Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.917
Revised: November 22, 2012
Accepted: December 15, 2012
Published online: February 14, 2013
Processing time: 142 Days and 15.7 Hours
AIM: To investigate the effects of early enteral nutrition (EEN) on the immune function and clinical outcome of patients with severe acute pancreatitis (SAP).
METHODS: Patients were randomly allocated to receive EEN or delayed enteral nutrition (DEN). Enteral nutrition was started within 48 h after admission in EEN group, whereas from the 8th day in DEN group. All the immunologic parameters and C-reactive protein (CRP) levels were collected on days 1, 3, 7 and 14 after admission. The clinical outcome variables were also recorded.
RESULTS: Sixty SAP patients were enrolled to this study. The CD4+ T-lymphocyte percentage, CD4+/CD8+ ratio, and the CRP levels in EEN group became significantly lower than in DEN group from the 7th day after admission. In contrast, the immunoglobulin G (IgG) levels and human leukocyte antigen-DR expression in EEN group became significantly higher than in DEN group from the 7th day after admission. No difference of CD8+ T-lymphocyte percentage, IgM and IgA levels was found between the two groups. The incidences of multiple organ dysfunction syndrome, systemic inflammatory response syndrome, and pancreatic infection as well as the duration of intensive care unit stay were significantly lower in EEN group than in DEN group. However, there was no difference of hospital mortality between the two groups.
CONCLUSION: EEN moderates the excessive immune response during the early stage of SAP without leading to subsequent immunosuppression. EEN can improve the clinical outcome, but not decrease the hospital mortality of SAP patients.
- Citation: Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol 2013; 19(6): 917-922
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.917
Severe acute pancreatitis (SAP) is a systemic disease characterized by a high mortality because of multiple organ dysfunction syndrome (MODS) and infectious complications[1,2]. During recent years, numerous studies concluded that immune dysregulation might play an important role in the development of MODS and infectious complications in SAP patients[3-5]. In the early stage of SAP, immune imbalance mainly appears as excessive immune response, which is associated with systemic inflammatory response syndrome (SIRS) and MODS[3-6]. Therefore, some clinicians advocate using immunomodulatory therapy in the acute phase of SAP[4,7,8]. However, no consensus on the efficacy of immunotherapy has been reached due to the conflicting results of relevant experiments.
As an essential therapeutic modality for SAP, early enteral nutrition (EEN) could increase antioxidant activity, modulate inflammatory response, and decrease the incidence of SIRS and subsequent MODS[9]. Hence, we infer that EEN might exert some underlying influence on the immune function of SAP patients. However, there have been few trials investigating the effects of EEN on the immune function of the patients during the initial stage of SAP. Zou et al[10] reported that enteral immunonutrition enhanced the immune function in pigs with SAP. Belabed et al[11] found that the immune enhancing diets was efficient in preserving lymphocyte function in head-injured rats. Nevertheless, the impact of standard enteral nutrition on the immunologic function of SAP patients remains unclear.
Therefore, this study aimed to investigate the influence of EEN on the immune function and clinical outcome of early-stage SAP patients.
This is a single-center, prospective, and randomized controlled clinical trial. Patients were randomly allocated to receive either EEN or delayed enteral nutrition (DEN) on admission. The study protocol was approved by the Ethics Committee of the hospital, and informed consent was obtained from each patient or his/her first-degree relatives. This study was also registered at Clinical Trials.gov (Identifier: NCT01507766).
All adult SAP patients (aged 18-70 years) admitted within 3 d of symptom onset to the Surgical Intensive Care Unit (SICU), the Department of General Surgery, Jinling Hospital from September 2010 to September 2011 were enrolled into this study. SAP was defined as presence of one or more local complications (e.g., pseudocyst, necrosis or abscess) or/and organ failure and acute physiology and chronic health evaluation II (APACHE II) score > 8 according to the widely used Atlanta criteria formulated in 1992[12]. Patients with chronic organs dysfunction or immunodeficiency or malnutrition, patients who had received artificial nutrition (either enteral nutrition or parenteral nutrition) before admission, and patients with ileus or pancreatitis in pregnancy were all excluded. All the SAP patients received specialized medical therapy for SAP[2,13] such as intensive monitoring, oxygen administration, fluid resuscitation, stopping oral feeding, exocrine pancreatic suppression, and antibiotic prophylaxis.
Patients in EEN group received enteral nutrition within 48 h after admission, whereas patients in the DEN group received enteral nutrition beginning from the 8th day after admission.
A nasojejunal tube (size 10F, Flocare, Nutricia Ltd, Wuxi, China) was placed beyond the Treitz’ ligament endoscopically or radiologically, and the position of tip was confirmed by fluoroscopy. The tube was placed within 24 h after admission in EEN group, and enteral nutrition was started from the next 24 h. Patients in DEN group received feeding on the 8th day after admission, and a nasojejunal tube was placed on the 7th day. Peptide-based formula (Peptisorb, Nutricia Ltd) was used in the first 24-48 h of feeding, and if patients were tolerant, whole protein formula (Nutrison Fibre, Nutricia Ltd) would be established subsequently. The goal intake of enteral nutrition was calculated as 20-25 kcal/kg·per day and protein need was calculated as 1.5 g/kg·per day[14,15]. The feeding rate was initiated at 15-20 mL/h and increased gradually by 15-20 mL every 6-8 h, using a pump. If the patients were intolerant due to abdominal distension and so on, we would slow down the feeding rate, dilute the feedings concentration, and use prokinetic agents to improve the intestinal motility.
Total parenteral nutrition was given during the first week of admission in DEN group. The caloric intake of parenteral nutrition was determined as 20-25 kcal/kg·per day and the calorie: nitrogen ratio was calculated as 120-150:1[16,17]. Fifty to seventy percentages of total energy need were supplied by glucose, and the use of lipids was on the basis of serum triglyceride levels[16]. Furthermore, sufficient vitamins, electrolytes, trace elements, and insulin were also added into the intravenous solution.
The baseline characteristics of patients including age, sex, body mass index, and etiology were recorded on admission. The APACHE II score, sequential organ failure assessment score, and C-reactive protein (CRP) levels were also collected. The CRP levels and the immunologic parameters including CD4+, CD8+ T-lymphocyte percentage, CD4+/CD8+ ratio, human leukocyte antigen-DR (HLA-DR), immunoglobulin A (IgA), IgG and IgM in peripheral blood were collected on days 1, 3, 7 and 14 after admission[4,6,18]. T-lymphocyte subset percentage and HLA-DR expression were detected by flow cytometry (BD FACS Aria, United States). IgA, IgG and IgM were measured by enzyme-linked immunosorbent assay. The incidences of SIRS and MODS during the first 2 wk after admission, the incidence of surgical operation, the development of pancreatic infection, the duration of ICU stay, and the hospital mortality were also recorded.
All the data were presented as median (interquartile range), if not stated otherwise. Categorical variables were expressed as absolute numbers or in percentages, and were analyzed using χ2 test. Continuous variables were compared by the Mann-Whitney U test or Wilcoxon signed-rank test as appropriate. Statistical Package for the Social Sciences (SPSS, version 17.0, Chicago, IL, United States) software was used for statistical analysis. P < 0.05 was considered statistically significant.
A total of 60 eligible patients with SAP (30 in each group) were enrolled into this clinical trial during the study period. The principal etiological factors of SAP were biliary origin (60%, 36/60) and hyperlipidemia (23%, 14/60). The demographic data and clinical parameters of the patients on admission are presented in Table 1. Three patients (5%, 3/60) died of MODS and septic shock during hospital stay.
| DEN group | EEN group | P value | |
| (n = 30) | (n = 30) | ||
| Age (yr) | 43 (34.5-51) | 45 (35-52) | 0.594 |
| Sex (male:female) | 18:12 | 20:10 | 0.287 |
| Etiology n (%) | |||
| Biliary origin | 17 (57) | 19 (63) | 0.278 |
| Hyperlipidemia | 8 (27) | 6 (20) | 0.373 |
| Alcohol abuse | 3 (10) | 4 (13) | 1.000 |
| Idiopathic | 2 (7) | 1 (3) | 1.000 |
| BMI | 24.6 (23.5-26.8) | 25.8 (23.9-28.8) | 0.158 |
| APACHEII score | 9.5 (8.5-11) | 10 (8-11.5) | 0.994 |
| SOFA score | 4.5 (3.5-5.5) | 4 (3-5) | 0.880 |
| CRP (mg/L) | 203.5 (188-253) | 195 (161-247.5) | 0.214 |
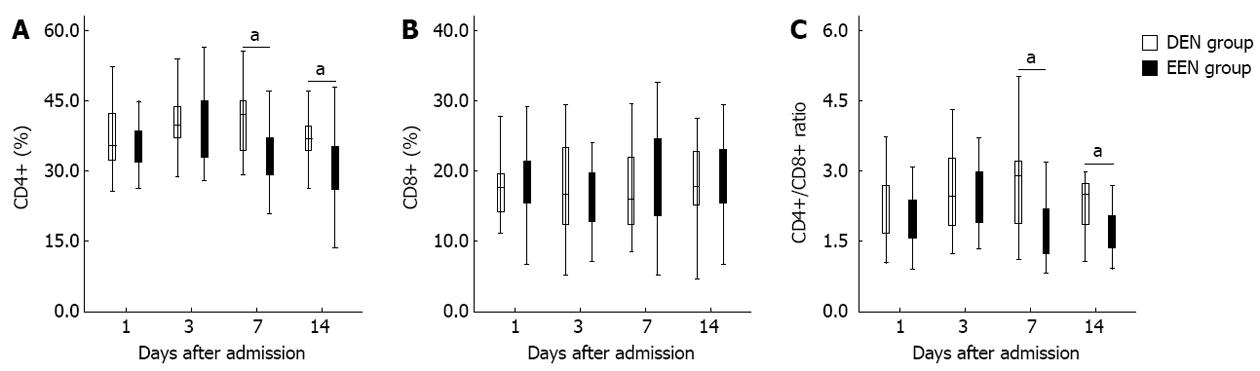
Figure 1 shows the differences of T-lymphocyte subsets percentage between DEN and EEN groups. As shown in Figure 1A, patients in EEN group had significantly lower CD4+ T-lymphocyte percentage from the 7th day (P < 0.05) after admission. Similar results were detected in the CD4+/CD8+ ratio (Figure 1C). However, there was no difference of CD8+ T-lymphocyte percentage between the two groups during the two weeks after admission (Figure 1B).
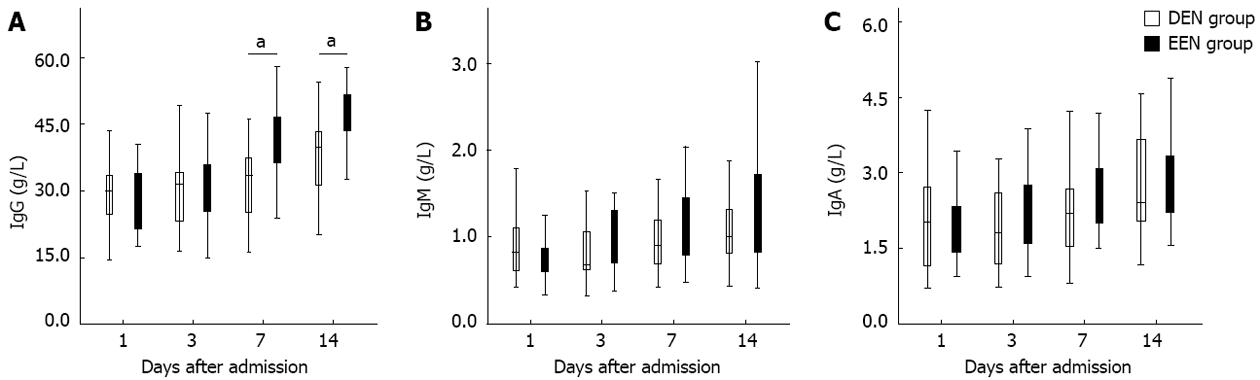
Figure 2 presents the disparities of immunoglobulin subtypes between the two groups. As shown in Figure 2A, patients in EEN group had significantly higher IgG levels from the 7th day (P < 0.05) after admission. Nevertheless, no difference of IgM and IgA between the two groups was found during the two weeks after admission (Figure 2B and C).
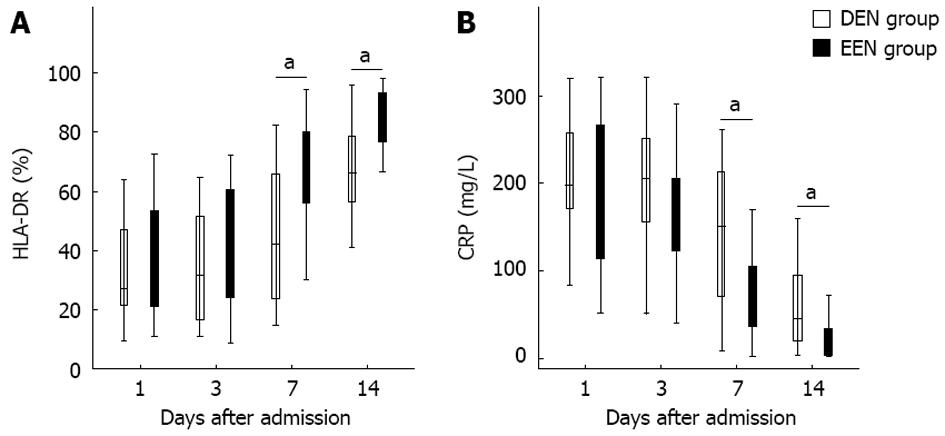
As shown in Figure 3A, patients in EEN group had significantly higher HLA-DR expression from the 7th day (P < 0.05) after admission. In contrast, the CRP levels of patients in EEN group were significantly lower than in patients of the DEN group from the 7th day (P < 0.05) after admission (Figure 3B).
As presented in Table 2, patients in EEN group had significantly lower incidences of MODS, SIRS and pancreatic infection as well as shorter ICU stay during hospital stay. However, no difference of hospital mortality and operation incidence was found between the two groups.
| DEN group (n = 30) | EEN group (n = 30) | P value | |
| Hospital mortality | 1 (3) | 2 (7) | 1.000 |
| ICU stay (d) | 12 (8-21) | 9 (5-14) | 0.033 |
| Pancreatic infection | 10 (33) | 3 (10) | 0.028 |
| MODS | 13 (43) | 5 (17) | 0.024 |
| SIRS | 22 (73) | 12 (40) | 0.009 |
| Surgical operation | 4 (13) | 2 (7) | 0.671 |
This clinical study investigated the effects of EEN on the immune function as well as on the clinical outcome of SAP patients. We found that EEN could moderate the excessive immune response during the early stage of SAP without leading to subsequent immunosuppression. Moreover, EEN administration could improve the clinical outcome, but not decrease the hospital mortality of SAP patients.
Immune dysregulation is one of the main causes responsible for a high mortality of SAP. Recent studies have shown that excessive immune response might play an important role in the development of SIRS and MODS in the early stage of SAP[3-6,19]. The imbalance of Th1/Th2 cells (differentiated from the Th0 cells which were derived from CD4+ T-lymphocytes) is the principal mechanism of excessive immune response[4,19]. Th1 cells mainly produce some anti-inflammatory factors, such as interleukin 10 (IL-10) and IL-4, whereas Th2 cells mainly release the pro-inflammatory factors, such as IL-6, and tumor necrosis factor-α (TNF-α)[3,4,19]. Pietruczuk et al[19] found that Th1 cells were suppressed more strongly than Th2 cells in the acute phase of SAP, thus the pro-inflammatory factors produced by Th2 cells were over-activated and then triggered the strong inflammatory reaction. For this reason, some researchers considered using immunosuppressive therapy for the patients with early-stage SAP. Up till now, a number of immunosuppressive agents (e.g., dexamethasone, IL-10, and anti-tumor necrosis factor monoclonal antibodies) have been studied in SAP animal experiments[4,8]. Unfortunately, the findings were inconsistent or even conflicting, and very few of these studies involved SAP patients. Moreover, inappropriate immunosuppression might be associated with subsequent infectious complications during the late stage of SAP[3,4,6]. Therefore, the effects of immunomodulatory therapy in SAP were still unclear, and further clinical trails are extremely needed.
Gut is the primary immune organ providing an initial barrier against extraneous antigens and microbes[20,21]. The previous studies reported that gut immune response was highly associated with enteral nutrition, and deficiency of enteral stimulation might induce immune suppression[20,21]. In addition, enteral nutrition (especially EEN) had been confirmed to modulate inflammatory response, maintain gut integrity, and release immunomodulating agents[9,22]. Based on the above findings, we postulated that EEN might have some underlying impacts on the immune function of SAP patients, however, few clinical trials about it have been conducted. Therefore, the principal purpose of this clinical study was to investigate the influence of EEN on the immune function of early-stage SAP patients.
CD4+, CD8+ T-lymphocyte percentage and CD4+/CD8+ ratio were closely related to the cellular immune function of SAP[23,24]. Uehara et al[5] observed that both CD4+ percentage and CD4+/CD8+ ratio were significantly reduced in patients with acute pancreatitis, whereas Liu et al[6] reported that the CD4+ percentage was increased significantly on the 7th day in SAP patients after admission. In the present study, we found that EEN could reduce CD4+ T-lymphocyte percentage as well as CD4+/CD8+ ratio from the 7th day after admission. And patients in EEN group also had significantly lower CRP levels from the same day. Furthermore, the initial incidences of SIRS and MODS in EEN group were significantly lower than in DEN group. These results indicated that EEN might be able to suppress the excessive immune response during the early phase of SAP. It has been demonstrated that EEN can prevent the release of pro-inflammatory mediators (e.g., IL-6 and TNF-α) and promote the release of anti-inflammatory factors (e.g., IL-10)[8,9]. In other words, EEN might suppress the over-activation of Th2 cells and promote the production of Th1 cells, and then moderate the imbalance of Th1/Th2 cells. This may be the underlying mechanisms of our conclusion.
In addition, since excessive or prolonged immunosuppressive therapy might induce the development of infectious complications, whether EEN could lead to subsequent immunosuppression still need to be further investigated. Immunoglobulin concentrations and HLA-DR expression levels were also closely related to the immune function of SAP patients. Zou et al[10] reported that the serum IgG levels of SAP pigs were significantly decreased on day 2 after induction of SAP, and then were increased significantly on day 8. Yu et al[25] found that continuous HLA-DR suppression was highly associated with infectious complications and poor outcome in SAP patients, and the HLA-DR suppression was inversely correlated with CRP levels. In our study as shown in Figures 2A and 3A, patients in EEN group had significantly higher IgG levels and HLA-DR expression from the 7th day after admission. And the IgM and IgA levels of patients in EEN group were also not lower than that of patients in DEN group. Furthermore, the incidence of pancreatic infection in EEN group was significantly lower than in DEN group, and this result is also consistent with the findings of previous studies[9,22]. These phenomena suggested that EEN did not lead to immunosuppression during the late stage of SAP.
There are several limitations in this study. Due to the small sample size and the single-center design, our results might be uncertain for a definite conclusion, and the accuracy should be tested by further large-sized studies. Moreover, since this study did not base on a pathophysiological model, the precise mechanisms of EEN in SAP patients should be verified by more basic experiments.
In conclusion, EEN could moderate the excessive immune response during the early stage of SAP without leading to subsequent immunosuppression. Moreover, EEN could improve the clinical outcome, but not decrease the hospital mortality of SAP patients. However, the precise mechanisms of EEN for SAP are still not clear, and further studies are required to verify our conclusions.
The immune dysregulation might play an important role in the development of multiple organ dysfunction syndrome (MODS) and infectious complications in severe acute pancreatitis (SAP) patients. In the early stage of SAP, immune imbalance mainly appears as excessive immune response, therefore, some clinicians advocate using immunomodulatory therapy for SAP. However, no consensus on the efficacy of immunotherapy has been reached due to the conflicting results of relevant experiments.
Early enteral nutrition (EEN) could increase antioxidant activity, modulate inflammatory response, and decrease the incidence of systemic inflammatory response syndrome (SIRS) and subsequent MODS. Hence, the authors infer that EEN might have some underlying influence on the immune function of SAP patients. However, there have been few trials investigating the effects of EEN on the immune function of the patients with SAP at the initial stage.
The study found that EEN could moderate the excessive immune response during the early stage of SAP without leading to subsequent immunosuppression; and EEN could improve the clinical outcome, but not decrease the hospital mortality of SAP patients.
The results of this study indicate that enteral feedings should be provided as early as possible to correct the immune dysregulation of SAP patients.
MODS, is a progressive condition usually characterized by combined dysfunction of multiple organs or systems due to various etiological factors in critically ill patients; SIRS, is diagnosed when two or more of the following criteria are met: body temperature < 36 °C or > 38 °C; heart rate > 90 beats/min; tachypnea > 20 breaths/min, or an arterial partial pressure of carbon dioxide < 32 mmHg; white blood cell count less than 4 × 109/L or greater than 12 × 109/L, or the presence of > 10% immature neutrophils (band forms).
Overall, presented paper is of a very good quality. Study design is appropriate and clear, helps to answer a clinical relevant question. Data analysis is sufficient. Manuscript is well structured, language is of sufficient quality.
P- Reviewers Bradley EL, Dambrauskas Z, Pan WS, Pezzilli R, Twomey PJ S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 2. | Brisinda G, Vanella S, Crocco A, Mazzari A, Tomaiuolo P, Santullo F, Grossi U, Crucitti A. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Shen Y, Cui N, Miao B, Zhao E. Immune dysregulation in patients with severe acute pancreatitis. Inflammation. 2011;34:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Zhang XP, Chen HQ, Liu F, Zhang J. Advances in researches on the immune dysregulation and therapy of severe acute pancreatitis. J Zhejiang Univ Sci B. 2009;10:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Uehara S, Gothoh K, Handa H, Tomita H, Tomita Y. Immune function in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Liu Z, Shen Y, Cui N, Yang J. Clinical observation of immunity for severe acute pancreatitis. Inflammation. 2011;34:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Rau BM, Krüger CM, Hasel C, Oliveira V, Rubie C, Beger HG, Schilling MK. Effects of immunosuppressive and immunostimulative treatment on pancreatic injury and mortality in severe acute experimental pancreatitis. Pancreas. 2006;33:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol. 2010;16:2867-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 9. | Oláh A, Romics L. Early enteral nutrition in acute pancreatitis--benefits and limitations. Langenbecks Arch Surg. 2008;393:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Zou XP, Chen M, Wei W, Cao J, Chen L, Tian M. Effects of enteral immunonutrition on the maintenance of gut barrier function and immune function in pigs with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2010;34:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Belabed L, Charrueau C, Besson V, Gupta S, Walrand S, Marchand-Verrecchia C, Richon S, Nafziger J, Plotkine M, Chaumeil JC. Impairment of lymphocyte function in head-injured rats: effects of standard and immune-enhancing diets for enteral nutrition. Clin Nutr. 2006;25:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1735] [Article Influence: 54.2] [Reference Citation Analysis (1)] |
| 13. | De Campos T, Braga CF, Kuryura L, Hebara D, Assef JC, Rasslan S. Changes in the management of patients with severe acute pancreatitis. Arq Gastroenterol. 2008;45:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Meier R, Ockenga J, Pertkiewicz M, Pap A, Milinic N, Macfie J, Löser C, Keim V. ESPEN Guidelines on Enteral Nutrition: Pancreas. Clin Nutr. 2006;25:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 15. | Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Ebner C. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 821] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 16. | Gianotti L, Meier R, Lobo DN, Bassi C, Dejong CH, Ockenga J, Irtun O, MacFie J. ESPEN Guidelines on Parenteral Nutrition: pancreas. Clin Nutr. 2009;28:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 17. | McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33:277-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 986] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 18. | Wang X, Li W, Zhang F, Pan L, Li N, Li J. Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: a randomized controlled trial. Inflammation. 2009;32:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol. 2006;12:5344-5351. [PubMed] |
| 20. | MacDonald TT. The mucosal immune system. Parasite Immunol. 2003;25:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Cunningham-Rundles S. Nutrition and the mucosal immune system. Curr Opin Gastroenterol. 2001;17:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Hegazi R, Raina A, Graham T, Rolniak S, Centa P, Kandil H, O’Keefe SJ. Early jejunal feeding initiation and clinical outcomes in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2011;35:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Vaccari M, Boasso A, Fenizia C, Fuchs D, Hryniewicz A, Morgan T, Weiss D, Doster MN, Heraud JM, Shearer GM. Fatal pancreatitis in simian immunodeficiency virus SIV(mac251)-infected macaques treated with 2’,3’-dideoxyinosine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol. 2012;86:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Uchida K, Kusuda T, Koyabu M, Miyoshi H, Fukata N, Sumimoto K, Fukui Y, Sakaguchi Y, Ikeura T, Shimatani M. Regulatory T cells in type 1 autoimmune pancreatitis. Int J Rheumatol. 2012;2012:795026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Yu WK, Li WQ, Li N, Li JS. Mononuclear histocompatibility leukocyte antigen-DR expression in the early phase of acute pancreatitis. Pancreatology. 2004;4:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |