Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1158
Revised: June 13, 2012
Accepted: June 28, 2012
Published online: February 28, 2013
Although much is known about how fat accumulates in the liver, much remains unknown about how this causes sustained hepatocellular injury. The consequences of injury are recognized as nonalcoholic steatohepatitis (NASH) and progressive fibrosis. The accumulation of fat within the hepatocytes sensitizes the liver to injury from a variety of causes and the regenerative capacity of a fatty liver is impaired. An additional stressor is sometimes referred to as a “second hit” in a paradigm that identifies the accumulation of fat as the “first hit”. Possible candidates for the second hit include increased oxidative stress, lipid peroxidation and release of toxic products such as malondialdehyde and 4-hydroxynonenal, decreased antioxidants, adipocytokines, transforming growth factor (TGF)-β, Fas ligand, mitochondrial dysfunction, fatty acid oxidation by CYPs (CYP 2E1, 4A10 and 4A14), and peroxisomes, excess iron, small intestinal bacterial overgrowth, and the generation of gut-derived toxins such as lipopolysaccharide and ethanol. Oxidative stress is one of the most popular proposed mechanisms of hepatocellular injury. Previous studies have specifically observed increased plasma and tissue levels of oxidative stress markers and lipid peroxidation products, with reduced hepatic and plasma levels of antioxidants. There is also some indirect evidence of the benefit of antioxidants such as vitamin E, S-adenosylmethionine, betaine, phlebotomy to remove iron, and N-acetylcysteine in NASH. However, a causal relationship or a pathogenic link between NASH and oxidative stress has not been established so far. A number of sources of increased reactive oxygen species production have been established in NASH that include proinflammatory cytokines such as tumor necrosis factor (TNF)-α, iron overload, overburdened and dysfunctional mitochondria, CYPs, and peroxisomes. Briefly, the pathogenesis of NASH is multifactorial and excess intracellular fatty acids, oxidant stress, ATP depletion, and mitochondrial dysfunction are important causes of hepatocellular injury in the steatotic liver.
- Citation: Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: A tale of “second hit”. World J Gastroenterol 2013; 19(8): 1158-1165
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1158
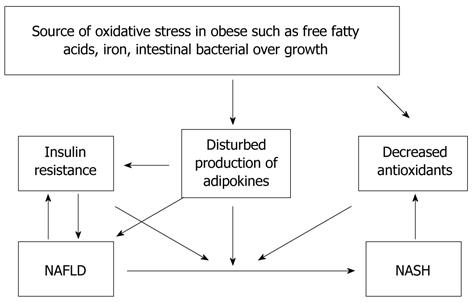
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent forms of chronic liver disease in the United States[1]. Contributing factors to this may include the increasingly sedentary lifestyle of the population and increased consumption of a high-fat (HF) diet and high fructose corn syrup (HFCS)[2]. In the setting of excessive central adiposity, insulin resistance is the major underlying cause of fat accumulation in the liver[3-7]. Nonalcoholic steatohepatitis (NASH), as a subgroup of NAFLD, is characterized by chronic and progressive liver pathology and may lead to advanced fibrosis, cirrhosis, end-stage liver disease, hepatocellular carcinoma and liver-related death[8-10]. One of the important and unresolved problems in NASH is the pathogenesis of hepatocyte injury. One hypothesis for the pathogenesis of NAFLD is the “two-hit” hypothesis[11]. According to this paradigm, the primary abnormality (“first hit”) is most likely insulin resistance, which leads to the accumulation of triglycerides within the hepatocytes. Then, a “second hit” induces hepatocyte injury and inflammation (NASH). The mechanisms that cause hepatocyte injury in fatty liver have not been fully elucidated to date. Oxidative stress is one of the most popular proposed mechanisms of hepatocellular injury (Figure 1). It was reported that obesity correlated with systemic oxidative stress in humans and mice[12-14]. Obese adults with metabolic syndrome (MS) have higher plasma concentration of oxidative stress biomarkers than obese adults without MS. Increased reactive oxygen species (ROS) production has been selectively shown in adipose tissue of obese mice[15,16]. Previous studies also specifically observed increased plasma and tissue levels of oxidative stress markers and lipid peroxidation products with reduced hepatic and plasma levels of antioxidants in patients with NASH[2-6,12-16].
Obesity is associated with low-grade chronic inflammation in humans, and this chronic inflammation is a link between obesity and insulin resistance[17-19]. Indeed, obesity is strongly associated with chronic macrophage accumulation within increased adipose tissue in obese humans. Xu et al[16] showed that inflamed macrophages are active within white adipose tissue and this activation occurs after increased adiposity and before insulin resistance. Macrophages secrete cytokines which promote insulin resistance in adipose tissue and eventually increase adipose tissue lipolysis, which causes insulin resistance in both muscle and the liver, besides significant amount of inducible NO synthase and interleukin-6[15]. Moreover, a positive correlation between adipocyte size and the content of accumulated macrophages in adipose has been reported. Increased fatty acids or accumulated macrophages might be the reason for this increased ROS production within adipose tissue. These data indicate localized inflammation and systemic consequences such as insulin resistance and increased circulating free fatty acids. Additional evidence that this chronic inflammation causes insulin resistance comes from restoring insulin sensitivity by various anti-inflammatory agents, such as high-dose salicylates via IKK-β inhibition or anti-tumor necrosis factor (TNF)-α antibody infusion[20].
A logical and attractive hypothesis is that oxidative stress in TG-loaded hepatocytes is the cause of sustained injury with consequent NASH, fibrosis and cirrhosis[11] (Figure 2). The imbalance between the increased ROS and decreased antioxidants leads to lipid peroxidation of polyunsaturated fatty acids, cellular membranes, mitochondrial membranes, and DNA[21]. Lipid peroxidation products have longer half-lives and the capability to reach extracellular targets. Lipid peroxidation produces cytotoxic aldehydes such as malondialdehyde (MDA) and 4-hydroxynonenal. ROS and these aldehydes further contribute to oxidative stress, decreased ATP production, and increased proinflammatory cytokine release. These events promote hepatocyte injury, necroinflammation and hepatocytes apoptosis. Despite the attractiveness of this hypothesis, most clinical studies only provide correlations between the presence of NASH and elevated indices of oxidant stress, without establishing a causal relationship[22-27]. The lipid peroxidation product 4-hydroxynonenal is found more in perivenular zone (zone 3), correlating with the histological lesions of NASH that are predominantly in zone 3[21]. Lipid peroxidation is greater in patients with NASH than in patients with simple steatosis. The same study has also shown that increased 4-hydroxynonenal strongly correlates with both the grade of necroinflammation and the stage of NASH, but not with the grade of steatosis, while increased evidence of oxidant damage to DNA as measured by 8-hydroxydeoxyguanosine only correlates with the grade of necroinflammation in patients with NASH.
Oxidative stress might play a role in the pathogenesis of hepatocyte dysfunction and inflammation in NAFLD. Oxidative stress can result from either excess ROS production and/or deficient antioxidant capacity[12-15,21]. The enzyme NAD(P)H oxidase catalyzes the transfer of a single electron to molecular oxygen to produce superoxide. Upregulation of NAD(P)H oxidase can raise ROS production, and thereby contribute to the pathogenesis of oxidative stress in HF and high-sucrose-fed mice. Formation of ROS is enhanced by different mechanisms, including xanthine oxidase activation, NADH auto-oxidation, and superoxide dismutase (SOD) inactivation. Experimental evidence supports the concept of diverse ROS, such as superoxide anion, hydrogen peroxide, hydroxyl radical, nitric oxide and peroxynitrite.
Assessment of: (1) NAD(P)H oxidase (a major source of ROS) in liver; (2) downregulation of the main antioxidant enzymes, SOD, glutathione peroxidase (GPX), catalase, and heme oxygenase (HO) in liver tissue; (3) level of lipid peroxidation products, MDA + 4-hydroxyalkenals in the liver; (4) plasma concentration of 8-isoprostanes, lipoperoxides in plasma samples by measuring MDA via HPLC; and (5) immunoblotting to quantify NADPH oxidase, Mn SOD, Cu Zn SOD, GPX, catalase, and HO-2 levels in liver will help to understand the underlying mechanisms of oxidative stress in the pathogenesis of NASH[12,13,22-28]. A number of sources of increased ROS production have been established in NASH that include proinflammatory cytokines, such as TNF-α, iron overload, overburdened and dysfunctional mitochondria, CYPs, and peroxisomes.
Mitochondria and mitochondrialβ-oxidation: The hepatocyte is a cell rich in mitochondria. Each hepatocyte contains approximately 800 mitochondria[28]. The hepatocyte mitochondria are the main site of β-oxidation of free fatty acids. The electrons removed from free fatty acids during β-oxidation are shuttled through the mitochondrial respiratory chain (MRC), eventually leading to ATP synthesis and generation of CO2 and water. Inherent in this process is the dissociation of partially reduced molecular oxygen in the form of superoxide, hydrogen peroxide and the hydroxyl radical, species collectively termed ROS. About 1%-5% of oxygen consumed during cellular respiration is not fully reduced to water during this process under physiological conditions, and the production of these ROS is further increased in dysfunctional mitochondria. Thus, mitochondria have been proposed to play a central role in the pathogenesis of NASH[29]. Mitochondria also increase their oxidation capacity for the increased fatty acid flux as observed in obesity and insulin resistant states in humans. However, this increase has its limits and excess free fatty acids are metabolized at other sites in hepatocytes such as peroxisomes (β-oxidation) and the smooth endoplasmic reticulum (ω-oxidation). Acyl-CoA oxidase catalyzes the initial reaction of fatty acid oxidation in peroxisomes; a process that generates hydrogen peroxide and thus may contribute to oxidant stress.
Mitochondrial β-oxidation of short-, medium- and long-chain fatty acids involves multiple steps that include entry of long-chain fatty acids into the mitochondria; a process dependent on carnitine shuttle enzymes carnitine palmitoyltransferase (CPT)-I (an outer membrane enzyme) and CPT-II, and the β-oxidation of fatty acids to form progressively shorter acyl-CoA moieties, acetyl-CoA[29]. These oxidation processes are associated with the reduction of oxidized NAD+ and FAD to NADH and FADH2. Reoxidation of NADH and FADH2 to NAD+ and FAD produces electrons that transfer to the MRC[29,30]. Partially reduced oxygen molecules, termed ROS, are constitutively generated during this process when the electrons of NADH and FADH2 directly react with oxygen and may contribute to oxidant stress if endogenous protective mechanisms are overwhelmed[30]. Consistent with the increased flux of nonesterified fatty acid to the liver in obese patients with NAFLD, mitochondrial β-oxidation of fatty acids in the liver is also increased and as such may contribute to increased generation of ROS and oxidant stress.
Excessive fatty acids might use alternative pathways other than mitochondrial β-oxidation to be metabolized and cause mitochondrial injury. These include peroxisomal and cytochrome P450 (microsomal CYP) oxidation systems regulated by mainly fatty acids and insulin[31]. These alternative fatty acid oxidation systems produce more ROS and thus their utilization may be a source of oxidant stress.
Peroxisomal fatty acidβ-oxidation: Peroxisomal oxidation of fatty acids is the normal route of metabolism of very long chain fatty acids and dicarboxylic acids[31]. Peroxisomal oxidation is a four-step pathway in which electrons from FADH2 and NADH are transferred directly to oxygen. Although this increases the production of hydrogen peroxide, peroxisomes are uniquely endowed with the enzyme catalase that eliminates this reactive oxygen molecule. Fatty acids not oxidized by mitochondria are mainly oxidized by CYP2E1; a process that further increases ROS production within the hepatocytes[32]. Increased endogenous substrate burden such as increased levels of free fatty acids and ketone bodies induce CYP2E1 expression in humans. In normal conditions, CYP2E1 oxidation produces oxygen radicals, but the balance between these ROS and the abundance of endogenous antioxidants determines the extent of resulting oxidant stress. Increased hepatic CYP2E1 expression has been demonstrated by immunostaining of paraffin-embedded liver biopsy sections in patients with NASH. In contrast, hepatic content of CYP3A was decreased in all liver sections from patients with NASH. These studies also showed that weight loss decreased hepatic CYP2E1 activity. In parallel, Leclercq et al[33] previously had reported that dietary sugar restriction decreased CYP2E1 activity in humans. These novel studies pointed out that insulin rather than ketone bodies, with or without glucose contribution, regulate the expression and activity of hepatic CYP2E1. These metabolic abnormalities increase hepatic CYP2E1 activity and subsequent pro-oxidant production in patients with NAFLD.
Cytochrome P450 fatty acidω(omega)-oxidation: Fatty acids can undergo oxidation by the CYP enzymes of the smooth endoplasmic reticulum which is a relatively minor pathway. CYP2E1 and CYP4A isoforms are involved in fatty acid oxidation in conditions with substrate overload such as increased ketone bodies in type 2 diabetes mellitus. CYP4A upregulation particularly occurs in conditions with decreased CYP2E1 activity. The expression of both CYP2E1 and CYP4A mRNA and their protein levels are increased in both obese and diabetic humans[32,33]. Their hepatic activity and expression are also reported to be increased in patients with NASH due to the increased substrates, mainly fatty acids and ketone bodies, irrespective of the underlying clinical condition of diabetes or obesity. Nonetheless, the capacity of this enzyme system is very low to handle fatty acids. Oxidation reactions by the CYP enzymes can be major producers of ROS because of a low degree of coupling between substrate binding and their weak affinity to molecular oxygen, leading to the release of species such as superoxide anion radical, hydroxyl radicals, and hydrogen peroxide.
Mitochondrial dysfunction and ATP depletion: Mitochondria are the organelles primarily responsible for fatty acid β-oxidation and oxidative phosphorylation; the process responsible for the production of ATP[30]. Several observations including decreased mitochondrial enzyme activities and increased fat concentration of skeletal muscle cells in obese or diabetic patients have suggested mitochondrial dysfunction in these disorders. Such abnormalities may increase ROS production and promote both oxidative stress and lipid peroxidation within hepatocytes. Mitochondrial dysfunction is frequently due to a combination of genetic abnormalities, physical inactivity, aging, lipotoxicity (free fatty acids), lipid peroxidation (mitochondrial DNA alterations), and TNF-α[29,30,34]. Hepatic mitochondrial abnormalities have been identified in NAFLD, suggesting that mitochondria may be the source or target of injury and that ineffective mitochondrial function resulting in cellular ATP depletion may be important pathophysiological processes in NAFLD and NASH. The presence of megamitochondria, or mitochondrial swelling, is a microscopically detectable structural abnormality of hepatocyte mitochondria found in a variety of liver diseases including NAFLD[28]. Crystalline inclusions within the mitochondrial matrix have been documented by electron microscopy in patients with NASH. Hepatic mitochondrial DNA levels and the protein products of the mitochondrial genes are also decreased in patients with NASH. Impaired hepatic MRC function increases ROS production, and if ROS production exceeds antioxidant capabilities, oxidative stress and injury, lipid peroxidation of macromolecules and cellular membranes, mitochondrial DNA damage, direct damage of several mitochondrial enzymes, and further MRC dysfunction with more pro-oxidant production are observed. Mixed macro- and microvesicular steatosis due to β-oxidation defects in the mitochondria was the predominant type of steatosis in this study, and CYP 2E1 expression was upregulated, and levels of the antioxidant glutathione were decreased. Carnitine and CPT-I and CPT-II are required to transfer long-chain free fatty acids into the mitochondria for β-oxidation. Some investigators have reported the role of carnitine deficiency in NAFLD development, while others have observed normal hepatic content of total and free carnitine in patients with NASH.
Iron, oxidant stress and NASH: Iron can play a central role in promoting oxidant stress and this is proposed to be the mechanism of progressive liver disease. A large-population based study reported a correlation between elevated serum alanine aminotransferase levels and increased serum transferrin and iron concentrations[35,36]. After initial measurements, investigators induced iron depletion to a level of near-iron deficiency by phlebotomy. Interestingly, they observed improvements in both insulin sensitivity and serum alanine aminotransferase activity in some of the patients, indicating that iron may play a role not only in oxidant stress but also in the initial predisposing factor of insulin resistance.
Free fatty acid toxicity: In addition to insulin resistance and hyperinsulinemia, obesity and type 2 diabetes mellitus are strongly associated with increased concentrations of free fatty acids in the circulation[24,26]. Fatty acids are involved in many important cellular events such as synthesis of cellular membranes, energy storage, and intracellular signaling pathways. However, chronically elevated free fatty acids have the capability to disturb diverse metabolic pathways and induce insulin resistance in many organ systems[37-39]. In addition to their metabolic effects, fatty acids could induce cellular apoptosis, also called lipotoxicity, in two ways: direct toxicity and an indirect effect. One proposed mechanism of fatty acid toxicity in hepatocytes is that fatty acids induce translocation of Bax (which is a mitochondrial protein and a member of the Bcl-2 family) to lysosomes and cause lysosomal destabilization, which promotes the release of cathepsin B (a specific lysosomal enzyme), from lysosomes to the cytosol. Subsequently, a cathepsin-B-dependent process induces nuclear factor (NF)-κB activation and TNF-α overexpression in the liver[26]. TNF-α might further increase lysosomal destabilization and cathepsin-B-dependent hepatocyte apoptosis. Then, cytochrome c release from dysfunctional mitochondria may occur. Mitochondrial dysfunction causes energy depletion, which activates proteolytic caspases and induces DNA fragmentation and chromatin condensation. NF-κB is a transcriptional factor and has both apoptotic and antiapoptotic effects. In healthy hepatocytes, activation of NF-κB by TNF-α induces Bcl-2 synthesis, which prevents the release of cytochrome c from the mitochondria and subsequent apoptosis. Moreover, while cathepsin B has been demonstrated in hepatocyte lysosomes of healthy control individuals, the majority of hepatocytes in patients with NAFLD show diffuse distribution of cathepsin B in the cytosol, with a positive correlation with the stage of NASH[40-44].
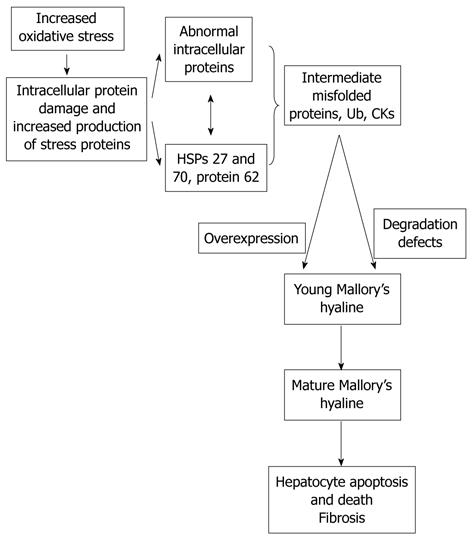
Hepatic steatosis is the most frequent and initially observed morphological feature of these processes[45-53]. Steatosis, inflammation, glycogen nuclei, lipogranulomas, ballooning of hepatocytes, Mallory bodies, and fibrosis are the major features of NAFLD.
Genetic and environmental factors may affect the development of liver fibrosis in NAFLD[54-62]. Age, severity of obesity, presence of diabetes, and hyperglycemia are the major nongenetic factors. Elevated plasma glucose, free fatty acids and adipocytokines activate both Kupffer cells and hepatic stellate cells (HSCs) and stimulate fibrogenesis[63-65]. Hepatic connective tissue growth factor (CTGF) mRNA was overexpressed in all NASH patients, while hepatic CTGF mRNA and its protein were upregulated in fa/fa rats (obese and diabetic) compared with their lean littermates. The same study also demonstrated upregulation of both CTGF mRNA and its protein in HSCs after exposure to high concentrations of either glucose or insulin. After activation, HSCs proliferate and express α-smooth muscle actin. Activated HSCs express myogenic markers such as c-myb and myocyte enhancer factor-2, exhibit proinflammatory and profibrogenic properties, migrate and secrete extracellular matrix components (ECM) such as collagen, and regulate the degradation of ECM. Activation of HSCs is the crucial step in liver fibrogenesis. A study of NAFLD patients (16 patients with steatosis alone and 60 with NASH) demonstrated that activation of HSCs was positive in almost all cases, and markedly in two thirds of patients, and it was correlated with the degree and location of hepatic fibrosis. HSC activation and upregulation of profibrogenic genes were observed in rats on an HF diet. Lipid-peroxidation-associated inflammation and HSC activation with increased TGF-β1 mRNA expression in methionine-choline-deficient steatohepatitis models have also been reported.
Oxidative stress may also participate in the activation of HSCs and the development of fibrosis in NAFLD. The intracellular NADPH oxidase pathway produces ROS, and the disruption of NADPH oxidase protects mice from developing severe liver injury. Lipid peroxidation products enhance the production of both TGF-β and collagen.
Currently, proposed mechanisms for the transformation from NASH to NASH-associated hepatocellular carcinoma are severe and cumulative oxidative stress to the hepatocytes, production of damaged DNA, defective or inhibited DNA repair systems, chronic continued hepatocyte injury and inflammatory infiltration, impaired antioxidant systems, and increased cell cycle of hepatocytes[65-67]. Animal and human studies have also indicated that a connection between age, sex and the disease might be possible[66-70].
In conclusion, a liver with excess fat may be more vulnerable to stressors than a normal liver. The factors that play key roles in the development of NASH from NAFLD remain uncertain.
P- Reviewer Liu HK S- Editor Lv S L- Editor Kerr C E- Editor Zhang DN
| 1. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] |
| 2. | Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987-G995. [PubMed] |
| 3. | Canbakan B, Tahan V, Balci H, Hatemi I, Erer B, Ozbay G, Sut N, Hacibekiroglu M, Imeryuz N, Senturk H. Leptin in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:249-254. [PubMed] |
| 4. | Canbakan B, Senturk H, Tahan V, Hatemi I, Balci H, Toptas T, Sonsuz A, Velet M, Aydin S, Dirican A. Clinical, biochemical and histological correlations in a group of non-drinker subjects with non-alcoholic fatty liver disease. Acta Gastroenterol Belg. 2007;70:277-284. [PubMed] |
| 5. | Basaranoglu M, Basaranoglu G. Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol. 2011;17:4055-4062. [PubMed] |
| 6. | Basaranoglu M, Turhan N, Sonsuz A, Basaranoglu G. Mallory-Denk Bodies in chronic hepatitis. World J Gastroenterol. 2011;17:2172-2177. [PubMed] |
| 7. | Sonsuz A, Basaranoglu M, Bilir M, Senturk H, Akin P. Hyperinsulinemia in nondiabetic, both obese and nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2002;97:495. [PubMed] |
| 8. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 751] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 12. | Keaney JF, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434-439. [PubMed] |
| 13. | Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963-4971. [PubMed] |
| 14. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [PubMed] |
| 15. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [PubMed] |
| 16. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-1830. [PubMed] |
| 17. | Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. [PubMed] |
| 19. | Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522-2525. [PubMed] |
| 20. | Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321-1326. [PubMed] |
| 21. | Seki S, Kitada T, Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res. 2005;33:132-134. [PubMed] |
| 22. | Marchesini G, Ridolfi V, Nepoti V. Hepatotoxicity of fast food? Gut. 2008;57:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Milagro FI, Campión J, Martínez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity (Silver Spring). 2006;14:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1027] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 25. | Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502-509. [PubMed] |
| 26. | Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 614] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 27. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [PubMed] |
| 28. | Caldwell SH, Chang CY, Nakamoto RK, Krugner-Higby L. Mitochondria in nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:595-617, x. [PubMed] |
| 30. | Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193-199. [PubMed] |
| 31. | Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin Liver Dis. 2004;8:639-671, xi. [PubMed] |
| 32. | Lieber CS. CYP2E1: from ASH to NASH. Hepatol Res. 2004;28:1-11. [PubMed] |
| 33. | Leclercq I, Horsmans Y, Desager JP, Pauwels S, Geubel AP. Dietary restriction of energy and sugar results in a reduction in human cytochrome P450 2E1 activity. Br J Nutr. 1999;82:257-262. [PubMed] |
| 34. | Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999-1007. [PubMed] |
| 35. | Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124:1821-1829. [PubMed] |
| 36. | Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179-187. [PubMed] |
| 37. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [PubMed] |
| 38. | Tarcin O, Basaranoglu M, Tahan V, Tahan G, Sücüllü I, Yilmaz N, Sood G, Snyder N, Hilman G, Celikel C. Time course of collagen peak in bile duct-ligated rats. BMC Gastroenterol. 2011;11:45. [PubMed] |
| 39. | Canbakan B, Senturk H, Canbakan M, Toptas T, Tuncer M. Reliability of caspase activity as a biomarker of hepatic apoptosis in nonalcoholic fatty liver disease. Biomark Med. 2011;5:813-815. [PubMed] |
| 40. | Canbakan B, Senturk H, Canbakan M, Toptas T, Tabak O, Balci H, Olgac V, Ozbay G. Is alanine aminotransferase level a surrogate biomarker of hepatic apoptosis in nonalcoholic fatty liver disease? Biomark Med. 2010;4:205-214. [PubMed] |
| 41. | Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G, Hatemi I, Olgac V, Sonsuz A, Ozbay G. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology. 2008;55:1433-1438. [PubMed] |
| 42. | Ipekci SH, Basaranoglu M, Sonsuz A. The fluctuation of serum levels of aminotransferase in patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2003;36:371. [PubMed] |
| 43. | Sonsuz A, Basaranoglu M, Ozbay G. Relationship between aminotransferase levels and histopathological findings in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:1370-1371. [PubMed] |
| 44. | Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31:384. [PubMed] |
| 45. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] |
| 46. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2196] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 47. | Trinchet JC, Hartmann DJ, Pateron D, Laarif M, Callard P, Ville G, Beaugrand M. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol. 1991;12:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Teare JP, Sherman D, Greenfield SM, Simpson J, Bray G, Catterall AP, Murray-Lyon IM, Peters TJ, Williams R, Thompson RP. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Gallorini A, Plebani M, Pontisso P, Chemello L, Masiero M, Mantovani G, Alberti A. Serum markers of hepatic fibrogenesis in chronic hepatitis type C treated with alfa-2A interferon. Liver. 1994;14:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Yabu K, Kiyosawa K, Mori H, Matsumoto A, Yoshizawa K, Tanaka E, Furuta S. Serum collagen type IV for the assessment of fibrosis and resistance to interferon therapy in chronic hepatitis C. Scand J Gastroenterol. 1994;29:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999;44:624-630. [PubMed] |
| 52. | Mas VR, Fisher RA, Archer KJ, Maluf DG. Proteomics and liver fibrosis: identifying markers of fibrogenesis. Expert Rev Proteomics. 2009;6:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Juran BD, Lazaridis KN. Applying genomics to the study of complex disease. Semin Liver Dis. 2007;27:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Hamid S, Aquilina JW, Davidson W, Dhabuwala CB. Arteriovenous malformation of scrotum: a case report. J Urol. 1992;147:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Juran BD, Lazaridis KN. Genomics and complex liver disease: Challenges and opportunities. Hepatology. 2006;44:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci. 2003;73:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Kuramitsu Y, Nakamura K. Current progress in proteomic study of hepatitis C virus-related human hepatocellular carcinoma. Expert Rev Proteomics. 2005;2:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Tian Q, Stepaniants SB, Mao M, Weng L, Feetham MC, Doyle MJ, Yi EC, Dai H, Thorsson V, Eng J. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol Cell Proteomics. 2004;3:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 612] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 59. | Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, Jeffrey SS, Botstein D, Brown PO. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 60. | Sellick GS, Longman C, Tolmie J, Newbury-Ecob R, Geenhalgh L, Hughes S, Whiteford M, Garrett C, Houlston RS. Genomewide linkage searches for Mendelian disease loci can be efficiently conducted using high-density SNP genotyping arrays. Nucleic Acids Res. 2004;32:e164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1260] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 62. | Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1055] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 63. | Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, Kuroki T, Yoshizato K. Proteome analysis of rat hepatic stellate cells. Hepatology. 2000;32:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 64. | Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem. 2001;276:25318-25323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 268] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 65. | Deng X, Liang J, Lin ZX, Wu FS, Zhang YP, Zhang ZW. Natural taurine promotes apoptosis of human hepatic stellate cells in proteomics analysis. World J Gastroenterol. 2010;16:1916-1923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 67. | Makridakis M, Vlahou A. Secretome proteomics for discovery of cancer biomarkers. J Proteomics. 2010;73:2291-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 68. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2160] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 69. | Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Fléjou JF, Degott C, Belghiti J, Bernades P, Valla D. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 294] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 70. | Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |