Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1247
Revised: November 18, 2012
Accepted: December 25, 2012
Published online: February 28, 2013
Processing time: 199 Days and 9.3 Hours
AIM: To identify key variables associated with colon cancer testing using the 2009 California Health Inventory Survey (CHIS).
METHODS: The CHIS has been conducted biennially since 2001 using a two-stage, geographically stratified random-digit-dial sample design to produce a representative sample of the entire State. For this study we used survey data from 2001-2009 inclusive. We restricted our analysis to White, Black, and Hispanic/Latinos aged 50-80 years. Weighted data was used to calculate the proportion of participants who underwent some form of colon cancer testing (colonoscopy, flexible sigmoidoscopy or fecal occult blood testing) within the previous 5 years stratified by race/ethnicity. For inferential analysis, boot-strapping with replacement was performed on the weighted sample to attain variance estimates at the 95%CI. For mean differences among categories we used t-tests and for comparisons of categorical data we used Pearson’s χ2. Binary logistic regression was used to identify independent variables associated with undergoing some form of testing. Trend analysis was performed to determine rates of testing over the study period stratified by race.
RESULTS: The CHIS database for 2009 had 30 857 unique respondents corresponding to a weighted sample size of 10.6 million Californians. Overall, 63.0% (63.0-63.1) underwent a colon cancer test within the previous 5 years; with 70.5% (70.5%-70.6%) of this subset having undergone colonoscopy. That is 44.5% (44.4%-44.5%) of all individuals 50-80 underwent colonoscopy. By multivariable regression, those tested were more likely to be male (OR = 1.06; 95%CI: 1.06-1.06), Black (OR = 1.30; 95%CI: 1.30-1.31), have a family member with colon cancer (OR = 1.71; 95%CI: 1.70-1.72), and have health insurance (OR = 2.71; 95%CI: 2.70-2.72). Progressive levels above the poverty line were also associated with receiving a test (100%-199%: 1.21; 1.20-1.21), (200%-299%:1.41; 1.40-1.42), (> 300:1.69; 1.68-1.70). The strongest variable was physician recommendation (OR = 3.90; 95%CI: 3.88-3.91). For the Hispanic/Latino group, additional variables associated with testing were success of physician-patient communication (OR = 2.44; 95%CI: 2.40-2.48) and naturalized citizenship status (OR = 1.91; 95%CI: 1.89-1.93). Trend analysis demonstrated increased colon cancer testing for all racial/ethnic subgroups from 2001-2009 although the rate remained considerably lower for the Hispanic/Latino subgroup.
CONCLUSION: Using CHIS we identified California citizens most likely to undergo colon cancer testing. The strongest variable associated with testing for all groups was physician recommendation.
- Citation: Modiri A, Makipour K, Gomez J, Friedenberg F. Predictors of colorectal cancer testing using the California Health Inventory Survey. World J Gastroenterol 2013; 19(8): 1247-1255
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1247
Colorectal cancer is the fourth leading cause of new cancer cases diagnosed in the United States and is the second leading cause of cancer death[1]. Population-based surveys such as the Behavior Risk Factor Surveillance System (BRFSS) and the National Health Interview Survey (NHIS) suggest that colon cancer screening tests are underutilized in the United States[2]. Based on Surveillance, Epidemiology and End Results (SEER) data, adults over the age of 50 demonstrate a low prevalence of colon cancer screening[2-5]. One study reported that only 59% of adults aged ≥ 50 years were screened for colon cancer[6].
Colon cancer can be prevented in most cases by strict adherence to accepted colon cancer screening guidelines[7]. A position paper co-authored by several specialty societies listed flexible sigmoidoscopy, Computed tomography colonography, barium enema every 5 years, or colonoscopy every 10 years in average risk adults ≥ 50 years of age as acceptable for the detection of adenomatous polyps and cancer[7-10]. Annual fecal occult blood testing (FOBT), preferably with immunochemical methodology, as an acceptable alternative method for cancer screening[7,8]. Cancer screening using stool DNA remains under investigation[8,10]. Despite a lack of evidence from randomized controlled trials, colonoscopy has been shown to be a more sensitive test for the detection of adenomas and colon cancer than flexible sigmoidoscopy +/- FOBT[11,12].
Factors identified as positive predictors of colon cancer screening participation have included older age, male gender, married status, negative smoking history, higher education, white non-Hispanic race, family history of colon cancer, regular medical care, physician recommendation, participation in other preventive health care services, and health insurance coverage[2,4,13-26].
The California Health Inventory Survey (CHIS) is the nation’s largest state health survey. A random-dial telephone survey is conducted every two years on a wide range of health topics. CHIS data gives a detailed picture of the health care needs of California’s large and diverse population. More than 50 000 Californians including adults, teenagers and children, are surveyed[27]. The specific aim of this study was to explore the CHIS database to identify demographic, socioeconomic, health and behavioral factors associated with participation in colon cancer testing. In addition, another aim was to perform a detailed analysis of the Latino population.
CHIS is a collaborative project of the University of California at Los Angeles Center for Health Policy Research, the California Department of Health Services, and the Public Health Institute. (http://www.chis.ucla.edu/methodology.html). The survey has been conducted biennially since 2001 using a two-stage, geographically stratified random-digit-dial sample design to produce a representative sample of the State. At the first stage, telephone numbers are drawn within predefined geographic areas or “strata”. Telephone numbers are screened to determine if they are households and thus eligible for the survey. At the second stage, one adult is randomly selected among all adults living in a household.
Questionnaires in CHIS include, among others, health status, health conditions, physical disability, mental health, health behaviors, women’s health, cancer history and prevention, food environment, neighborhood and housing, access to and use of health care, health insurance, public program eligibility, interpersonal violence, employment, income and demographics including preferred spoken language, language of TV, radio, and newspaper. Participant citizenship, immigration status, country of birth and English language proficiency is also ascertained.
For this study CHIS data from 2001-2009 was downloaded in SPSS v19 (IBM, Armonk, NY, United States ) format. Each survey was individually weighted using the final sample weight (RAKEDW0). RAKEDW0 accounts for sample selection probabilities and statistical adjustments for possible under coverage and nonresponse biases. It is essential to weigh the data for valid variance estimation otherwise it will be underestimated.
We first analyzed the group aged 50-80 years from the most recent survey, 2009. Racial/ethnic analysis was limited to White, Black, and Hispanic/Latino groups as classified by the UCLA CHPR definition. For descriptive and inferential analyses, boot-strapping with replacement (iterations = 1000) was performed to attain variance estimates at the 95%CI[28,29]. This was performed using the bootstrapping module for SPSS. For mean differences among categories we used t-tests and for comparisons of categorical data we used Pearson’s χ2. We performed multivariable logistic regression using colon cancer testing (colonoscopy, flexible sigmoidoscopy or FOBT) as the dependent variable. Independent predictor variables entered into the model were those judged to be of clinical importance in univariate analysis. The output was expressed as odds ratios and their bootstrapped 95% confidence intervals. We subsequently stratified our sample and repeated the regression analysis for those in the Hispanic/Latino sub-group.
We used weighted CHIS data for 2001-2009 to calculate the proportion of participants who underwent some form of colon cancer testing within the previous 5 years stratified by race/ethnicity. The proportion was derived by dividing the number of individuals age 50-80 in each race who underwent testing in a given year by the total number of 50-80 year olds in that particular racial/ethnic category for that year. A two-tailed P value < 0.05 was considered significant for hypothesis testing.
There were 47 614 individual participants in the 2009 CHIS survey. This corresponded to a full weighted sample size of 27.6 million individuals from the State of California. There were 30 857 unique respondents between the ages of 50-80 years corresponding to a weighted sample of 10.6 million citizens. Table 1 categorizes these participants as to whether they received at least one colon cancer test within the previous 5 years and further identifies those who received colonoscopy. Overall, 63.0% (63.0-63.1) underwent a colon cancer test; with 70.5% (70.5-70.6) of this subset having undergone colonoscopy. That is 44.5% (44.4-44.5) of all individuals 50-80 underwent colonoscopy. The mean age of CHIS participants between the ages of 50-80 years was 63.33 (63.32-63.33). Those who underwent colonoscopy were approximately three years older. Whites comprised the largest racial group at 60.8% (60.8-60.9) followed by Hispanic/Latinos 14.8% (CI 14.8-14.9). Hispanic/Latinos were least likely to undergo a colon cancer test. There were minimal differences in body weight among the test categories; however current smokers were far less likely to undergo a colon cancer test. There appeared to be no association between alcohol use and testing status.
| Totaln = 10 596 208 | Colonoscopy/Flexible sigmoidoscopy/Fecal occult blood testn = 6 678 773 | Colonoscopyn = 4 711 189 | No colonoscopy/Flexible sigmoidoscopy/Fecal occult blood testn = 3 917 435 | |
| Mean age, yr | 63.33 (63.32-63.33) | 64.19 (64.18-64.20) | 64.69 (64.68-64.70) | 61.86 (61.85-61.87) |
| Males, % | 46.6 (46.5-46.6) | 46.8 (46.7-46.8) | 47.2 (47.1-47.2) | 46.3 (46.2-46.3) |
| Body mass index, kg/m2, % | ||||
| < 18.5 | 1.7 (1.6-1.7) | 1.5 (1.5-1.5) | 1.5 (1.5-1.6) | 2.0 (1.9-2.0) |
| 18.5-24.99 | 36.1 (36.0-36.1) | 35.1 (35.0-35.1) | 36.3 (36.3-36.4) | 37.8 (37.8-37.9) |
| 25.0-29.99 | 37.2 (37.1-37.2) | 38.6 (38.6-38.7) | 37.8 (37.8-37.9) | 34.7 (34.7-34.8) |
| > 30.0 | 25.1 (25.1-25.1) | 24.8 (24.8-24.9) | 24.3 (24.3-24.3) | 25.5 (25.5-25.6) |
| Ethnicity1, % | ||||
| White | 60.8 (60.8-60.9) | 63.7 (63.7-63.8) | 66.1 (66.1-66.2) | 55.9 (55.8-56.0) |
| African American | 6.1 (6.1-6.1) | 6.3 (6.3-6.3) | 6.0 (6.0-6.0) | 5.8 (5.8-5.8) |
| Latino | 14.8 (14.8-14.9) | 12.2 (12.2-12.3) | 10.6 (10.6-10.7) | 19.2 (19.2-19.3) |
| Asian | 11.3 (11.2-11.3) | 10.7 (10.7-10.7) | 10.5 (10.4-10.5) | 12.2 (12.2-12.2) |
| Other | 7.0 (7.0-7.0) | 7.0 (7.0-7.0) | 6.7 (6.6-6.7) | 5.8 (5.7-5.8) |
| Smoking status, % | ||||
| Current | 11.1 (11.1-11.2) | 8.2 (8.2-8.3) | 8.0 (8.0-8.0) | 16.1 (16.1-16.1) |
| Former | 35.9 (35.8-35.9) | 37.8 (37.8-37.9) | 38.4 (38.3-38.4) | 32.5 (32.5-32.6) |
| Never | 53.0 (52.9-53.0) | 53.9 (53.9-54.0) | 53.6 (53.6-53.7) | 51.3 (51.3-51.4) |
| Did not complete high school, % | 15.7 (15.7-15.8) | 12.9 (12.9-13.0) | 10.7 (10.7-10.8) | 20.4 (20.4-20.5) |
| Marital status, % | ||||
| Married | 65.0 (65.0-65.0) | 67.9 (67.8-67.9) | 68.8 (68.7-68.8) | 60.1 (60.0-60.1) |
| Living w/partner | 4.1 (4.1-4.1) | 3.7 (3.7-3.8) | 3.6 (3.6-3.6) | 4.6 (4.6-4.7) |
| Widow/separated/divorced | 25.1 (25.1-25.2) | 23.7 (23.6-23.7) | 23.2 (23.2-23.2) | 27.7 (27.6-27.7) |
| Never married | 5.8 (5.8-5.8) | 4.7 (4.7-4.7) | 4.4 (4.4-4.4) | 7.6 (7.6-7.7) |
| Household income (United States $) | 73 671 (73 630-73 720) | 79 191 (79 151-79 238) | 81 798 (81 728-81 876) | 64 260 (64 157-64 336) |
| Income relative to poverty level, % | ||||
| 0-99 | 10.8 (10.8-10.8) | 7.7 (7.7-7.8) | 7.1 (7.1-7.1) | 16.5 (16.4-16.5) |
| 100-199 | 17.1 (17.1-17.2) | 14.9 (14.8-14.9) | 14.3 (14.3-14.3) | 21.3 (21.2-21.3) |
| 200-299 | 13.7 (13.6-13.7) | 13.2 (13.2-13.3) | 13.5 (13.5-13.5) | 14.4 (14.4-14.5) |
| > 300 | 58.4 (58.4-58.4) | 64.2 (64.1-64.2) | 65.1 (65.1-65.2) | 47.8 (47.6-47.9) |
| Dwelling2, % | ||||
| Urban | 39.7 (39.6-39.7) | 39.5 (39.4-39.5) | 37.6 (37.6-37.7) | 40 (40.0-40.1) |
| Secondary city | 20.3 (20.2-20.3) | 19.5 (19.5-19.6) | 19.4 (19.3-19.4) | 21.4 (21.4-21.5) |
| Suburban | 25.1 (25.1-25.1) | 26.9 (26.9-27.0) | 28.2 (28.2-28.3) | 22.1 (22.0-22.1) |
| Rural | 15.0 (14.9-15.0) | 14.1 (14.0-14.1) | 14.8 (14.8-14.8) | 16.4 (16.4-16.5) |
| Insurance status, % | ||||
| Uninsured | 9.5 (9.5-9.5) | 4.6 (4.6-4.6) | 3.3 (3.3-3.3) | 17.8 (17.8-17.9) |
| Medicare and medicaid | 8.9 (8.9-9.0) | 8.0 (8.0-8.0) | 8.5 (8.5-8.6) | 10.5 (10.5-10.5) |
| Medicare and others | 27.2 (27.2-27.2) | 31.8 (31.8-31.9) | 33.9 (33.9-34.0) | 19.3 (19.3-19.4) |
| Medicare only | 3.4 (3.4-3.4) | 3.1 (3.1-3.1) | 3.4 (3.4-3.4) | 3.8 (3.8-3.8) |
| Medicaid | 3.6 (3.6-3.6) | 2.3 (2.3-2.3) | 2.1 (2.0-2.1) | 5.8 (5.7-5.8) |
| Employer-based | 41.1 (41.0-41.1) | 43.6 (43.6-43.6) | 42.2 (42.1-42.2) | 36.7 (36.7-36.7) |
| Privately purchased | 4.6 (4.6-4.6) | 4.6 (4.6-4.6) | 4.6 (4.6-4.6) | 4.6 (4.5-4.6) |
| Other | 1.8 (1.8-1.8) | 2.0 (2.0-2.0) | 2.1 (2.0-2.1) | 1.5 (1.5-1.5) |
| Alcohol use, % | ||||
| Never | 93.3 (93.2-93.4) | 93.5 (93.4-93.6) | 93.5 (93.4-93.6) | 93.0 (93.0-93.1) |
| Yes | 6.7 (6.2-6.8) | 6.6 (6.5-6.9) | 6.5 (6.5-6.8) | 6.9 (6.8-7.0) |
| Working status, % | ||||
| Employed full time | 41.4 (41.4-41.4) | 39.9 (39.8-39.9) | 38.1 (38.1-38.2) | 44.0 (43.9-44.0) |
| Employed part time | 7.1 (7.1-7.1) | 7.0 (7.0-7.1) | 7.2 (7.2-7.2) | 7.1 (7.1-7.2) |
| Mod-Severe work impaired | 6.1 (6.1-6.1) | 5.4 (5.4-5.4) | 5.3 (5.3-5.4) | 7.4 (7.4-7.5) |
| Spouse Employed F/P Time | 32.6 (32.4-32.6) | 33.6 (33.5-33.6) | 33.4 (33.4-33.6) | 30.8 (30.7-30.8) |
There was an association between educational attainment and colon cancer testing. Those who underwent a colonoscopy were nearly twice as likely [20.4% (20.4-20.5) vs 10.7% (10.7-10.8)] to have graduated high school. Marital status and household income also appeared to be associated with testing. Those tested were far more likely to be living above the poverty line threshold as defined by the United States Department of Labor. Among those who underwent a test, 4.6% (4.6-4.6) were uninsured. In contrast, among those who did not undergo a test, 17.8% (17.8-17.9) were uninsured. Factors associated with colonoscopy (Table 2).Respondents who underwent colonoscopy reported more frequent doctor visits in the year prior to the survey. They also reported receiving most of their medical care in a doctor’s office setting compared to those who did not [78.1 (78.1-78.2) vs 65.1 (65.0-65.1)]. Those who underwent a colonoscopy had a higher prevalence of medical problems such as diabetes and were more likely to participate in health screening tests such as mammograms for females and prostate specific antigen (PSA) tests for males. The prevalence of having a family history of colorectal cancer (CRC) in a first degree relative, usually a parent, was two-fold higher in respondents who underwent colonoscopy [10.7 (10.7-10.7) vs 4.8 (4.8-4.8)]. Participants who underwent a colonoscopy were far more likely to receive a recommendation for this test from their physician. A substantial majority were performed for “routine screening”. Characteristics of Hispanic/Latino CHIS Participants (Table 3).
| Colonoscopyn = 4 711 189 | No colonoscopyn = 5 885 019 | |
| Number doctor visits in past year, % | 4.22 (4.22-4.22) | 3.28 (3.28-3.28) |
| Site of health provider, % | ||
| Doctor’s office | 78.1 (78.1-78.2) | 65.1 (65.0-65.1) |
| Community/government clinic | 18.1 (18.1-18.2) | 21.4 (21.3-21.4) |
| Emergency room | 0.2 (0.2-0.2) | 0.8 (0.8-0.8) |
| Random source/none | 0.5 (0.5-0.5) | 12.7 (12.6-12.7) |
| General health, % | ||
| Excellent | 17.8 (17.7-17.7) | 16.7 (16.6-16.7) |
| Very good | 31.0 (31.0-31.1) | 28.2 (28.2-28.3) |
| Good | 29.0 (28.9-29.1) | 29.0 (28.9-29.0) |
| Fair | 16.3 (16.3-16.4) | 19.2 (19.2-19.3) |
| Poor | 5.8 (5.8-5.8) | 6.9 (6.9-6.9) |
| Medical health, % | ||
| Elevated cholesterol | 12.1 (12.1-12.2) | 10.0 (9.9-10.0) |
| Hypertension | 43.8 (43.8-43.9) | 35.9 (35.8-35.9) |
| Diabetes | 15.9 (15.9-15.9) | 15.0 (14.9-15.0) |
| Heart disease | 14.2 (14.1-14.2) | 10.8 (10.8-10.8) |
| Mammograms in past 6 yr, n | 2.23 (2.23-2.34) | 1.64 (1.63-1.64) |
| PSA ever tested, % | 34.3 (34.3-34.4) | 22.1 (22.0-22.1) |
| Physician recommended “colon test”, % | 67.3 (67.3-67.4) | 39.6 (39.5-39.6) |
| Reason for colonoscopy, % | ||
| Routine screening | 75.4 (75.3-75.4) | |
| Because of a problem | 16.8 (16.7-16.8) | |
| Other | 7.9 (7.9-7.9) | |
| Reason no colonoscopy in 10 yr, % | ||
| Never thought about it | 11.9 (11.9-12.0) | |
| Doctor did not recommend | 10.0 (10.0-10.1) | |
| No problems/not necessary | 9.6 (9.5-9.6) | |
| Too expensive/no insurance | 4.5 (4.5-4.6) | |
| Procrastination | 3.8 (3.8-3.8) | |
| Too painful/embarrassing | 2.9 (2.9-2.9) | |
| Other | 57.3 (57.3-57.4) | |
| Family history of CRC, % | ||
| Sibling | 2.8 (2.8-2.8) | 1.6 (1.6-1.6) |
| Father | 4.1 (4.1-4.1) | 1.8 (1.8-1.8) |
| Mother | 3.8 (3.8-3.8) | 1.4 (1.4-1.4) |
| Total | 10.7 (10.7-10.7) | 4.8 (4.8-4.8) |
| Total | Colonoscopy/Flexible sigmoidoscopy/Fecal occult blood test | Colonoscopy | No Colonoscopy/Flexible sigmoidoscopy/Fecal occult blood test | |
| Weighted | Weighted | Weighted | Weighted | |
| n = 1 487 447 | n = 801 832 | n = 492 646 | n = 685 615 | |
| Latino/Hispanic sub-type, % | ||||
| Mexican | 80.6 (80.5-80.6) | 79.8 (79.7-79.9) | 79.2 (79.1-79.4) | 81.4 (81.3-81.5) |
| Salvadoran | 8.2 (8.1-8.2) | 6.4 (6.4-6.5) | 8.0 (7.9-8.1) | 10.3 (10.3-10.4) |
| Guatemalan | 2.4 (2.3-2.4) | 2.0 (2.0-2.0) | 1.6 (1.6-1.7) | 2.7 (2.7-2.8) |
| Central American | 2.8 (2.7-2.8) | 3.5 (3.4-3.5) | 3.0 (2.9-3.0) | 1.9 (1.9-1.9) |
| South American | 2.1 (2.0-2.1) | 2.7 (2.7-2.8) | 2.3 (2.3-2.3) | 1.4 (1.3-1.4) |
| Other Latino | 3.9 (3.8-3.9) | 5.6 (5.5-5.6) | 5.9 (5.9-6.0) | 2.3 (2.3-2.4) |
| Citizen status, % | ||||
| United states born citizen | 31.2 (31.1-31.2) | 37.1 (37.0-37.2) | 39.3 (39.1-39.4) | 24.2 (24.1-24.3) |
| Naturalized citizen | 43.5 (43.4-43.6) | 47.4 (47.3-47.6) | 50.2 (50.1-50.3) | 38.9 (38.8-39.0) |
| Non-citizen | 25.3 (25.2-25.4) | 15.4 (15.4-15.5) | 10.5 (10.4-10.6) | 36.9 (36.8-37.0) |
| Year lived in United States, % | ||||
| ≤ 1 | 0.2 (0.2-0.2) | 0.0 (0.0-0.1) | 0.0 (0.0-0.2) | 0.5 (0.4-0.5) |
| 2-4 | 1.4 (1.3-1.4) | 1.1 (1.1-1.1) | 0.8 (0.8-0.8) | 1.6 (1.6-1.7) |
| 5-9 | 1.0 (1.0-1.1) | 0.5 (0.5-0.5) | 0.3 (0.2-0.3) | 1.6 (1.6-1.7) |
| 10-14 | 3.9 (3.8-3.9) | 3.1 (3.1-3.2) | 1.3 (1.2-1.3) | 4.8 (4.7-4.8) |
| ≥ 15 | 62.4 (62.3-62.4) | 58.1 (58.1-58.3) | 58.4 (58.3-58.6) | 67.3 (67.2-67.5) |
| Not ascertained | 31.2 (31.2-31.3) | 37.2 (37.2-37.3) | 39.2 (39.1-39.4) | 24.2 (24.1-24.5) |
| Ability to speak English, % | ||||
| Very good | 13.0 (12.9-13.1) | 15.5 (15.4-15.5) | 17.7 (16.9-17.1) | 10.1 (10.1-10.2) |
| Good | 19.1 (19.0-19.1) | 21.7 (21.6-21.8) | 26.9 (26.7-27.0) | 16.1 (16.0-16.2) |
| Not good | 33.4 (33.3-33.5) | 29.6 (29.5-29.8) | 23.1 (23.0-23.3) | 37.8 (37.7-37.9) |
| Not at all | 18.8 (18.7-18.8) | 14.6 (14.5-14.7) | 15.3 (15.2-15.) | 23.6 (23.5-23.7) |
| Language of TV/radio/newspaper, % | ||||
| English | 17.2 (17.1-17.3) | 19.9 (19.8-20.0) | 24.3 (24.2-24.5) | 14.0 (14.0-14.2) |
| English and Spanish | 33.0 (32.9-33.1) | 32.9 (32.7-33.0) | 31.1 (30.9-31.2) | 33.2 (33.1-33.3) |
| Only Spanish | 34.1 (34.0-34.2) | 28.7 (28.6-28.8) | 27.6 (27.5-27.8) | 40.4 (40.3-40.6) |
| Not ascertained | 15.7 (15.7-15.7) | 18.6 (18.5-18.7) | 17.0 (16.9-17.1) | 12.3 (12.2-12.4) |
| Language spoken at home, % | ||||
| English | 15.7 (15.7-15.8) | 18.6 (18.5-18.7) | 17.0 (16.9-17.1) | 12.3 (12.2-12.4) |
| English and Spanish | 46.6 (46.5-46.7) | 48.4 (48.3-48.5) | 54.0 (53.9-54.3) | 44.5 (44.4-44.7) |
| Only Spanish | 37.0 (36.9-37.1) | 32.2 (32.2-32.3) | 28.1 (27.9-28.2) | 42.6 (42.5-42.8) |
| Other | 0.7 (0.7-0.7) | 0.8 (0.8-0.9) | 0.9 (0.8-0.9) | 0.6 (0.6-0.7) |
| Doctor conducted last interview in, % | ||||
| English | 32.0 (31.9-32.1) | 41.0 (40.8-41.1) | 46.4 (46.2-46.5) | 21.5 (21.4-21.6) |
| Spanish | 39.7 (39.6-39.8) | 34.5 (34.4-34.6) | 29.6 (29.4-29.7) | 45.7 (45.6-45.9) |
| Not ascertained | 28.3 (28.2-28.4) | 24.5 (24.4-24.6) | 24.0 (23.9-24.3) | 32.8 (32.7-32.9) |
| Hard time understanding your doctor, % | ||||
| Yes | 5.3 (5.2-5.3) | 3.5 (3.5-3.6) | 4.0 (3.9-4.1) | 7.3 (7.2-7.4) |
| No | 87.0 (87.0-87.1) | 94.1 (94.0-94.2) | 92.4 (92.3-92.5) | 78.8 (78.7-78.9) |
| Not ascertained | 7.7 (7.7-7.8) | 2.4 (2.2-2.6) | 3.6 (3.5-3.7) | 13.9 (13.8-14.0) |
The largest number of Hispanic/Latino CHIS participants were of Mexican origin. The prevalence of testing was higher for naturalized citizens compared to those born in the United States. Non-citizens were least likely to undergo testing. Those who did not undergo testing lived within the United States longer than those who were tested.
The ability to speak English and the language spoken at home was associated with testing status. The prevalence of testing in those who spoke “very good” or “good” English compared to their less proficient counterparts was [37.2% (37.0-37.3) vs 26.2% (26.1-26.4)]. Those tested were more likely to speak English in their home. In addition, the language of TV/radio/newspaper was also associated with testing. Among Hispanic/Latino respondents who underwent colon testing, rates were higher for those who listened and watched media in English. Testing rates were lowest for those who listened and watched only in Spanish. Respondents were considerably more likely to have undergone a colon test if their doctor conducted the interview in English [41.0% (40.8-41.1) vs 21.5% (21.4-21.6)]. Lastly, participants who did not have a test were twice as likely to have “Had hard time understanding their doctor at the last visit” [7.3 (7.2-7.4) vs 3.5 (3.5-3.6)]. Multivariable analysis (Table 4).
| Variable | n | All participants adjustedOR1 (95%CI) | White adjustedOR1 (95%CI) | Black adjustedOR1 (95%CI) | Hispanic adjustedOR1 (95%CI) |
| Gender | |||||
| Female | 5 658 375 | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 4 937 833 | 1.06 (1.06-1.06) | 1.03 (1.03-1.04) | 1.07 (1.06-1.08) | 1.25 (1.24-1.26) |
| High school educated | |||||
| No | 1 663 605 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 8 932 603 | 1.01 (1.00-1.01) | 0.96 (0.95-0.99) | 1.03 (0.98-1.05) | 1.28 (1.27-1.29) |
| Family history of colon cancer | |||||
| No | 9 809 630 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 786 578 | 1.71 (1.70-1.72) | 1.88 (1.87-1.89) | 1.54 (1.53-1.56) | 1.29 (1.26-1.33) |
| Income relative to poverty level | |||||
| 0%-99% | 1 144 390 | 1.00 | 1.00 | 1.00 | 1.00 |
| 100%-199% | 1 811 952 | 1.21 (1.20-1.21) | 1.33 (1.30-1.36) | 1.14 (1.12-1.15) | 0.88 (0.87-0.89) |
| 200%-299% | 1 451 680 | 1.41 (1.40-1.42) | 1.44 (1.40-1.48) | 1.31 (1.27-1.40) | 1.50 (1.49-1.52) |
| > 300% | 6 188 185 | 1.69 (1.68-1.70) | 1.77 (1.72-1.86) | 1.66 (1.64-1.69) | 1.48 (1.46-1.50) |
| Health insured | |||||
| No | 1 006 640 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 9 589 568 | 2.71 (2.70-2.72) | 2.88 (2.87-2.89) | 2.14 (2.11-2.23) | 3.52 (3.48-3.56) |
| Physician Recommended colon test | |||||
| No | 5 095 110 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 5 501 098 | 3.90 (3.88-3.91) | 3.77 (3.74-3.80) | 3.99 (3.96-4.02) | 4.37 (4.32-4.42) |
| Citizenship status1 | |||||
| United States born citizen | 464 083 | 1.00 | |||
| Naturalized citizen | 647 039 | 1.91 (1.89-1.93) | |||
| Years lived in United States1 | |||||
| ≤ 1 | 29 749 | 1.00 | |||
| 2-4 | 20 824 | 0.98 (0.96-1.02) | |||
| 5-9 | 14 874 | 1.38 (1.33-1.44) | |||
| 10-14 | 58 010 | 0.78 (0.75-0.82) | |||
| ≥ 15 | 928 167 | 1.57 (1.54-1.62) | |||
| Hard time understanding physician1 | |||||
| Yes | 78 835 | 1.00 | |||
| No | 1 294 079 | 2.44 (2.40-2.48) | |||
The output from the regression model demonstrated that Blacks had an odds ratio 30% higher than Whites for undergoing a colon cancer test. Male gender, family history of colon cancer, and progressive levels above the poverty line were also factors independently associated with testing. Having health insurance and a physicians’ recommendation were the strongest predictors of testing. For the Hispanic/Latino group, additional independent factors associated with testing included citizenship status and ability to understand their physician during their most recent office visit.
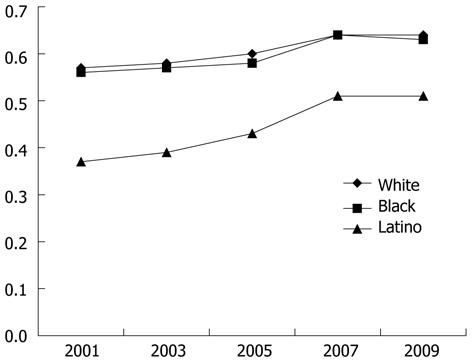
Colon Cancer Testing Trend Data (Figure 1). Figure 1 displays the proportion of CHIS participants age 50-80 who underwent colon cancer testing (colonoscopy, flexible sigmoidoscopy or FOBT) within the previous 5 years stratified by race/ethnicity. The proportion tested increased in all groups between 2001 and 2007 and then appeared to plateau. Overall, the prevalence of testing was nearly identical among Whites and Blacks. The proportion of Hispanic/Latinos who underwent a test rose from approximately 35% in 2001 to 50% in 2009.
According to the 2009 CHIS survey, approximately 63.0% of participants aged 50-80 had a colon cancer test such as FOBT, flexible sigmoidoscopy or colonoscopy within the previous 5 years. Of these, approximately 70% underwent colonoscopy as the colon cancer test. Regression analysis demonstrated that those who underwent at least one colon cancer test were more likely to be male, Black, have a family history of CRC, and have health insurance. Progressive levels above the poverty line were also associated with receiving a test. The strongest variable associated with undergoing a test was physician recommendation. For the Hispanic/Latino group, those at increased risk for no testing were those born in the United States and those who “Had hard time understanding their doctor at the last visit”.
Trend analysis demonstrated that the proportion of Blacks in California undergoing a colon cancer test is increasing in parallel with other racial/ethnic groups. This is encouraging as prior studies had identified white, non-Hispanic race as a predictor of colon cancer testing[29]. The increased participation by Blacks may be due to the more widespread use of colonoscopy as a colon cancer test. One survey revealed that this is the preferred colon cancer test by this group[13]. We were surprised to find no association between colon cancer testing and educational level. Prior studies suggested educational attainment was a positive predictor of colon cancer screening[27,30]. Similar to prior large population-based studies, our study also identified males, a family history of colon cancer, having health insurance, progressive levels above the poverty line, and having undergone screening with PSA or mammography as predictors of adherence to colon cancer testing[21]. Most importantly, our study confirms physician recommendation as the strongest variable associated with testing.
Several population-based studies looking at predictors of colorectal cancer screening participation have been performed[30]. BRFSS is a nationwide population-based telephone survey designed to measure preventive health practices, such as adherence to colon cancer testing and risk factors for adherence or non-adherence. A study of 52 754 respondents over age 50 in 1997 demonstrated low participation in colon cancer screening protocols with 19.8% for FOBT, 30.5% for sigmoidoscopy and 41.1% for either[21]. Respondents who underwent other preventive testing such as mammography or PAP smear demonstrated a higher adherence to screening[21]. Similarly, responses of 64 084 BRFSS participants over the age of 50 in 1999 demonstrated that colon cancer screening was reported by 43.4%, with 22.8% undergoing colonoscopy or sigmoidoscopy and 9.9% undergoing FOBT[30]. Negative predictors of screening included < college education and lack of health care coverage[30]. An examination of the 2002 BRFSS demonstrated a disparity in colon cancer testing between Hispanic and non-Hispanic Whites. Only, 41.9% of Hispanic respondents aged > 50 underwent colon cancer testing in the past year by either FOBT or colonoscopy/sigmoidoscopy compared to 55.2% of non-Hispanic whites[19].
The NHIS is an in-person survey conducted by the Centers for Disease Control and Prevention. The 2000 NHIS survey revealed that less than half of the United States population > 50 years underwent colon cancer screening at the recommended time intervals[23]. The strongest predictor of adherence was a physician visit within the previous year. A usual source of care, having undergone recent health preventive services such as a mammogram or PAP smear, and physician recommendation were also important predictors[23].
An analysis of health behaviors of Medicare enrollees living in the United States was conducted using the SEER database[3]. A random sample of Medicare claims from 14 states for the period 1995 to 2003 were reviewed and demonstrated increased use of colonoscopy for colon cancer testing as well as a decline in the use of FOBT and sigmoidoscopy. The transition to use of colonoscopy was more rapid in Whites than non-Whites[3]. A similar study of Medicare claims in SEER regions from 1996-2005 showed a persistence of racial and ethnic disparities in the use of colonoscopy for screening as Whites were more likely to undergo this test as the physician preferred modality than Blacks or Hispanics[29]. Data collected in 2003 from 11 SEER regions demonstrated racial/ethnic disparities in colon cancer screening that varied significantly by geography[5]. A telephone survey of Medicare consumers living in three urban counties in North Carolina and 17 urban and rural counties in South Carolina was performed in 2001[17]. Overall, 56.8% of Whites adhered to Medicare-covered intervals for colon cancer testing compared to only 39.1% of Blacks[17]. Blacks preferred to undergo a colonoscopy rather than FOBT.
A major strength of our study is the sampling methodology incorporated into CHIS. Once weighted, the sample is representative of the entire population of the State of California including recent immigrants. Bootstrap methodology with resampling allows for accurate variance estimates. The repeated nature of the survey allows for the identification of trends in the use of health care services for the population. The incorporation of health behaviors, income, education, insurance coverage and other integral variables allows for adjusted estimates of healthcare utilization.
There are important weaknesses of our results. First, the indication for colon cancer testing is not available for every biennial survey. Other than 2009 (Table 2), we were not able to determine whether testing was performed for screening or was indicated for a sign/symptom of colonic disease. Therefore we were careful to use the terms “testing” and “screening” separately in our analysis and discussion. The dependent variable in this manuscript is clearly colon cancer testing. Erroneous conclusions about the presented trend data may be drawn if this is not kept in mind. For example, Blacks may be undergoing more colon tests over time because they are more willing to see their doctor for blood in their stools.
Another important weakness is that medical records are unavailable to confirm testing. Without records, we are left with the possibility of recall bias by the participants which could lead to misclassification. Examples include inability to remember that a doctor recommended a colon cancer test, or even that the participant had a test. Another shortcoming without records is that we are unable to separate whether patients underwent a sigmoidoscopy or FOBT as these results are reported in aggregate. Finally, without personal knowledge of the physician-patient interaction, we cannot further explain the variable “Had hard time understanding their doctor at the last visit”. According to CHIS documentation for 2009, only 1.67% of those responding yes to this question answered that this was due to language differences. Other possibilities may include visual, auditory, or intellectual disabilities.
In conclusion, approximately 63% of California citizens between the ages of 50 and 80 have some form of colon cancer testing. Males, Blacks, insured, those with a family history of colorectal cancer, and those living above the poverty line were more likely to undergo a test. The strongest variable associated with testing was physician recommendation. For Hispanic/Latinos, those born in the United States and those with difficulty understanding their doctor are at increased risk for no testing. Our data is encouraging in that the level of participation in colon cancer testing appears to be increasing over the past decade for all studied racial/ethnic groups.
Colorectal cancer is the fourth leading cause of new cancer cases diagnosed in the United States and is the second leading cause of cancer death. Population-based surveys such as the Behavior Risk Factor Surveillance System (BRFSS) and the National Health Interview Survey (NHIS) suggest that colon cancer screening tests are underutilized in the United States. Colon cancer can be prevented in most cases by strict adherence to accepted colon cancer screening guidelines. Despite a lack of evidence from randomized controlled trials, colonoscopy has been shown to be a more sensitive test for the detection of adenomas and colon cancer than flexible sigmoidoscopy +/- fecal occult blood test (FOBT). The California Health Inventory Survey (CHIS) is the nation’s largest state health survey. CHIS data gives a detailed picture of the health care needs of California’s large and diverse population. The specific aim of this study was to explore the CHIS database to identify demographic, socioeconomic, health and behavioral factors associated with participation in colon cancer testing. In addition, another aim was to perform a detailed analysis of the Latino population.
Colorectal cancer is one of the leading causes of cancer diagnosed in the United States. Prior population-based studies reported positive and negative predictors of adherence to colon cancer testing with mixed results. This large population-based study seeks to clarify these predictors and to provide a detailed analysis of the Latino population.
A major strength of our study is the sampling methodology incorporated into CHIS. Once weighted, the sample is representative of the entire population of the State of California including recent immigrants. Bootstrap methodology with resampling allows for accurate variance estimates. The repeated nature of the survey allows for the identification of trends in the use of health care services for the population. The incorporation of health behaviors, income, education, insurance coverage and other integral variables allows for adjusted estimates of healthcare utilization.
The study indicates approximately 63% of California citizens between the ages of 50 and 80 have some form of colon cancer testing. Males, Blacks, insured, those with a family history of colorectal cancer, and those living above the poverty line were more likely to undergo a test. The strongest variable associated with testing was physician recommendation. For Hispanic/Latinos, those born in the United States and those with difficulty understanding their doctor are at increased risk for no testing. Author’s data is encouraging in that the level of participation in colon cancer testing appears to be increasing over the past decade for all studied racial/ethnic groups. Most importantly, the data can be used to remove barriers to colon cancer testing amongst the general population and minorities such as the Latino population.
CHIS is the nation’s largest state health survey. A random-dial telephone survey is conducted every two years on a wide range of health topics. CHIS data gives a detailed picture of the health care needs of California’s large and diverse population. Colon cancer testing includes annual FOBT, flexible sigmoidoscopy +/- FOBT, and/or colonoscopy.
This large population-based study provides insight into predictors of undergoing colon cancer testing. This data can be used to reduce the prevalence of colorectal cancer in the United States by removing barriers to colon cancer testing in the general population which includes minorities.
P- Reviewers Leitman M, Coleman H, Tanaka T S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | American Cancer Society. Cancer Facts and Figures 2010. Atlanta, Georgia: American Cancer Society 2010; . |
| 2. | Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Fenton JJ, Cai Y, Green P, Beckett LA, Franks P, Baldwin LM. Trends in colorectal cancer testing among Medicare subpopulations. Am J Prev Med. 2008;35:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Fenton JJ, Tancredi DJ, Green P, Franks P, Baldwin LM. Persistent racial and ethnic disparities in up-to-date colorectal cancer testing in medicare enrollees. J Am Geriatr Soc. 2009;57:412-418. [PubMed] |
| 5. | Semrad TJ, Tancredi DJ, Baldwin LM, Green P, Fenton JJ. Geographic variation of racial/ethnic disparities in colorectal cancer testing among medicare enrollees. Cancer. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Available from: http://www.cdc.gov/nchs/nhis.htm.. |
| 7. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3126] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 8. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1457] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 9. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 10. | Selby JV, Friedman GD, Quesenberry CP, Weiss NS. Effect of fecal occult blood testing on mortality from colorectal cancer. A case-control study. Ann Intern Med. 1993;118:1-6. [PubMed] |
| 11. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 12. | Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1202] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 13. | Ata A, Elzey JD, Insaf TZ, Grau AM, Stain SC, Ahmed NU. Colorectal cancer prevention: adherence patterns and correlates of tests done for screening purposes within United States populations. Cancer Detect Prev. 2006;30:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, Marcus AC, Steiner JF, Ahnen DJ. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 15. | Green AR, Peters-Lewis A, Percac-Lima S, Betancourt JR, Richter JM, Janairo MP, Gamba GB, Atlas SJ. Barriers to screening colonoscopy for low-income Latino and white patients in an urban community health center. J Gen Intern Med. 2008;23:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Heo M, Allison DB, Fontaine KR. Overweight, obesity, and colorectal cancer screening: disparity between men and women. BMC Public Health. 2004;4:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Pollack LA, Blackman DK, Wilson KM, Seeff LC, Nadel MR. Colorectal cancer test use among Hispanic and non-Hispanic U.S. populations. Prev Chronic Dis. 2006;3:A50. [PubMed] |
| 20. | Rosen AB, Schneider EC. Colorectal cancer screening disparities related to obesity and gender. J Gen Intern Med. 2004;19:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Schenck AP, Klabunde CN, Davis WW. Racial differences in colorectal cancer test use by Medicare consumers. Am J Prev Med. 2006;30:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, Coates RJ. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Shelton RC, Jandorf L, Ellison J, Villagra C, DuHamel KN. The influence of sociocultural factors on colonoscopy and FOBT screening adherence among low-income Hispanics. J Health Care Poor Underserved. 2011;22:925-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Thompson B, Coronado G, Neuhouser M, Chen L. Colorectal carcinoma screening among Hispanics and non-Hispanic whites in a rural setting. Cancer. 2005;103:2491-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 562] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 27. | Available from: http://www.chis.ucla.edu/about.html. |
| 28. | Krewski D, Rao JNK. Inference From Stratified Samples: Properties of the Linearization, Jackknife and Balanced Repeated Replication Methods. Ann Statist. 1981;9:1010-1019. [RCA] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | White A, Vernon SW, Franzini L, Du XL. Racial and ethnic disparities in colorectal cancer screening persisted despite expansion of Medicare’s screening reimbursement. Cancer Epidemiol Biomarkers Prev. 2011;20:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ioannou GN, Chapko MK, Dominitz JA. Predictors of colorectal cancer screening participation in the United States. Am J Gastroenterol. 2003;98:2082-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |