense homogeneous enhancement, suggesting a lesion with an abundant blood supply and an intact overlying mucosa on an early- (Figure 1B) and delayed-phase (Figure 1C) contrast-enhanced CT. The endoscope examination showed a submucosal gastric mass that was approximately 2.0 cm and was identified at the lesser curvature of the antrum (Figure 2A). A solid mass originated from the superficial layer of the muscular propria, measuring 20 mm × 16 mm, with a marginal halo. The mass appeared to be a round, hypoechoic lesion with homogenous echogenicity (Figure 2B).
Endoscopic submucosal dissection (ESD) was initially planned, although it was unsuccessful due to significant bleeding. As such, the patient was treated via open-abdomen partial surgical excision of the stomach. After 7 mo, an abdominal CT follow-up showed no sign of tumor recurrence.
Pathologic findings indicated that this was a glomus tumor, which is characterized by many vessels that are lined with endothelial cells and surrounded by a solid proliferation of round or cuboidal cells with a round nucleus and eosinophilic cytoplasm (Figure 2C). Immunohistochemical studies revealed that the tumor cells were positive for smooth muscle actin (40%) and muscle-specific actin (100%++) but negative for desmin and CD34/CD31.
Gastric tumors are either epithelial or mesenchymal in origin. Radiologic findings enable a straightforward differentiation between mucosal or submucosal tumors. However, the identification of benign or malignant tumors depends on their biological behavior. Approximately 85% to 90% of all gastric tumors are benign, and 50% of these are mesenchymal in origin. This subgroup of gastric tumors can be further divided into two groups. The first group arises from the somatic tissue, which includes smooth muscle tumors, neural tumors, lipocystic tumors, and tumors originating from vascular or perivascular tissues (e.g., glomus tumors). In contrast, more than 95% of all malignant tumors of the stomach are adenocarcinomas. The remaining malignant tumor types include lymphomas, sarcomas (e.g., malignant gastrointestinal stromal tumors), and carcinoid tumors, among others. The second group is larger and includes GISTs[1]. Glomus tumors in the stomach are rare and are almost always benign; therefore, the most important role of imaging in diagnostic and therapeutic approaches is the differentiation of GISTs from other gastric submucosal tumors.
In 1935, Masson published a monograph entitled “Les glomus cutanes del’Homme.” The classic location of a glomus tumor is the subungual region of a digit, although it may occur anywhere in the body, including the skin, soft tissues, nerves, stomach, nasal cavity, trachea, and liver. Glomus tumors of the stomach typically appear as a submucosal nodule or mass on the antrum[2]. Although a glomus tumor of the stomach is a pathologic diagnosis, it should be included as a differential diagnosis if a solitary, hypervascular submucosal tumor is present in the stomach. Other mesenchymal tumors, such as carcinoid tumors, GISTs, neurilemmomas, and hemangiomas, may show a similar pattern. The overlap of radiologic findings in many gastric tumors makes their differentiation difficult. However, some unusual gastric tumors have characteristic radiologic features that may suggest a specific diagnosis. The incidence of gastric carcinoid tumors is increasing, and multiple tumors may occur from the fundus to the antrum. The imaging features of GISTs are variable, ranging from a small nodule with signal changes similar to the gastric muscle to a large mass with a cystic change, an intratumoral hemorrhage, necrosis, or ulceration with air-fluid levels. Hemangiomas are characterized by color changes in the vessels that appear blue or red in an endoscopy and may contain phleboliths that can be detected by CT. Neurilemmomas have a moderately or markedly higher hyperintensity with T2-weighted imaging. In combination with tumor size, location, enhanced pattern, and the intrinsic soft-tissue content, the high resolution provided by an magnetic resonance imaging may constitute one the noninvasive diagnostic or therapeutic options for gastric glomus tumors, in addition to surgery, endoscopic mucosal resectioning, and fine-needle aspiration biopsy[1,3,4].
Our report describes a method of discerning between gastric glomus tumors and other SMTs via CT and EUS imaging findings. Endoscopic submucosal enucleation is a feasible and safe procedure for the treatment of this lesion. However, further investigation and comparative studies are required to confirm the safety and efficacy of this method. Radiologically, glomus tumors appear as smooth submucosal masses with or without ulceration and may contain tiny flecks of calcification. Imaging results may suggest suspicious malignant findings, such as a large lesion measuring more than 3-5 cm, a lesion with irregular margins, a cystic space, a heterogeneous echo, or an interval change on follow-up. However, in the absence of such findings, close surveillance is recommended along with an operation and postoperative follow-ups at undefined time intervals[4-6].
In conclusion, CT and EUS findings that correlate with clinical presentations can contribute to the early detection and identification of gastric glomus tumors, particularly in terms of assessing the tumor blood supply. Endoscopists prefer ESD to open surgery, although the former necessitates caution due to complications that may occur due to significant bleeding.
P- Reviewer Kita H S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Park SH, Han JK, Kim TK, Lee JW, Kim SH, Kim YI, Choi BI, Yeon KM, Han MC. Unusual gastric tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Murray MR, Stout AP. The glomus tumor: Investigation of its distribution and behavior, and the identity of its “epithelioid” cell. Am J Pathol. 1942;18:183-203. [PubMed] |
| 3. | Maffei M, Andereggen E, Genevay M, Huber O, Dumonceau JM. Clinical challenges and images in GI. A gastric glomus tumor. Gastroenterology. 2006;131:1380, 1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Kang JH, Lim JS, Kim JH, Hyung WJ, Chung YE, Choi JY, Park MS, Kim MJ, Kim KW. Role of EUS and MDCT in the diagnosis of gastric submucosal tumors according to the revised pathologic concept of gastrointestinal stromal tumors. Eur Radiol. 2009;19:924-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Canzonieri V, Bidoli E. Differential diagnosis of malignant gastric tumors. A pathological appraisal with reference to locally advanced gastric carcinoma. Suppl Tumori. 2003;2:S5-S9. [PubMed] |
| 6. | Ferrozzi F, Tognini G, Marchesi G, Spaggiari E, Pavone P. Gastric tumors with fatty components. CT findings and differential diagnosis. Radiol Med. 2000;100:343-347. [PubMed] |