Published online Feb 21, 2013. doi: 10.3748/wjg.v19.i7.1140
Revised: November 26, 2012
Accepted: December 15, 2012
Published online: February 21, 2013
Processing time: 124 Days and 22.4 Hours
A pleural effusion containing chylomicrons is termed chylothorax and results from leakage of lymph fluid into the pleural cavity. We report on the case of a 59-year-old woman with severe dyspnea due to a large chylothorax. She was known to have liver cirrhosis but no ascites. There was no history of trauma, cardiac function was normal and thorough diagnostic work-up did not reveal any signs of malignancy. In summary, no other etiology of the chylothorax than portal hypertension could be found. Therapy with diuretics as well as parenteral feeding failed to relieve symptoms. After a transjugular intrahepatic portosystemic shunt (TIPS) had successfully been placed, pleural effusion decreased considerably. Eight months later, TIPS revision had to be performed because of stenosis, resulting in remission from chylothorax. This case shows that even in the absence of ascites, chylothorax might be caused by portal hypertension and that TIPS can be an effective treatment option.
- Citation: Lutz P, Strunk H, Schild HH, Sauerbruch T. Transjugular intrahepatic portosystemic shunt in refractory chylothorax due to liver cirrhosis. World J Gastroenterol 2013; 19(7): 1140-1142
- URL: https://www.wjgnet.com/1007-9327/full/v19/i7/1140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i7.1140
Chylothorax is defined as a pleural effusion containing chylomicrons. Triglyceride levels of at least 110 mg/dL have been found to reliably indicate chylothorax[1], originating from a lesion in the lymphatic vessels, mostly the thoracic duct. In about 40% of patients with chylothorax, these lesions are caused by trauma or surgery, whereas in about 30%, a malignant tumor is found. The remaining cases are attributed to a variety of conditions, including congenital, infectious and cardiac diseases, or are considered to be idiopathic[2]. Chylothorax rarely occurs in patients with liver cirrhosis. In such cases, concomitant ascites is usually present[3]. We report on the effective treatment of massive chylothorax in a female patient with liver cirrhosis but no ascites by placement of a transjugular intrahepatic portosystemic shunt (TIPS).
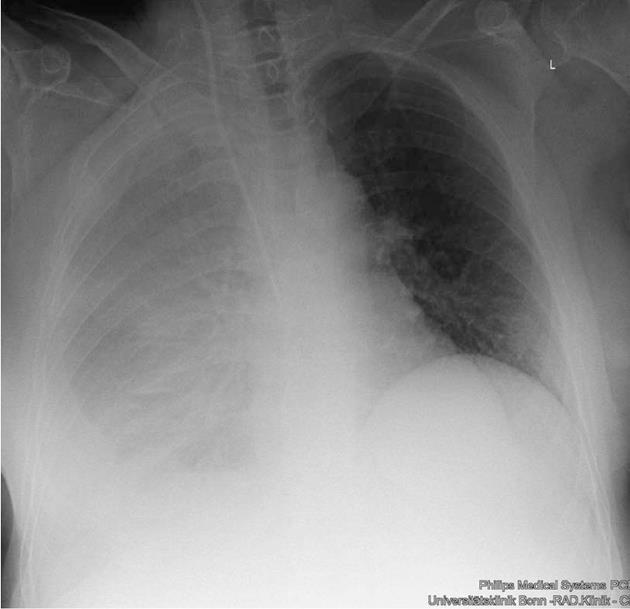
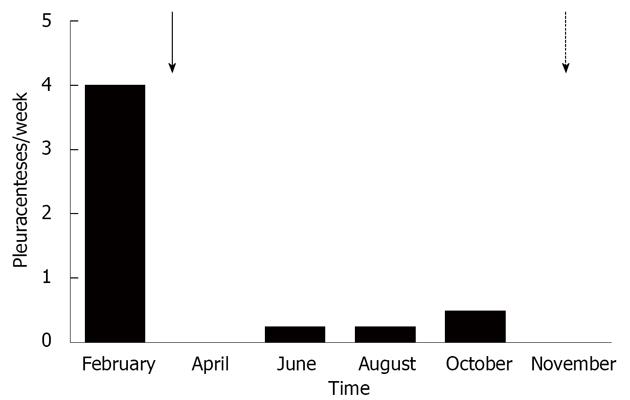
The 59-year-old woman was referred to our university hospital due to chylothorax which could not be sufficiently managed in a peripheral hospital. Since 2008, a right sided pleural effusion had to be drained occasionally despite diuretic therapy. In recent weeks, however, the pleural effusion had turned milky and had to be drained more frequently. Three weeks prior to admission, nearly 1.5 L/d had to be removed (Figure 1). The patient presented no further symptoms apart from dyspnea. Her past medical history included diagnosis of alcoholic liver cirrhosis (Child B), diabetes mellitus type II and resection of a local ovarian carcinoma 15 years ago. The exact tumor staging was unknown. The patient had received adjuvant chemotherapy and the carcinoma has been in remission since then. One year ago, she was treated for bleeding gastric ulcer related to infection with Helicobacter pylori. There was no history of trauma.
Apart from the signs of right-sided pleural effusion, some spider naevi and a small umbilical hernia, physical examination was unremarkable. Blood analysis showed slightly elevated bilirubin (1.2 mg/dL, reference range up to 1 mg/dL), increased gamma-glutamyl transferase (112 U/L, reference range up to 38 U/L), international normalized ratio (of prothrombin time) above normal (1.3) and decreased serum albumin (31 g/L, reference range 35-52 g/L). Model for End-stage Liver Disease score was 10. Mild hyperglycemia was due to diabetes mellitus. Urine analysis was unremarkable. Analysis of the pleural fluid showed 95 polymorphonuclear cells/μL, 294 mononuclear cells/μL, low albumin of 4.2 g/L, no cholesterol, but markedly increased triglycerides (386 mg/dL). Bacterial cultures were negative, microscopic examination did not reveal malignant cells. No tumor was detected in a computed tomography (CT) scan carried out at the referring hospital. Cardiac evaluation by echocardiography and transthoracic echocardiography revealed no signs of heart failure or pulmonary hypertension. Abdominal ultrasound showed a liver morphology consistent with cirrhosis and concomitant splenomegaly, but neither portal vein thrombosis nor signs of hepatocellular carcinoma nor ascites were present. To further characterize the degree of portal hypertension, we performed endoscopy of the upper gastrointestinal tract. Esophageal varices grade II were detected.
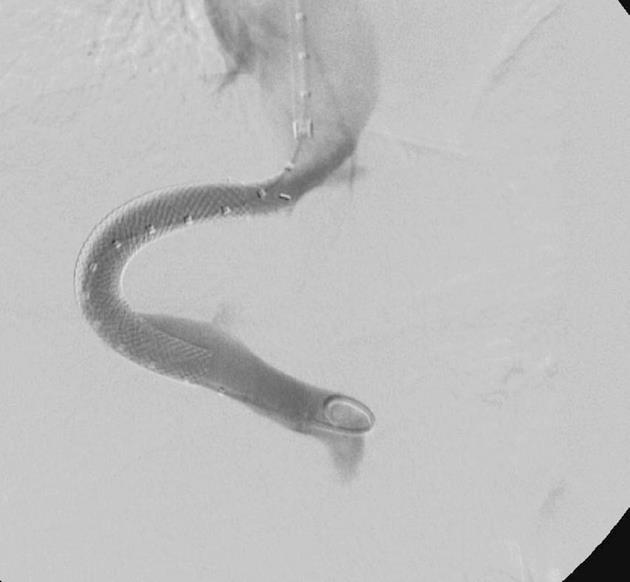
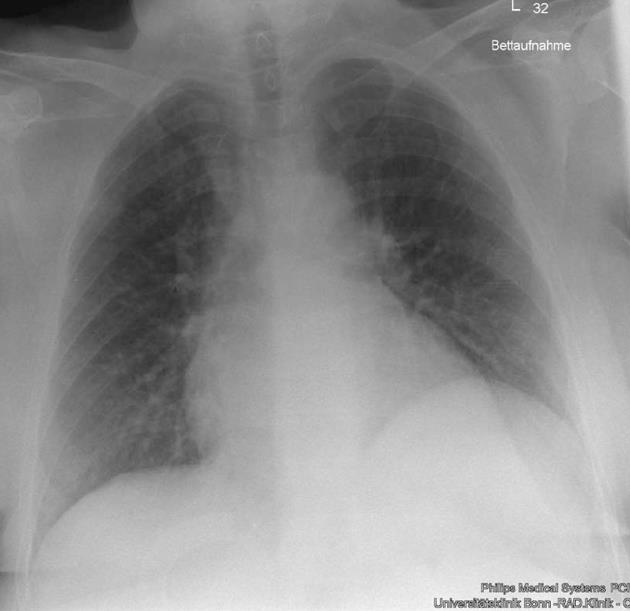
In summary, we confirmed the diagnosis of chylothorax with a daily production of 2-4 liters. However, it was uncertain whether this was caused by liver cirrhosis. As diuretic therapy failed to drain the chylothorax, we put the patient on parenteral feeding for a total of nine days to reduce the flow in the lymphatic ducts and to promote healing of a suspected lymphatic lesion. As expected, the milky aspect of the pleural effusion vanished, but the daily amount of secretion from the pleural drainage increased even further (about 1.5-3 L/d). Since high renin blood levels are often associated with decompensated portal hypertension[4], we determined renin blood levels, revealing a value of 1680 μU/mL (reference range up to 46 μU/mL) in conjunction with an elevated level of aldosterone of 946 pg/mL (reference range up to 310 pg/mL). Thus, we proposed TIPS as treatment option and this was placed successfully in March 2010 (Figure 2). Fifteen days later, the pleural drain could be removed; another week later, the patient could be dismissed with a small, stable pleural effusion under diuretic medication. During the following months, the pleural effusion had to be drained about once per month. TIPS revision due to stenosis had to be performed by inserting a new stent in November 2010. Since then, no further pleural drainage was needed (Figure 3). After TIPS placement, the patient had several episodes of hepatic encephalopathy grade I-II according to the West Haven criteria, but responded to treatment with lactulose and rifaximin. Levels of bilirubin did not increase significantly after the intervention. To date, more than two years after TIPS placement, the patient is still without recurrence of chylothorax (Figure 4).
Liver cirrhosis, together with portal hypertension is a rare cause of chylothorax[3]. Diagnosis was difficult in this patient because she presented with almost normal laboratory values and without ascites. However, tiny amounts of abdominal fluid had been present in the past. Conservative management of chylothorax failed. To the best of our knowledge, TIPS as successful treatment of chylothorax in cirrhotic patients has been described before in only two cases: in one patient with traumatic injury of the thoracic duct[5] and in a second patient with concomitant refractory ascites[6]. High renin levels in our patient suggested that activation of the renin angiotensin aldosterone system (RAAS), causing sodium and fluid retention, was due to portal hypertension, complicated by the leakage of chyle in the pleural cavity. This activated RAAS argued against idiopathic etiology, which is described in 10% of patients with chylothorax. Indeed, renin values in our patient decreased considerably from 1680 to 317 μU/mL after TIPS placement, along with regression of excessive chyle formation. Other etiologies of chylothorax were not very plausible: there was no history of recent trauma or surgery and a malignant process - bearing the history of ovarian cancer in mind - was improbable due to negative CT scan and pleural fluid cytology. Furthermore, the patient developed no malignancy during the follow-up of more than two years. In the end, placement of TIPS improved the quality of life of this patient enormously. After failure of conservative treatment, the main alternative to TIPS would have been surgical procedures, such as pleurodesis or ligation/embolization of the thoracic duct[7], which, however, do not address portal hypertension as underlying disease. Fasting and parenteral nutrition have been described as effective in chylothorax particularly following surgery or trauma. In most other forms, lymphatic obstruction or increased venous pressure in the superior vena cava cause lymphangiectasia and chylothorax[7]. In these cases, parenteral feeding is not effective. Somatostatin has been used in chylothorax of various etiologies with some success[8]. Due to its characteristic contents, drainage of a chylothorax can lead to malnutrition and immunosuppression caused by loss of lipids, immunoglobulins and lymphocytes. However, this characteristic content seems to reduce the risk of infection of a pleural drain[9].
The success of the TIPS placement in our patient confirms the hepatic origin of the chylothorax. It has been suggested that in liver cirrhosis, chyle flow increases substantially due to increased formation of hepatic lymph and due to portal hypertension. Since drainage into the venous system is limited by a valve at the junction of the thoracic duct and the subclavian vein, pressure in the lymphatic vessels is increased, leading to an elevated risk of spontaneous rupture[10]. Thus, in our patient, such a spontaneous leak might have persisted as long as portal hypertension was high, maintaining the pleural effusion via small gaps in the diaphragm as in hepatic hydrothorax, which occurs in about 5%-12% of liver cirrhosis patients[11]. A recent review described a clinical response rate of about 70% in 198 patients with hepatic hydrothorax after TIPS placement. However, controlled studies are still missing. No case of chylothorax was described in these patients[12].
In summary, this case shows that massive chylothorax can develop in patients with otherwise well compensated liver cirrhosis and that it can be treated effectively by TIPS placement.
P- Reviewers Durandy Y, Neuberger J, Bordas JM S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Staats BA, Ellefson RD, Budahn LL, Dines DE, Prakash UB, Offord K. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980;55:700-704. [PubMed] |
| 2. | Agrawal V, Sahn SA. Lipid pleural effusions. Am J Med Sci. 2008;335:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Romero S, Martín C, Hernandez L, Verdu J, Trigo C, Perez-Mateo M, Alemany L. Chylothorax in cirrhosis of the liver: analysis of its frequency and clinical characteristics. Chest. 1998;114:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Arroyo V, Ginès P. Mechanism of sodium retention and ascites formation in cirrhosis. J Hepatol. 1993;17 Suppl 2:S24-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Vignaux O, Gouya H, Dousset B, Mazuir E, Buffet C, Calmus Y, Legmann P. Refractory chylothorax in hepatic cirrhosis: successful treatment by transjugular intrahepatic portosystemic shunt. J Thorac Imaging. 2002;17:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kinney TB, Ferrara SL, Miller FJ, Roberts AC, Hassanein T. Transjugular intrahepatic portosystemic shunt creation as treatment for refractory chylous ascites and chylothorax in a patient with cirrhosis. J Vasc Interv Radiol. 2004;15:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Doerr CH, Miller DL, Ryu JH. Chylothorax. Semin Respir Crit Care Med. 2001;22:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med. 2006;12:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007;32:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Aalami OO, Allen DB, Organ CH. Chylous ascites: a collective review. Surgery. 2000;128:761-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 279] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Garcia N, Mihas AA. Hepatic hydrothorax: pathophysiology, diagnosis, and management. J Clin Gastroenterol. 2004;38:52-58. [PubMed] |
| 12. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |