Published online Feb 21, 2013. doi: 10.3748/wjg.v19.i7.1068
Revised: December 4, 2012
Accepted: December 27, 2012
Published online: February 21, 2013
Processing time: 217 Days and 14.9 Hours
AIM: To clarify the specific roles and mechanisms of long interspersed nuclear element-1 ORF-1 protein [human long interspersed nuclear element-1 (LINE-1), ORF-1p] in chemotherapeutic drug resistance and cell proliferation regulation in hepatocellular carcinoma (HCC) cells.
METHODS: MTT assays were performed to identify the effect of the chemotherapeutic drug toxicity on HepG2 cells. Cell proliferation inhibition and the IC50 were calculated by the Origin 8.0 software. Western blotting assays were performed to investigate whether LINE-1 ORF-1p modulates the expression of some important genes, including p53, p27, p15, Bcl-2, mdr, and p-gp. To corroborate the proliferation and anchor-independent growth results, the HepG2 cells were analyzed by flow cytometry to investigate the effect of LINE-1 ORF-1p on the apoptosis regulation.
RESULTS: LINE-1 ORF-1p contributed to the resistance to several chemotherapeutic drugs (cisplatin and epirubicin) in HepG2 cells. The IC50 of the epirubicin and cisplatin increased from 36.04 nmol/L to 59.11 nmol/L or from 37.94 nmol/L to 119.32 nmol/L. Repression of LINE-1 ORF-1p expression by the siRNA could markedly enhance the response of HepG2 cells to the epirubicin and cisplatin. The IC50 correspondingly decreased from 28.06 nmol/L to 3.83 nmol/L or from 32.04 nmol/L to 2.89 nmol/L. Interestingly, down-regulation of LINE-1 ORF-1p level by siRNA could promote the response of HepG2 cells to the paclitaxel. The IC50 decreased from 35.90 nmol/L to 7.36 nmol/L. However, overexpression of LINE-1 ORF-1p did not modulate the paclitaxel toxicity in HepG2 cells. Further Western blotting revealed that LINE-1 ORF-1p enhanced mdr and p-gp gene expression. As a protein arrested in the nucleus, LINE-1 ORF-1p may function through modulating transcriptional activity of some important transcription factors. Indeed, LINE-1 ORF-1p promoted HepG2 cell proliferation, anchor-independent growth and protected the cells against apoptosis through modulating the expression of p15, p21, p53, and Bcl-2 genes.
CONCLUSION: LINE-1 ORF-1p promotes HepG2 cell proliferation and plays an important role in the resistance of chemotherapeutic drugs. By establishing novel roles and defining the mechanisms of LINE-1 ORF-1p in HCC chemotherapeutic drug resistance and cell proliferation regulation, this study indicates that LINE-1 ORF-1p is a potential target for overcoming HCC chemotherapeutic resistance.
- Citation: Feng F, Lu YY, Zhang F, Gao XD, Zhang CF, Meredith A, Xu ZX, Yang YT, Chang XJ, Wang H, Qu JH, Zeng Z, Yang JL, Wang CP, Zhu YF, Cui JJ, Yang YP. Long interspersed nuclear element ORF-1 protein promotes proliferation and resistance to chemotherapy in hepatocellular carcinoma. World J Gastroenterol 2013; 19(7): 1068-1078
- URL: https://www.wjgnet.com/1007-9327/full/v19/i7/1068.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i7.1068
Hepatocellular carcinoma (HCC) which is the most common tumor worldwide, and the third most common cause of cancer death, is often accompanied by a poor prognosis[1]. The majority of HCC patients are unresectable at the time of first diagnosis, and even in patients who are suitable for surgery, the risk of recurrence is high. Consequently, chemotherapy is an important alternative strategy for most HCC patients[2]. Cisplatin, paclitaxel and epirubicin are common chemotherapeutic agents used in the treatment of HCC. However, chemotherapy is often ineffective in HCC patients due to potent multidrug resistance (MDR)[3]. Many studies have indicated that alterations in target gene expression are correlated with drug resistance and may provide a new strategy for sensitizing cancer cells to chemotherapeutic drugs and to reverse MDR in cancer cells.
The human long interspersed nuclear element-1 (LINE-1) gene encodes two proteins: LINE-1 ORF-1p and LINE-1 ORF-2p[4]. It is reported that LINE-1 ORF-2p is mainly involved in the retrotransposition process through its endonuclease activity[4]. In human cancer cells, LINE-1 ORF-2p can be regulated by LINE-1 ORF-1p[4,5]. The expression of the LINE-1 ORF-1 protein is almost 1000-fold greater than the LINE-1 ORF-2 protein[5], which indicates that LINE-1 ORF-1p has many roles. Our previous results suggested that LINE-1 ORF-1p enhances the proliferation of several cancer cell lines[6]. LINE-1 ORF-1p also increased the proliferation of hepatocellular carcinoma cells Bel-7402, SMMC-7721, HepG2, and LO2 cells and was associated with a significant risk of HCC progression[7]. However, the detailed mechanisms and roles of LINE-1 ORF-1p in HCC development are still largely unknown.
Based on our previous study[7], we hypothesized that LINE-1 ORF-1p might also be involved in HCC development and chemotherapy resistance regulation. To address this hypothesis, we performed MTT assays and found that LINE-1 ORF-1p protects HepG2 cells against chemotherapy. More importantly, we also found that LINE-1 ORF-1p enhances HepG2 cell proliferation through the modulation of genes involved in cell proliferation and apoptosis such as p53 and Bcl-2.
The full length of LINE-1 ORF-1p cDNA was cloned into a pcDNA3.1 vector linked with fludarabine + high-dose cytarabine + G-CSF (FLAG) at the amino terminus by polymerase chain reaction using a cDNA library as the template[6,7]. The siRNA targeted to LINE-1 ORF-1p was cloned into the psilencer-2.1-U6 (neo) vector (Ambion). The sequence of siRNA is AAGGAGGTGCACTATAAGAAC[6,7]. The luciferase reporters, Luc-p21 (containing p53 binding element), Luc-Smad4 binding element (SBE), Luc-SP1 and Luc-androgen response element (ARE) were gifts from Dr. Cui JJ and Yang YT.
Antibodies against p27, p53, Bcl-2, and p15 were from Santa Cruz Biotechnology Inc., United States, and antibodies against MDR, p-gp and GAPDH were from Sigma-Aldrich, St. Louis, United States. The antibody against LINE-1 ORF-1p was described previously[6,7]. Polyclonal anti-rabbit IgG was from Qiagen, Beijing, China.
Epirubicin (Pfizer, NY, United States), Cisplatin (QILU Pharmaceutical Co., Jinan, China), paclitaxel (Roche, Basel, Swiss), Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) and other agents (Amersham Biosciences, Piscataway, NJ, United States) were used in this study. HepG2 cells (American Type Culture Collection, ATCC) were cultured in DMEM (GIBCO, Grand Island, NY, United States) medium containing 10% FBS.
HepG2 cells were transfected with FLAG-LINE-1 ORF-1p or siRNA plasmid, and cultured for 24 h at 37 °C and 5% CO2. Then, 10 μL CCK-8 reagent was added to each well and cultured at 37 °C and 5% CO2 for 4 h. The absorbance of the inhibition rate was measured using a multifunctional microplate reader at 490 nm. The inhibition rate = (A control group - A administration group)/(A control group - A blank group) × 100%. The assays were performed three times with similar results.
HepG2 cells which stably expressed FLAG-LINE-1 ORF-1p or siRNA were placed in 6-well plates, with a bottom layer of 0.7% low-melting-temperature agar in DMEM and a top layer of 0.25% agar in DMEM. Colonies were scored after 3 wk of growth. The assays were performed in three independent experiments with similar results.
HepG2 cells were seeded in 24-well plates. After 24 h, the cells were transfected with FLAG-LINE-1 ORF-1p and the indicated reporter gene using Lipofectamine 2000 (Invitrogen). Twenty-four hours later, the cells were harvested and analyzed for luciferase following the manual protocol (Promega Corp., Madison, WI, United States).
Assays were performed following the protocol provided by the apoptosis assay kit (Qiagen, Beijing, China). In brief, cells were treated with dehydrated alcohol for flow cytometry analysis. Before analysis, cells were treated with 0.5% RNase at 65 °C for 30 min. The assays were performed three times with similar results.
Plasmids were transfected into HepG2 cells using Lipofectamine 2000 (Invitrogen). Forty-eight hours later, transfected cells were cultured in 500 μg/mL G418 (Invitrogen) for approximately 2 mo. Individual clones were screened by Western blotting using anti-FLAG or anti-LINE-1 ORF-1p antibodies. Similar results were observed with stable transfection or transient transfection with individual clones or pool clones.
Total protein in the samples was measured by SDS-PAGE and trans-printed to a nitrocellulose (NC) membrane. The NC membranes were blocked with 5% BSA in TBST buffer and incubated with the antibodies. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies after washing with TBST buffer. Membranes were visualized using the appropriate kit (Qiagen).
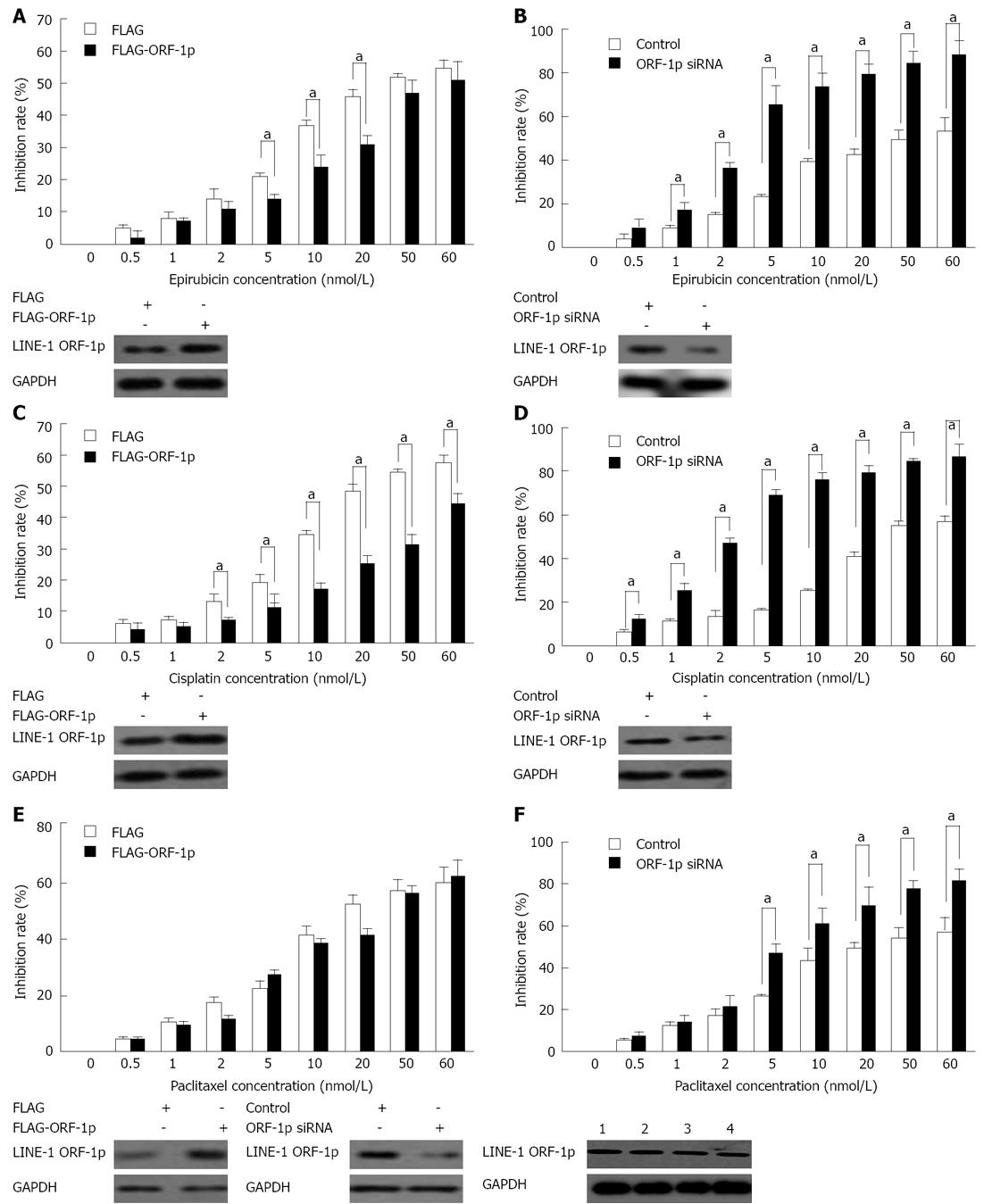
To determine whether LINE-1 ORF-1p modulates the cytotoxic effects of chemotherapeutic agents, MTT assays were performed. The results showed that overexpression of LINE-1 ORF-1p significantly reduced the cytotoxicity of epirubicin and cisplatin on HepG2 cells (Figure 1) and the corresponding IC50 values increased significantly (Table 1). Reduction of LINE-1 ORF-1p expression by siRNA markedly promoted the sensitivity of HepG2 cells to epirubicin and cisplatin (Figure 1). The IC50 values correspondingly decreased significantly (Table 1).
| Drugs | IC50 (nmol/L) | |||
| FLAG | FLAG-ORF-1p | Control | siRNA | |
| Epirubicin | 36.04 ± 4.48 | 59.11 ± 3.37 | 28.06 ± 5.31 | 3.84 ± 0.48 |
| Cisplatin | 37.94 ± 2.90 | 119.32 ± 21.99 | 32.04 ± 3.71 | 2.89 ± 0.44 |
| Paclitaxel | 27.01 ± 3.91 | 29.01 ± 2.95 | 35.90 ± 7.03 | 7.36 ± 0.69 |
Interestingly, our data also showed that neither overexpression of LINE-1 ORF-1p plasmids nor the empty vector protected HepG2 cells from the cytotoxicity of paclitaxel (Figure 1E). Knockdown of LINE-1 ORF-1p increased the cytotoxic effect of paclitaxel on HepG2 cells (Figure 1F). The IC50 values correspondingly decreased from 35.90 nmol/L to 7.36 nmol/L (Table 1). Taken together, these results suggest that LINE-1 ORF-1p mediates chemotherapeutic drug resistance in HepG2 cells.
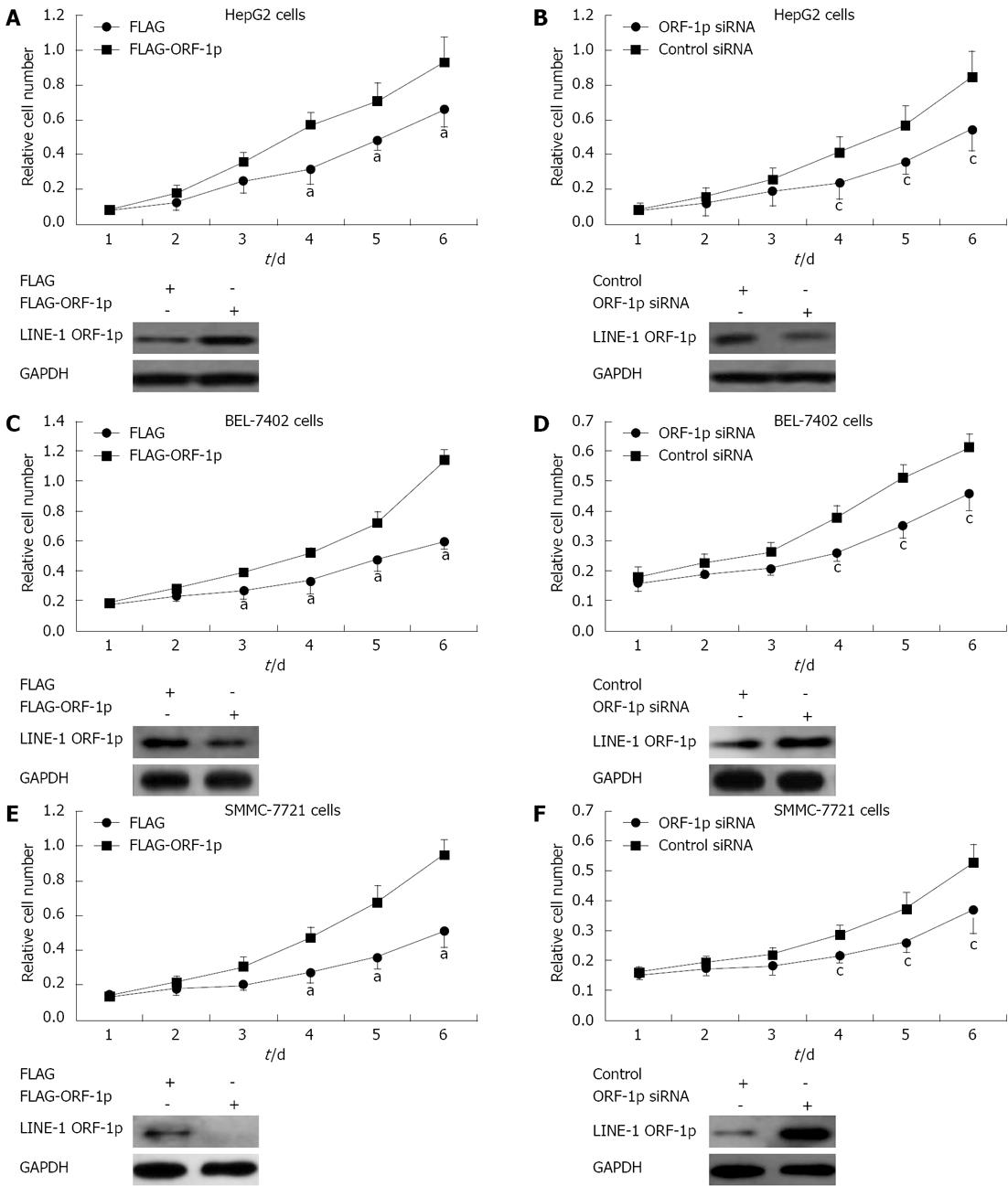
To determine whether LINE-1 ORF-1p affected HepG2 cell proliferation, MTT assays were performed. HepG2 cells transfected with FLAG-LINE-1 ORF1-p had an increased proliferation rate compared with those transfected with FLAG empty vector (Figure 2A); whereas cells transfected with LINE-1 ORF-1p siRNA, grew more slowly than those transfected with the control siRNA (Figure 2B). In addition, LINE-1 ORF-1p also promoted HCC SMMC-7721 (Figure 2C and D) and BEL-7402 cell growth (Figure 2E and F). These results indicated that LINE-1 ORF-1p affects HCC cell proliferation.
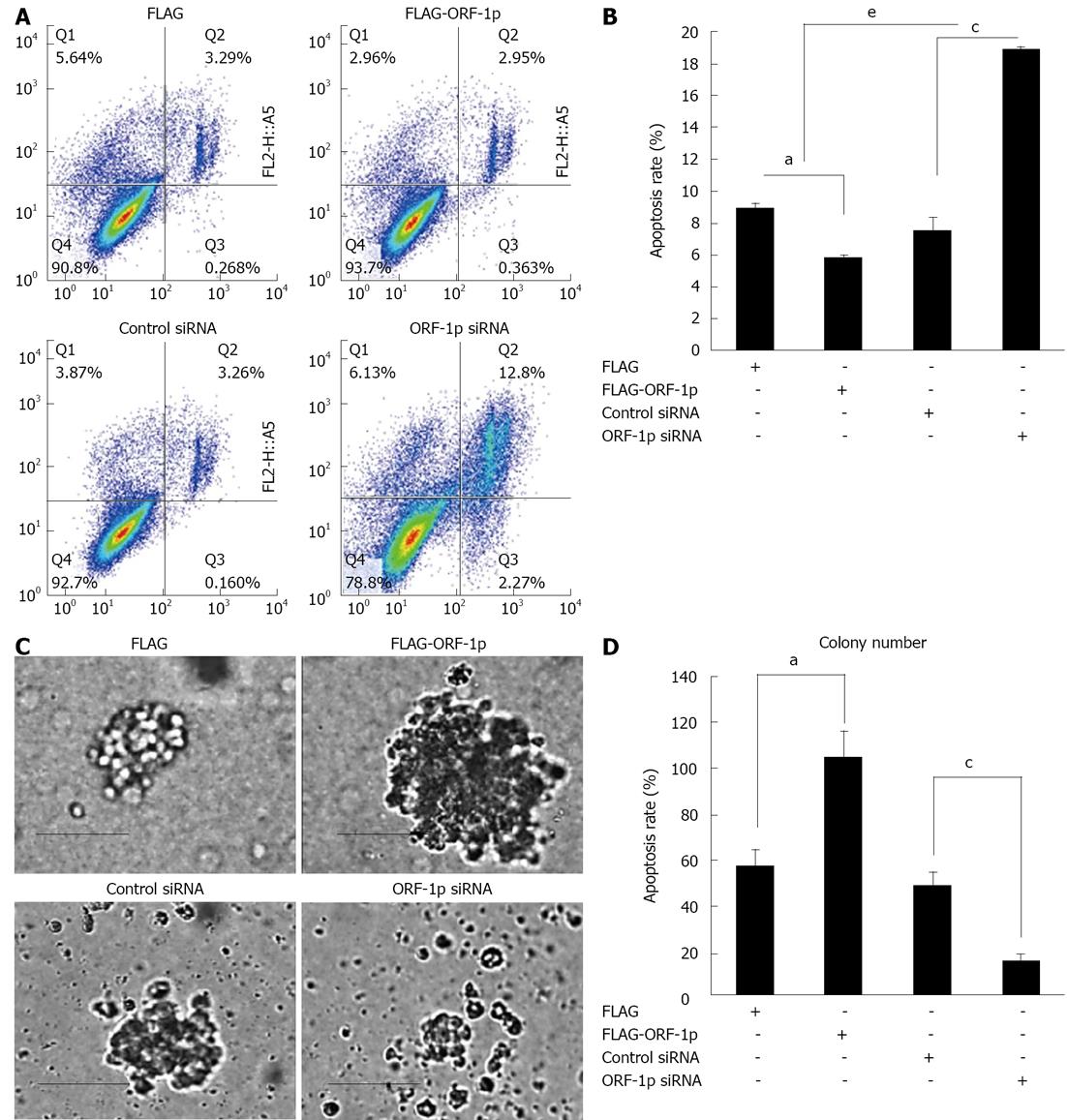
To investigate the role of LINE-1 ORF-1p in HepG2 cell apoptosis, apoptosis assays were performed. Overexpression of LINE-1 ORF-1p protected HepG2 cells from apoptosis (Figure 3), whereas knockdown of LINE-1 ORF-1p significantly promoted the apoptosis of HepG2 cells (Figure 3).
The effects of LINE-1 ORF1-p on anchorage-independent growth were tested in stable HepG2 cell lines expressing either FLAG-LINE-1 ORF1-p or siRNA. Overexpression of LINE-1 ORF-1p significantly increased the anchorage-independent growth of HepG2 cells (Figure 3); whereas knockdown of endogenous LINE-1 ORF1-p decreased anchorage-independent growth of HepG2 cells (Figure 3). These results suggest that LINE-1 ORF-1p also strongly promotes HCC cell growth.
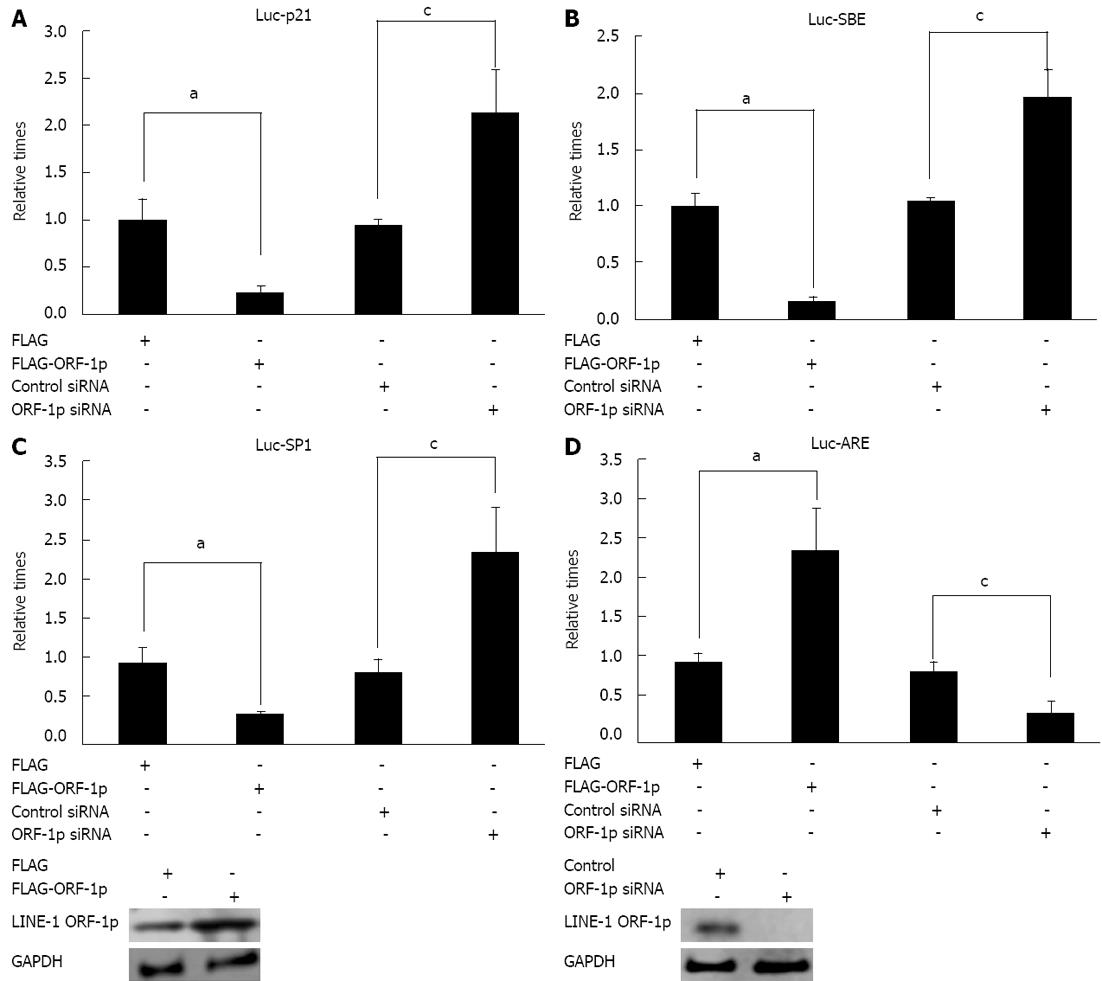
To determine the mechanism of LINE-1 ORF1-p, luciferase assays were performed. The results showed that overexpression of LINE-1 ORF-1p decreased Luc-p21, Luc-SBE and Luc-Sp1 activity. Knock-down of LINE-1 ORF-1p expression enhanced the activities of Luc-p21 (Figure 4A), Luc-SBE (Figure 4B) and Luc-Sp1 (Figure 4C).
In addition, overexpression of LINE-1 ORF-1p promoted the transcription of Luc-ARE reporter (Figure 4D). Down-regulation of LINE-1 ORF1-p expression reduced the activity of Luc-ARE (Figure 4D). Taken together, these results suggest that LINE-1 ORF-1p may regulate cell proliferation by modulating the transcription activities of these genes
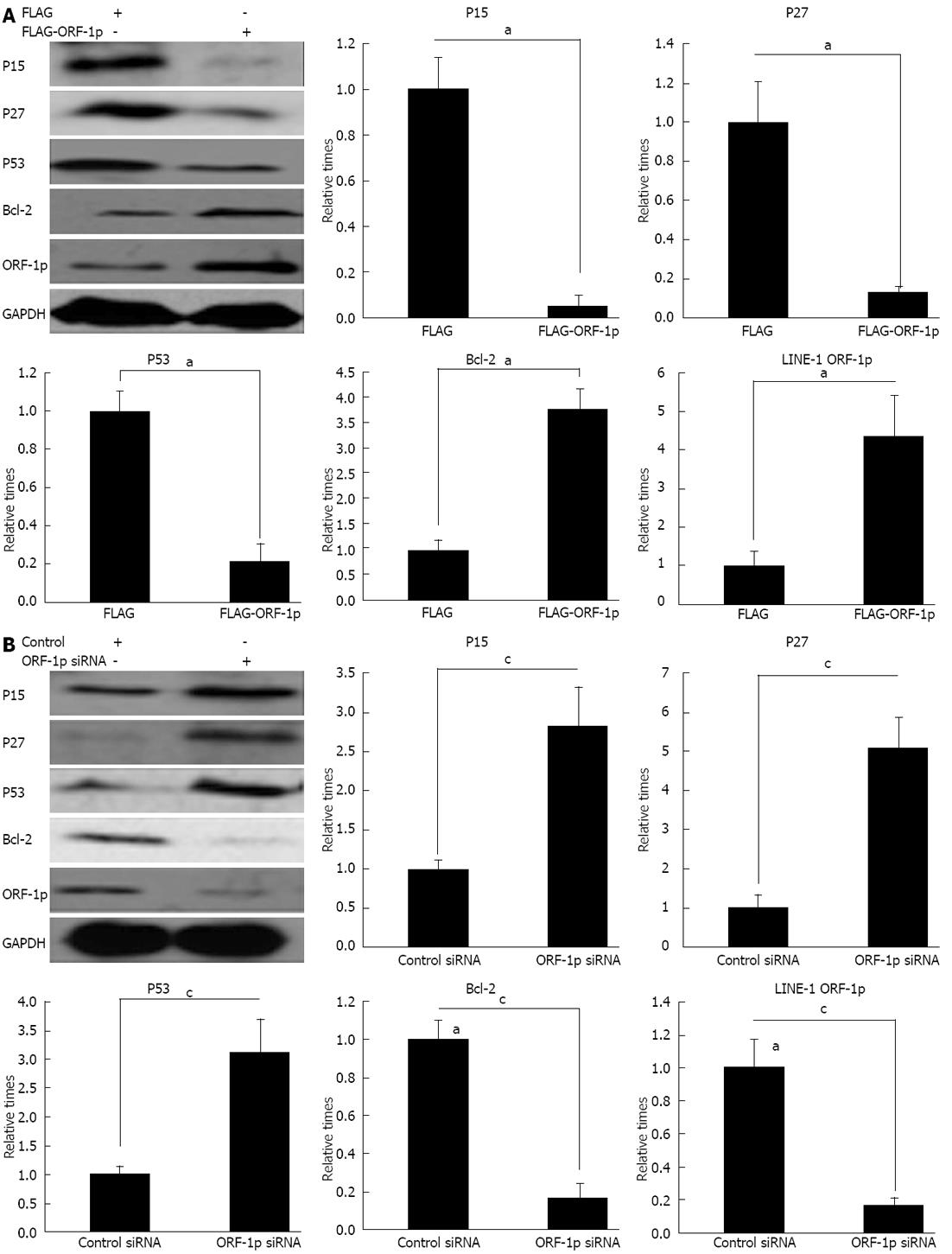
To corroborate the results of the luciferase assay, it is necessary to investigate whether LINE-1 ORF-1p regulates the protein levels of the genes related to cell proliferation and apoptosis. The results showed that overexpression of LINE-1 ORF-1p decreased the expression of p15, p53 and p27 genes (Figure 5A) and increased the expression of Bcl-2 (Figure 5A). However, reduction of LINE-1 ORF-1p expression with LINE-1 ORF1-p siRNA increased the protein level of p15, p53 and p27 (Figure 5B), and reduced the protein level of Bcl-2 (Figure 5B). These findings indicated that LINE-1 ORF-1p promotes cell proliferation by regulating the expression of these genes.
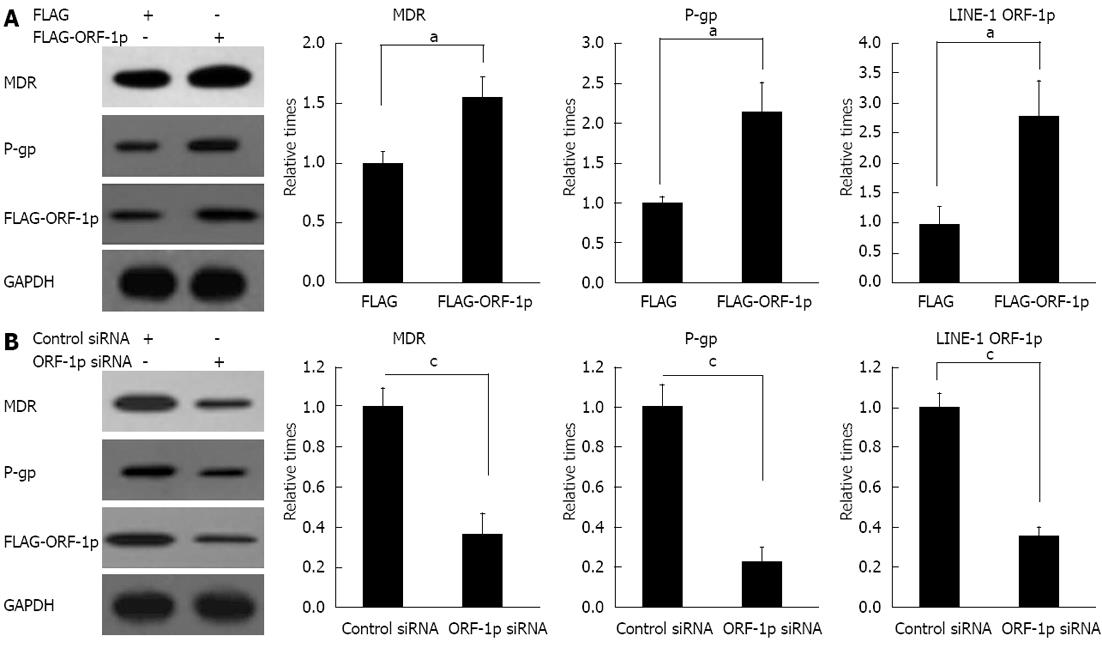
In order to investigate the mechanism of LINE-1 ORF-1p-mediated drug resistance, the effects of LINE-1 ORF-1p on the expression of genes involved in drug resistance were examined. The results showed that overexpression of LINE-1 ORF1-p increased MDR and p-gp protein levels (Figure 6A). Repression of LINE-1 ORF-1p expression through siRNA strongly reduced MDR and p-gp protein levels (Figure 6B). These results indicated that LINE-1 ORF-1p mediates drug resistance by modulating the expression of MDR and p-gp.
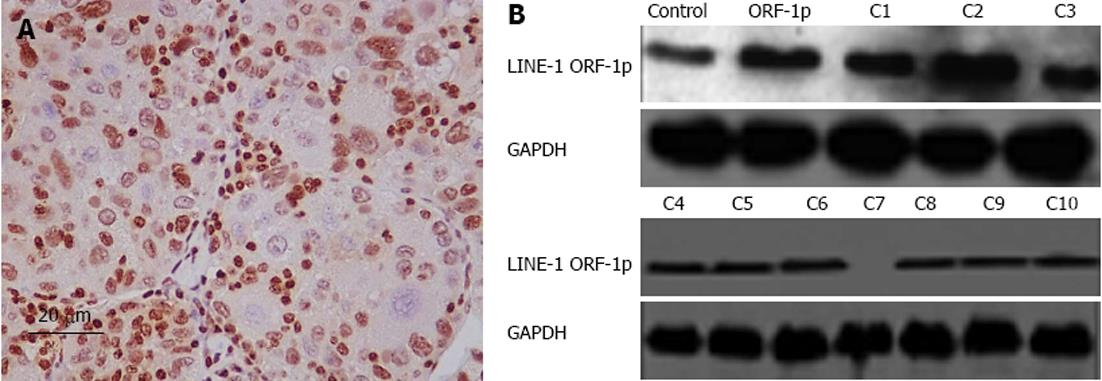
Next, we used immunohistochemistry and immunoblot assays to detect LINE-1 ORF-1p protein expression in clinical specimens and the HepG2 cell line. In most cases, the HepG2 cell line and liver tumors expressed high protein levels of LINE-1 ORF-1p (Figure 7).
In this study, we provide evidence of the novel roles of LINE-1 ORF-1p in chemotherapeutic drug response and proliferation of HepG2 cells. The results demonstrated that over-expreesion of LINE-1 ORF-1p significantly inhibited the cytotoxic effects of epirubicin and cisplatin on HepG2 cells. However, knockdown of LINE-1 ORF-1p with epirubicin, cisplatin or paclitaxel had a synergistic effect on HepG2 cells. Moreover, LINE-1 ORF-1p regulated the proliferation and anchor-independent growth of HepG2 cells. In addition, LINE-1 ORF-1p repressed Luc-SBE, Luc-SP1, and Luc-p21 transcription, and enhanced Luc-ARE transcription. More importantly, LINE-1 ORF-1p modulated the expression of important genes: p15, p27, p53 and Bcl-2, which are related to HepG2 cell proliferation and apoptosis. Finally, LINE-1 ORF-1p also affected mdr and p-gp gene expression, which is related to chemotherapeutic drug resistance in cancer cells.
LINE-1 comprises at least 17% of the human genome sequence and may significantly affect human genome stability and chromosome structure after de-methylation. LINE-1 displays a silenced state in normal adult tissues with high methylation[8]. It is activated by the de-methylation mechanism, and then frequently retrotransposes[9]. Frequent transposition of LINE-1 may lead to significant changes in the genome, eventually inducing tumorigenesis[10]. It was reported that genome hypomethylation was significantly increased in patients with HCC. The levels of serum LINE-1 hypomethylation at initial presentation correlated significantly with tumor size, tumor number and alpha-fetoprotein level. Moreover, high serum LINE-1 hypomethylation correlates significantly with poor survival[11,12]. Therefore, it is thought that overexpression of LINE-1-associated protein promotes the progression of HCC and affects the outcome of systemic chemotherapy.
Our previous studies indicated that LINE-1 ORF-1p was involved in the regulation of transformation, development and proliferation of several cancer cells[13,14]. We also established several tumor cell models of LINE-1 ORF-1p in human lung cancer, gastric cancer and breast cancer[15]. It is reported that LINE-1 ORF-1p interacts with its own DNA[16]. Thus, LINE-1ORF-1p may regulate important transcription factor activity by changing the structure of chromatin, and in turn promote the growth of cancer cells by modulating the expression of genes related to proliferation, cell cycling and apoptosis regulation[17-19]. LINE-1 ORF-1p may play a role in the regulation of cancer cell proliferation and chemotherapy resistance through various mechanisms.
In this study, we found that overexpression of LINE-1 ORF-1p significantly inhibited the cytotoxic effect of epirubicin and cisplatin on HepG2 cells, without affecting the role of paclitaxel, these results were expected. LINE-1 ORF-1p siRNA with epirubicin, cisplatin or paclitaxel had a synergistic effect on HepG2 cells. In HCC, the rapid growth of cells is caused by high levels of DNA replication and expression, and a high rate of metabolism. In addition, in HCC cells, chromatins are generally unfolded and not agglutinated[17-19]. Therefore, the DNA is more vulnerable than in normal somatic cells[12]. Some chemotherapeutic agents such as cisplatin and epirubicin target bio-macromolecules, such as DNA, and are naturally cytotoxic to HepG2 cells[12]. While LINE-1 ORF-1p binds to DNA, it is likely to play a protective role and act as a barrier to DNA. It is possible to protect DNA against the cytotoxic effects of epirubicin and cisplatin. Our previous study showed that LINE-1 ORF-1p promotes the cell cycling program. These previous results also confirmed the conclusions in this study. In addition, LINE-1 ORF-1p enhanced the protein level of mdr and p-gp genes[20-22]. Thus, LINE-1 ORF-1p also modulates the genes involved in multi-drug resistance.
We then found that LINE-1 ORF-1p promoted Bcl-2 gene expression which is involved in resistance to apoptosis, and inhibited the expression of the genes, p15, p27 and p53[13]. These genes promote apoptosis and arrest cell growth or cell cycling. These results were consistent with those from the luciferase assays. LINE-1 ORF-1p reduced the activity of Luc-p21, Luc-SBE, and Luc-Sp1 reporters and enhanced Luc-ARE reporter transcription. p53, Smad4, Sp1 and AR are important transcription factors, which modulate the transcription of many genes and play a role in the regulation of cancer progression. LINE-1 ORF-1p may interact with these transcriptional factors which are recruited to specific regulatory elements such as SP1 and p53 binding sites. They then interfere with their function through conformational alteration or by affecting their co-repressor recruitment. Further studies are required to address this hypothesis.
Paclitaxel targets microtubules. Overexpression of LINE-1 ORF-1p did not antagonize the cytotoxic effect of paclitaxel, however, knockdown of LINE-1 ORF-1p and paclitaxel had a synergistic effect and reduced its expression level and slowed cell growth by modulating p53, p15, p27, and Bcl-2 expression.
Taken together, these findings suggest that LINE-1 ORF-1p may have a role in the development of HCC, and is likely to be involved in the prediction of chemotherapeutic effect. In addition, these findings significantly advance our understanding of the functions and mechanisms of LINE-1 ORF-1p in cancer cell proliferation and may provide a novel potential therapeutic target in HCC.
Hepatocellular carcinoma (HCC) is the commonest form of cancer worldwide, and the third most common cause of cancer death. Chemotherapy is currently the most commonly adopted strategy for treating HCC patients; however, it also has shortcomings as HCC patients can exhibit robust multidrug resistance.
Long interspersed nuclear element-1 ORF-1 protein (LINE-1 ORF-1p) plays an important role in the development of several types of carcinoma. However, the involvement of LINE-1 ORF-1p in the pathogenesis of hepatocellular carcinoma and whether it is responsible for making these tumors resistant to chemotherapy has not been addressed. In this study, the authors demonstrate that the overexpression of LINE-1 ORF-1p, commonly observed in HCC patients, could be a potential mechanism for both promoting cell proliferation and for resistance to chemotherapy.
Recent reports have highlighted the importance of LINE-1 ORF-1p in different types of cancers. This is the first study to report that LINE-1 ORF-1p mediates resistance to chemotherapy in HCC patients. Furthermore, our in vitro studies suggest that LINE-1 ORF-1p mediates the regulation of cellular apoptotic and proliferative pathways, and aids in the metastatic progression of HCC.
By understanding the mechanism of LINE-1 ORF-1p-mediated induction of resistance to chemotherapy, this may represent a future strategy for therapeutic intervention in the treatment of patients with hepatocellular carcinoma. In addition, the expression levels of LINE-1 ORF-1p in hepatocellular carcinoma patients may serve as a predictive marker of chemotherapy outcome.
LINE-1 is an autonomous non-LTR retrotransposon in mammals. Retrotransposition requires the function of the two L1-encoded polypeptides, ORF1p and ORF2p. LINE-1 ORF-1p, which comprises at least 17% of the human genome sequence, is silenced (non-active) in normal adult tissues. However, in cancer cells, LINE-1 ORF-1p is active and affects genomic stability and chromosome structure through its retrotransposonal activity, eventually leading to tumorigenesis.
In this work, the authors investigated the roles of LINE-1 ORF-1p in the drug resistance and proliferation of HCC with interesting findings.
P- Reviewer Tong QS S- Editor Zhai HH L- Editor Ma JY E- Editor Lu YJ
| 1. | Guo XL, Li D, Hu F, Song JR, Zhang SS, Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ. Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett. 2012;320:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Lee JH, Chung YH, Kim JA, Shim JH, Lee D, Lee HC, Shin ES, Yoon JH, Kim BI, Bae SH. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Sauer G, Kafka A, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Basal expression of the multidrug resistance gene 1 (MDR-1) is associated with the TT genotype at the polymorphic site C3435T in mammary and ovarian carcinoma cell lines. Cancer Lett. 2002;185:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Goodier JL, Zhang L, Vetter MR, Kazazian HH. LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex. Mol Cell Biol. 2007;27:6469-6483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Evans JD, Peddigari S, Chaurasiya KR, Williams MC, Martin SL. Paired mutations abolish and restore the balanced annealing and melting activities of ORF1p that are required for LINE-1 retrotransposition. Nucleic Acids Res. 2011;39:5611-5621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Gao XD, Hu M, Zhu Y. The down-regulation of LINE-1 encoded ORF-1p impacts the characters of A549 cells. Zhongguo Shengwu Kexue Zazhi. 2010;30:14-20. |
| 7. | Fan F, Gao XD, Lu YY, Zhang F, Wang B, Chang XJ, Qu JH, Wang CP, Yang YP. Regulatory effect of LINE-1 ORF-1p on hepatocellular carcinoma cells and proliferation of immortalized hepatocellular cells. Jiefangjun Yixue Zazhi. 2012;37:213-216. |
| 8. | Sunami E, de Maat M, Vu A, Turner RR, Hoon DS. LINE-1 hypomethylation during primary colon cancer progression. PLoS One. 2011;6:e18884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet. 2011;12:187-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Ohka F, Natsume A, Motomura K, Kishida Y, Kondo Y, Abe T, Nakasu Y, Namba H, Wakai K, Fukui T. The global DNA methylation surrogate LINE-1 methylation is correlated with MGMT promoter methylation and is a better prognostic factor for glioma. PLoS One. 2011;6:e23332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Ramzy II, Omran DA, Hamad O, Shaker O, Abboud A. Evaluation of serum LINE-1 hypomethylation as a prognostic marker for hepatocellular carcinoma. Arab J Gastroenterol. 2011;12:139-142. [PubMed] |
| 12. | Hu MM, Zhu YF, Wang Y, Wang Y, Zhang L, Gao XD, Dong J, Yu JY. Prokaryotic expression of LINE-1 encoded protein LINE-1 ORF-1p and preparation of anti-LINE-1 ORF-1p antibody. Zhongguo Shengwu Kexue Zazhi. 2010;30:7-10. |
| 13. | Pivonková H, Pecinka P, Cesková P, Fojta M. DNA modification with cisplatin affects sequence-specific DNA binding of p53 and p73 proteins in a target site-dependent manner. FEBS J. 2006;273:4693-4706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Gao XD, Lu YY, Feng F, Wang CP, Wang H, Chang XJ, Yang YP. Effects of LINE-1 ORF-1p overexpression on proliferation and anchor- independent growth of SMMC7721 hepatoma cell line. Shiyong Ganzangbing Zazhi. 2011;14:323-326. |
| 16. | Chanel-Vos C, Giannakakou P. CENP-E checks in microtubule-drug resistance. Cell Cycle. 2010;9:1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Yan J, Fang Y, Ding L, Zhu J, Lu Q, Huang C, Yang X, Ye Q. Regulation of large-scale chromatin unfolding by Smad4. Biochem Biophys Res Commun. 2004;315:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Zhang F, Feng F, Yang PX, Li ZJ, You JH, Xie WX, Gao XD, Yang JL. Four-and-a-half-LIM protein 1 down-regulates estrogen receptor α activity through repression of AKT phosphorylation in human breast cancer cell. Int J Biochem Cell Biol. 2012;44:320-326. [PubMed] |
| 19. | Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, Dusonchet L, Gebbia N, Abbadessa V, Crosta L. Pterostilbene and 3’-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol. 2005;37:1709-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Qin Q, Chen XP, Yang ZS, Xiao YH, Min H, Li YJ. Neferine increases STI571 chemosensitivity via inhibition of P-gp expression in STI571-resistant K562 cells. Leuk Lymphoma. 2011;52:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Choi WI, Jeon BN, Yun CO, Kim PH, Kim SE, Choi KY, Kim SH, Hur MW. Proto-oncogene FBI-1 represses transcription of p21CIP1 by inhibition of transcription activation by p53 and Sp1. J Biol Chem. 2009;284:12633-12644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Cui J, Germer K, Wu T, Wang J, Luo J, Wang SC, Wang Q, Zhang X. Cross-talk between HER2 and MED1 regulates tamoxifen resistance of human breast cancer cells. Cancer Res. 2012;72:5625-5634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |