Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.866
Revised: December 12, 2012
Accepted: December 22, 2012
Published online: February 14, 2013
Processing time: 152 Days and 21.6 Hours
AIM: To investigate the role of suppressor of cytokine signaling 3 (SOCS3) silencing in epithelial-mesenchymal transition (EMT) involved in a human hepatocellular carcinoma MHCC97H cell line.
METHODS: MHCC97H cells were transiently transfected with SOCS3 small-interfering RNA (siRNA). Morphological changes of the transfected cells were observed under microscope. Expressions of E-cadherin, Vimentin and α-smooth muscle actin (α-SMA) were identified with immunofluorescence. Furthermore, protein expressions and mRNA levels of characteristic markers of EMT (E-cadherin, Vimentin, α-SMA and Snail) were detected by Western blotting, quantitative real-time polymerase chain reaction. Transforming growth factor-β1 (TGF-β1) levels in the supernatant were measured with enzyme-linked immunosorbent assay.
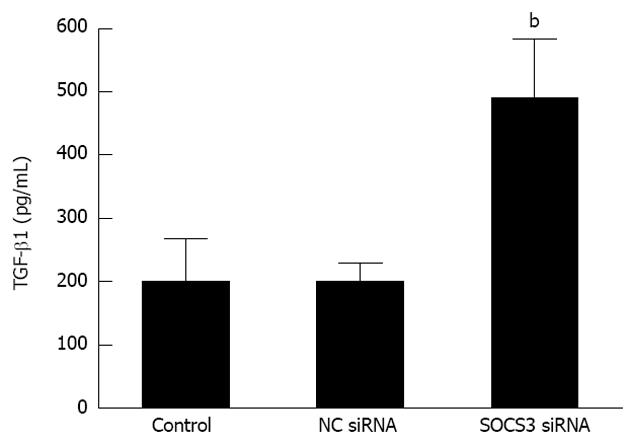
RESULTS: The transfected cells with SOCS3 siRNA showed a morphological alteration from a typical cobblestone morphology to mesenchymal spindle-shaped and fusiform features. SOCS3 siRNA lessened immunofluorescent expression of E-cadherin, but elicited immunofluorescent expressions of Vimentin and α-SMA in MHCC97H cells. More importantly, compared with the negative control, depletion of SOCS3 resulted in the decrease of the epithelial marker E-cadherin (P < 0.05), and the increase of the mesenchymal markers Vimentin and α-SMA and the transcription factor Snail in MHCC97H cells (P < 0.05). Moreover, compared with the negative control, SOCS3 siRNA evidently enhanced TGF-β1 secretion in MHCC97H cells (200.20 ± 29.02 pg/mL vs 490.20 ± 92.43 pg/mL, P < 0.05).
CONCLUSION: SOCS3 silencing is able to promote EMT in MHCC97H cells via changing the phenotypic characteristics and modulating the characteristic markers.
- Citation: Ji YY, Wang ZD, Li ZF, Li K. Interference of suppressor of cytokine signaling 3 promotes epithelial-mesenchymal transition in MHCC97H cells. World J Gastroenterol 2013; 19(6): 866-873
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/866.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.866
Hepatocellular carcinoma (HCC), the primary malignancy of the liver, ranks the sixth in incidence and the third in cancer-related deaths worldwide[1]. Poor prognosis of HCC is associated with a high potential of vascular invasion, metastasis, and recurrence even after curative surgical resection[2]. The main cause of death in HCC patients is intrahepatic metastasis, but the underlying mechanism is still not fully understood. Epithelial-mesenchymal transition (EMT) is the morphological and molecular change that occurs when epithelial cells lose their characteristics, gain mesenchymal properties and become motile, which is a key event in tumor invasion and metastasis[3-5]. The most common character of EMT is that the cells turn to spindle-like morphology from compact and well-arranged epithelial structure[6]. The epithelial marker E-cadherin plays a central role in cell-cell adhesion junctions in maintenance of cell polarity[7-9]. Loss of E-cadherin expression is commonly related to tumor invasiveness, metastasis and poor prognosis, including HCC[8]. Furthermore, the expression of mesenchymal markers such as vimentin and α-smooth muscle actin (α-SMA), along with the expression of transcription factors such as Snail is an essential molecular marker of EMT[10]. Snail is able to repress E-cadherin and overexpression of Snail has been reported to be correlated with HCC metastasis through the induction of EMT[11,12]. Moreover, the levels of transforming growth factor-β1 (TGF-β1) are very high in many cancer cells, which are related to EMT and a high incidence of metastasis[4]. In particular, TGF-β1 induced-EMT has been found to be associated with HCC invasion and metastasis[13]. EMT is being increasingly recognized as a crucial step that promotes tumor invasiveness and metastasis, thus understanding the influential factor of EMT could allow the development of novel therapies targeting at HCC invasion and metastasis.
Suppressor of cytokine signaling (SOCS) family proteins have been implicated in the negative regulation of various cytokines[14,15]. Recently, emerging evidence suggests that SOCS may be tumor suppressors. It has been postulated that SOCS can decelerate or inhibit the progression of cirrhosis and HCC[16,17]. Intriguingly, SOCS3 silencing is a significant predictor or poor survival indicating that SOCS3 might play a special role in limiting late-stage HCC progression[18]. Although SOCS3 may be involved in the suppression of tumor growth and metastasis of HCC[19,20], its role in hepatoma carcinoma cells has not been completely established. Thus, it will be of interest to learn more about whether SOCS3 depletion is able to promote EMT of HCC. The objectives of the present study were to primarily investigate the role of SOCS3 silencing in EMT involved in MHCC97H cells.
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Carlsbad, CA, United States). Polyclonal anti-human E-cadherin, Vimentin, Snail, SOCS3, anti-α-SMA and anti-β-SMA antibodies were products of Santa Cruz Biotechnology (Santa Cruz, CA, United States). Human TGF-β1 enzyme-linked immunosorbent assay (ELISA) kit was obtained from Invitrogen (Carlsbad, CA, United States). siRNA specific for SOCS3 (siGENOME SMARTpool, M-004299-08-0005) and negative control siRNA (siGENOME Non-Targeting siRNA Pool, D-001206-13-05), siGLO Green (6-FAM) Transfection Indicator (D-001630-01-05), DharmaFECT 4 transfection reagent (T-2002-04), and siGLO Green (6-FAM) Transfection Indicator (D-001630-01-05) were purchased from Dharmacon (Lafayette, CO, United States)
Human MHCC97H cell, a typical HCC cell line with a high metastatic potential[21], was obtained from Liver Cancer Institute of Fudan University (Shanghai, China). MHCC97H cells were cultured with DMEM supplemented with 10% FBS in a humidified incubator at 5% CO2 and 37 °C.
MHCC97H cells (1 × 105) were seeded into 6-well plates and were grown until 60%-80% confluent. The cells were transiently transfected with 25 nmol/L of SOCS3 small-interfering RNA (siRNA) or negative control siRNA (NC siRNA) using Dharma FECT 4 transfection reagents according to the manufacturer’s instructions. After 24 h, fluorescent images of transfected cells were observed under fluorescence microscope (Nikon, Japan). After 48 h, protein expression and mRNA levels of SOCS3 were detected by Western blotting, quantitative real-time polymerase chain reaction (PCR) and reverse transcription (RT)-PCR. Transfection rates of 60%-70% of the cells were accepted for all the experiments.
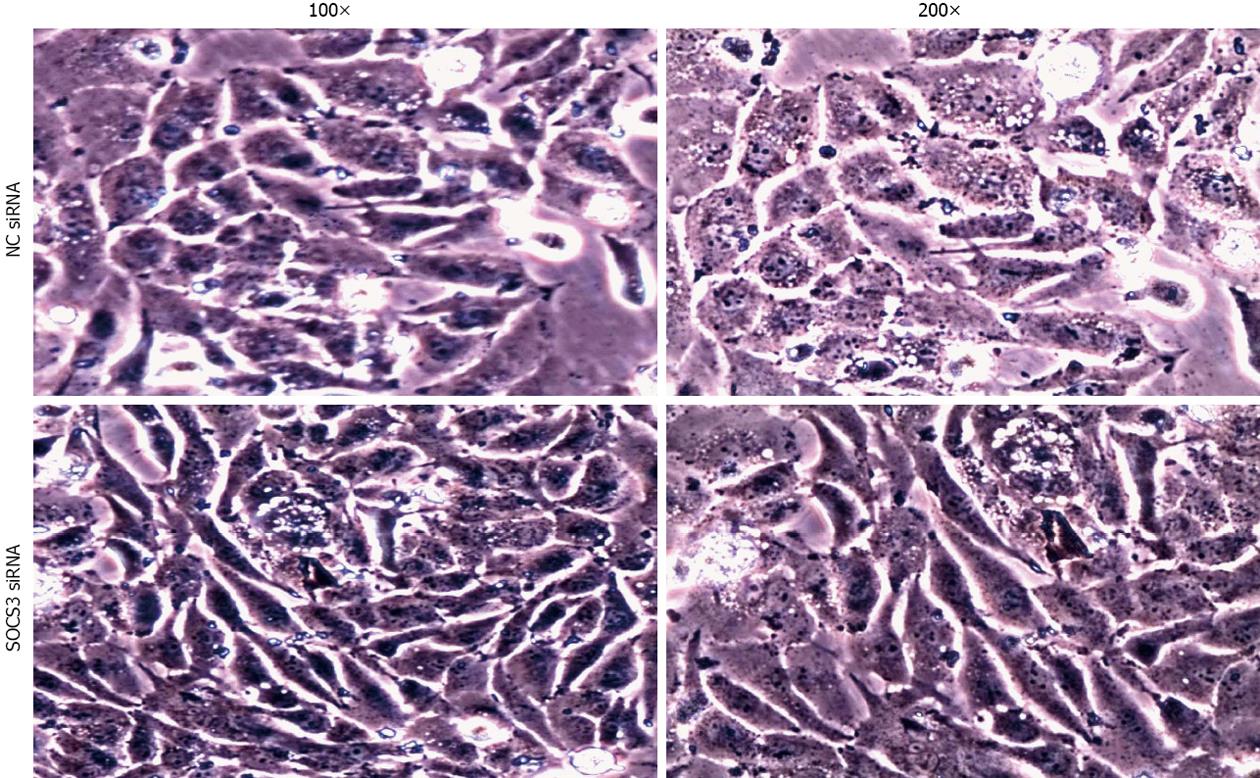
After application of NC siRNA or SOCS3 siRNA for 48 h, morphological changes of the transfected cells were observed under an inverted phase-contrast microscope (Nikon, Japan). The photographs were taken at 100× and 200× magnifications by a digital camera.
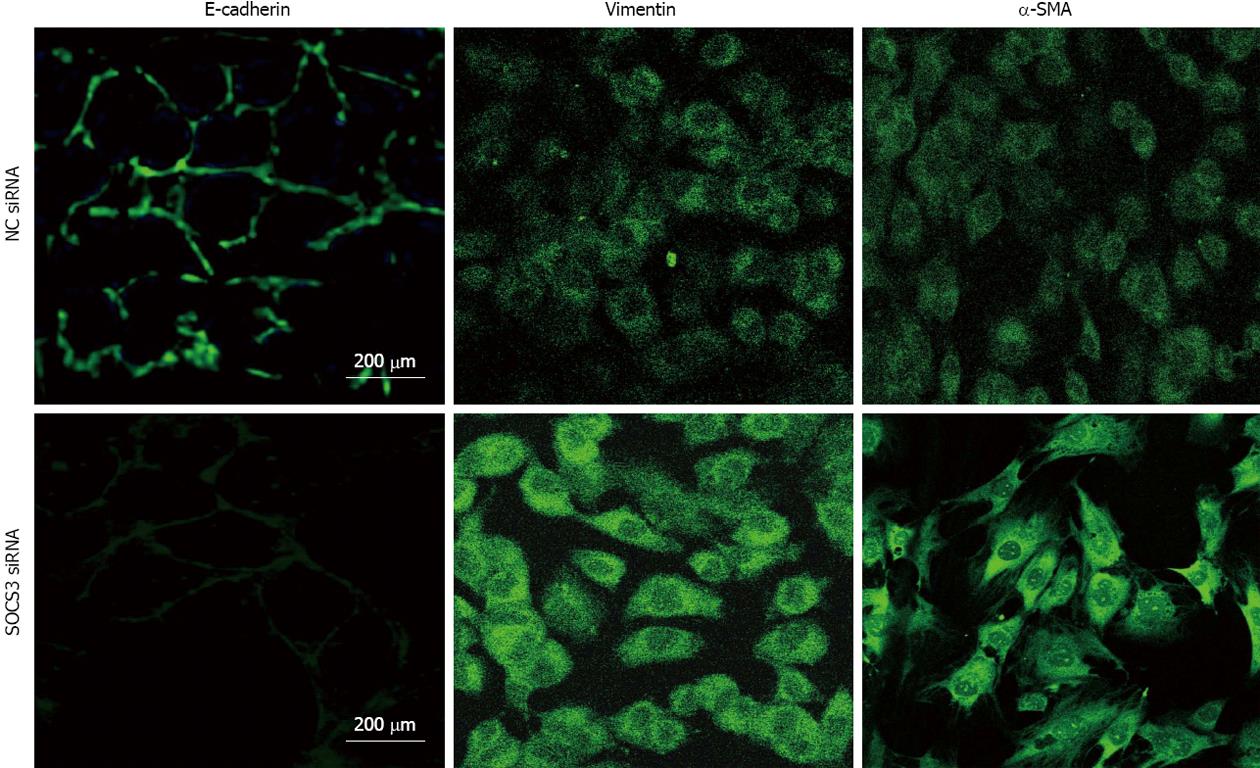
MHCC97H cells were plated on cover slips and grown to confluence, and were transiently transfected with 25 nmol/L of SOCS3 siRNA or NC siRNA for 48 h. After the treatment, the cells were fixed with 4% formaldehyde-PBS for 15 min. The cell membranes were fenestrated with 0.3% Triton-100-PBS, and nonspecific binding sites were blocked with 10% goat serum. The cells were incubated with anti-human antibody including E-cadherin (1:100) or Vimentin (1:200) or α-SMA (1:100) and then incubated with the secondary antibody conjugated to fluorescein isothiocyanate. The immunolabeled cells were observed under fluorescence microscope (Nikon, Japan).
After application of NC siRNA or SOCS3 siRNA for 48 h, concentration of TGF-β1 in the supernatant of the cells was measured by ELISA kits according to the manufacturer’s instructions.
As described previously[22], protein samples (25 μg) were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA, United States). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20, and incubated with specific antibodies against E-cadherin (1:200), Vimentin (1:200), Snail (1:100), SOCS3 (1:200), α-SMA (1:400) and β-actin (1:400). The expression of β-actin was used as a loading control. Reagents (Pierce Corp., Rockford, IL, United States) for the enhanced chemiluminescence were applied to the blots, and the light signals were detected by X-ray film. Optical densities of the bands were scanned and quantified with the Syngene Gene Tools (Syngene Corp., Cambridge, United Kingdom). Three independent experiments were carried out to study protein expression.
mRNA levels were determined by our previous method[22]. Total RNA was isolated using a TRIzol Kit (Invitrogen Corp., Carlsbad, CA, United States). cDNA was synthesized from 1 μg samples of total RNA using Revert AidTM First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) following the manufacturer’s instructions. Real-time PCR was performed with the SYBR Premix Ex Taq™ II Perfect Real Time kit (Takara, Japan) on an ABI QPCR System (Applied Biosystems, CA, United States) following the manufacturer’s instructions. The samples were run in triplicate. Primers for human E-cadherin, Vimentin, Snail, SOCS3, α-actin and β-actin were designed with Beacon designer v 4.0 (Premier Biosoft, United States) (Table 1). Traditional PCR was performed according to the manufacturer’s instructions. The RT-PCR products were analyzed by electrophoresis through 2% agarose gels containing ethidium bromide. A melting point dissociation curve generated by the instrument was used to confirm that only a single product was present. Quantization of relative gene expression was calculated by the comparative Ct method (2-ΔΔCT) as described by the manufacturer. Data were normalized to human β-actin mRNA levels. Three independent experiments were carried out to study mRNA levels.
| Gene | Primer sequence | Accession number | Expected size (bp) |
| E-cadherin | 5′-CCCGGGACAACGTTTATTAC-3′ | NM_004360.3 | 190 |
| 5′-GCTGGCTCAAGTCAAAGTCC-3′ | |||
| Vimentin | 5′-AAAGTGTGGCTGCCAAGAAC-3′ | NM_003380.2 | 200 |
| 5′-AGCCTCAGAGAGGTCAGCAA-3′ | |||
| α-SMA | 5′-GCGCAAATACTCGGTGTGGA-3′ | NM_001141945.1 | 170 |
| 5′-CCCCCCCATTGAGAAGATTC-3′ | |||
| Snail | 5′-TGGTTGCTTCAAGGACACAT-3′ | NM_003068.3 | 141 |
| 5′-GTTGCAGTGAGGGCAAGAA-3′ | |||
| SOCS3 | 5′-CAGGAATGTAGCAGCGATGGAA-3′ | NM_003955.3 | 125 |
| 5′-CCTGTCCAGCCCAATACCTGA-3′ | |||
| β-actin | 5′-ATCGTGCGTGACATTAAGGAGAAG-3′ | NM_001101 | 179 |
| 5′-AGGAAGGAAGGCTGGAAGAGTG -3′ |
All data were expressed as the mean ± SD from three different experiments. Statistical analysis were carried out with Student’s t test for independent samples. In all cases, a value of P < 0.05 was considered statistically significant.
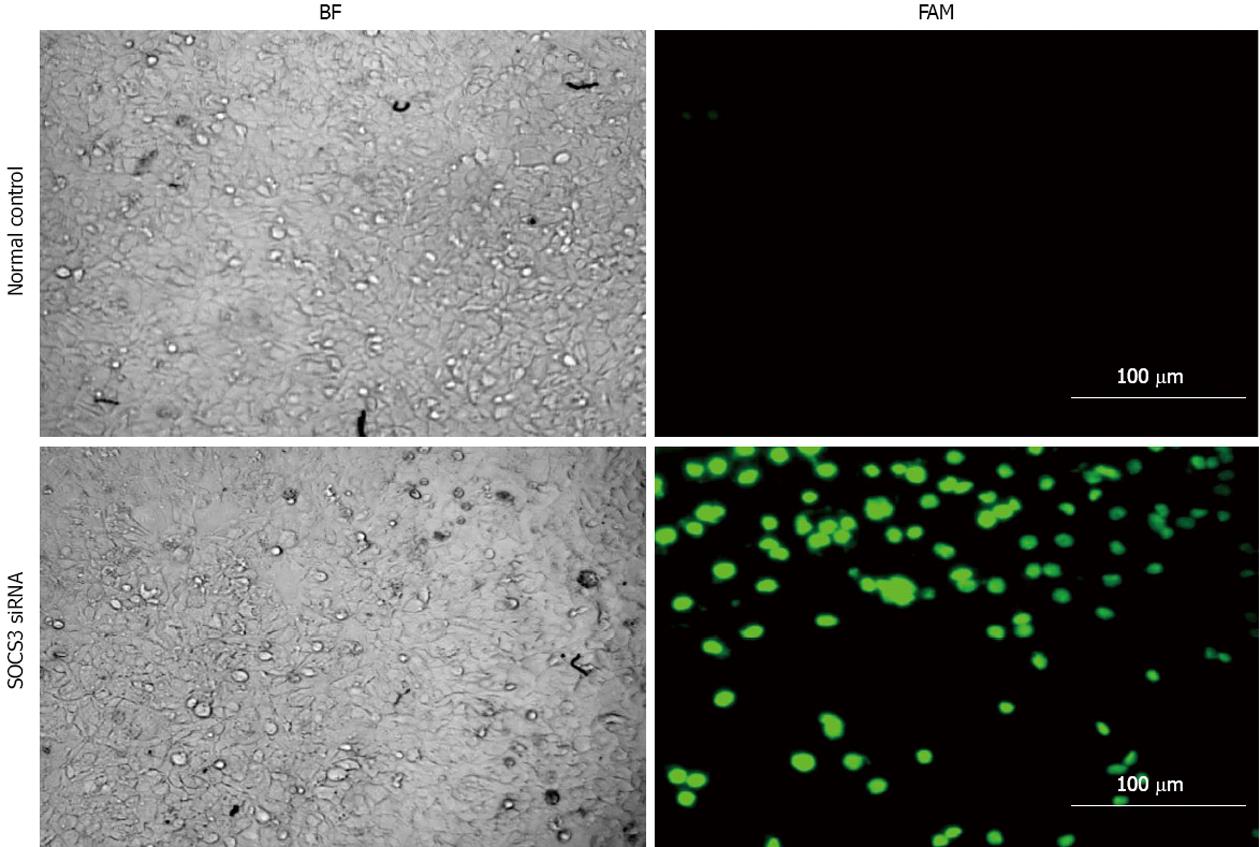
After MHCC97H cells were transiently transfected with SOCS3 siRNA (25 nmol/L) and siGLO Green Transfection Indicator (50 nmol/L) for 24 h, fluorescent expression was observed under fluorescence microscope. Compared with the control, the transfected cells with SOCS3 siRNA showed a green fluorescence (Figure 1). The results illustrated that SOCS3 siRNA had been successfully transfected into MHCC97H cells.
To investigate the role of SOCS3 in morphological changes of MHCC97H cells, the cells were transiently transfected with NC siRNA or SOCS3 siRNA for 48 h. As shown in Figure 2, knockdown of SOCS3 resulted in a significant change in cell morphology, as demonstrated by phase-contrast microscopy, with transition from a typical cobblestone morphology to mesenchymal spindle-shaped and fusiform features. The acquisition of a fibroblastic morphology suggested that MHCC97H cells could undergo the mesenchymal change after treated with SOCS3 siRNA.
To examine the effect of SOCS3 on immunofluorescent expressions of E-cadherin, Vimentin and α-SMA in MHCC97H cells, the cells were transiently transfected with NC siRNA or SOCS3 siRNA for 48 h, and expressions of E-cadherin, Vimentin and α-SMA were identified with immunocytofluorescence. The results showed that SOCS3 siRNA might lessen immunofluorescent expression of E-cadherin, but elicit immunofluorescent expressions of Vimentin and α-SMA in MHCC97H cells (Figure 3).
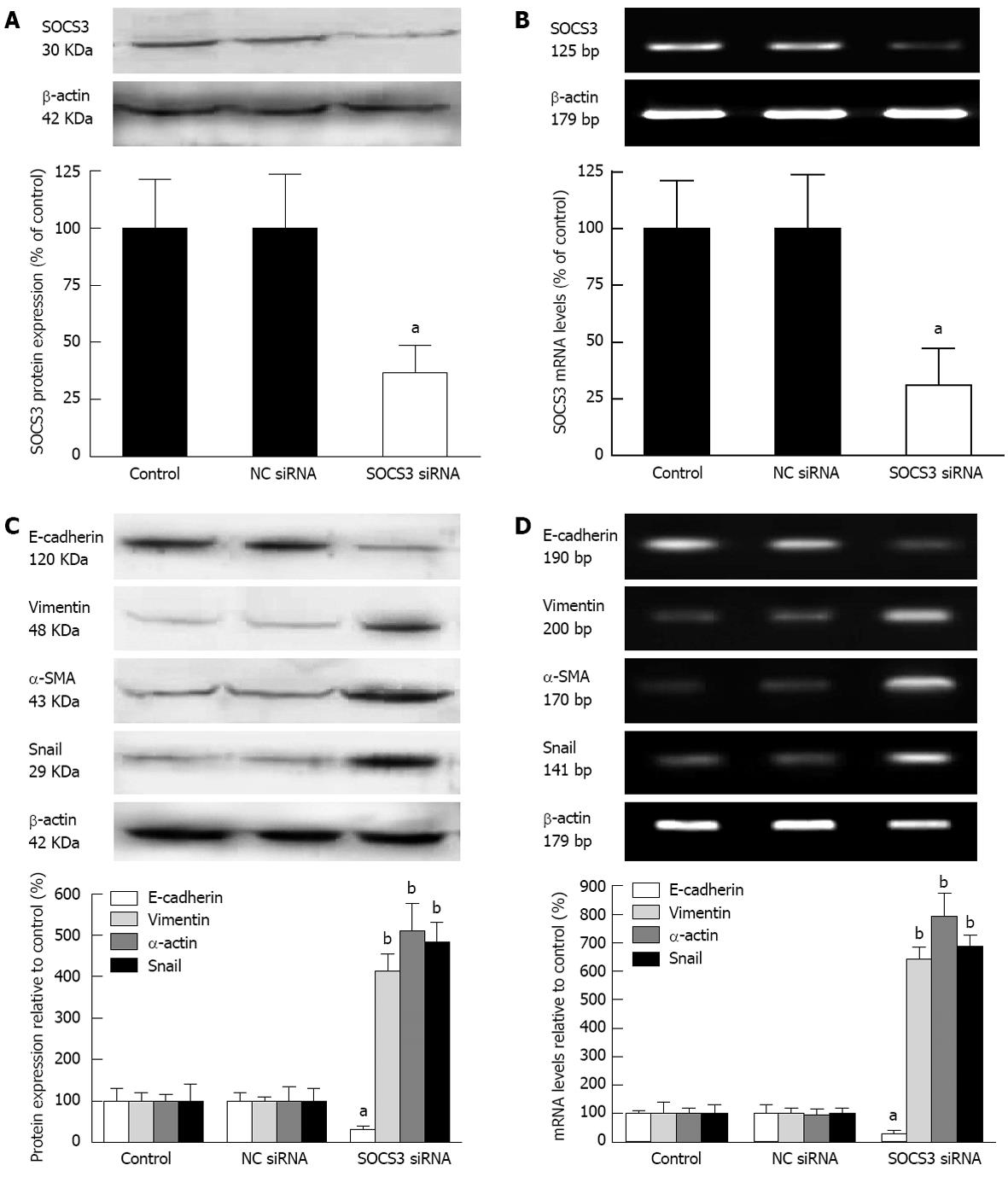
To further explore the impact of SOCS3 on characteristic markers of EMT in MHCC97H cells, the cells were transiently transfected with NC siRNA or SOCS3 siRNA for 48 h, and protein expression and mRNA levels of SOCS3 were detected. Knock down efficiency of SOCS3 was 69.2% or 63.5% as determined by Western blot or quantitative real-time PCR (P < 0.05) (Figure 4A and B). These results showed that SOCS3 siRNA could efficiently reduce protein expression and mRNA levels of SOCS3. Meanwhile, depletion of SOCS3 resulted in the decrease of the epithelial marker E-cadherin, and the increase of the mesenchymal markers Vimentin, α-SMA and Snail in MHCC97H cells (P < 0.05) (Figure 4C and D).
The above characteristic markers of EMT (E-cadherin, Vimentin, α-SMA and Snail) modulate the process of EMT, and TGF-β1 is believed to play a major role in this process. We then observed TGF-β1 levels in MHCC97H cells after SOCS3 silencing. As described in Figure 5, compared with the negative control, depletion of SOCS3 remarkably aggravated TGF-β1 secretion in MHCC97H cells (200.20 ± 29.02 pg/mL vs 490.20 ± 92.43 pg/mL, P < 0.05), suggesting that SOCS3 is able to decrease TGF-β1 generation of MHCC97H cells.
The present study demonstrated that the transfected cells with SOCS3 siRNA showed a morphological alteration from a typical cobblestone morphology to mesenchymal spindle-shaped and fusiform features, suggesting that MHCC97H cells might adopt the mesenchymal cell phenotype. More importantly, we found that lack of SOCS3 resulted in the decrease of the epithelial marker E-cadherin, and the increase of the mesenchymal markers Vimentin and α-SMA and the expression of transcription factor Snail in MHCC97H cells. In addition, SOCS3 siRNA evidently enhanced TGF-β1 secretion in MHCC97H cells. These results reveal that interference of SOCS3 is able to facilitate EMT involved in MHCC97H cells.
SOCS3 functions as a negative regulator of the Jak/Stat signal transduction pathway and has been described as a tumor suppressor in human cancers[23,24]. It has also been reported that SOCS3 silencing plays a role in human HCC and SOCS3 has a tumor suppressor role in mouse models of HCC[25]. So far, the relationship between SOCS3 and EMT in HCC is still elusive. As a result, our present study has focused on the effect of SOCS3 silencing on EMT in MHCC97H cells. We found for the first time that SOCS silencing was associated with EMT process in MHCC97H cells because of changes of the phenotypic characteristics.
EMT is a key and dynamic process that facilitates invasion of tumor cells. In addition to changes of the phenotypic characteristics, tumor cells are reported to gain the molecular characteristics of EMT including down-regulation of epithelial marker (E-cadherin) and up-regulation of mesenchymal markers (Vimentin and α-SMA)[26]. Loss of E-cadherin and gain of Vimentin or α-SMA are the most important consequence of EMT that facilitate invasion and metastasis of tumor cells[27-29]. In the present study, transfection of MHCC97H cells with SOCS3 siRNA resulted in down-regulation of epithelial marker E-cadherin and up-regulation of mesenchymal markers Vimentin and α-SMA, further confirming the promotion of EMT by SOCS3 silencing in MHCC97H cells.
Snail has been shown to be a strong repressor of transcription of the E-cadherin gene and evokes tumorigenic and invasive properties in epithelial cells upon overexpression[30]. It has been reported that the high expression of Snail leads to EMT and enhances motility and invasiveness of tumor cells[31]. Inverse correlation of Snail and E-cadherin was detected in HCC-derived cell lines[32]. In the present study, examination of Snail expression in MHCC97H cells with SOCS3 siRNA transfection indicated a significant increase in protein expression and mRNA levels of Snail, possibly leading to loss of E-cadherin resulting in the induction of EMT. These results are consistent with the mechanism of induction of EMT[11,12].
TGF-β1 is known to be the most potent inducer of EMT, and it initiates morphological transition of the cells from an epithelial to a fibroblastic appearance, accompanied by loss of epithelial cell marker such as E-cadherin and a gain of mesenchymal cell marker such as Vimentin[33,34]. During the late stages of HCC tumorgenesis, TGF-β1 stimulates cellular invasion through the EMT program[35]. A previous study also demonstrates that TGF-β1 expression is enhanced by SOCS3 gene deletion in HCC[25]. In this work, we further detected TGF-β1 levels in MHCC97H cells transfected with SOCS3 siRNA. The results displayed that depletion of SOCS3 remarkably aggravated TGF-β1 secretion in MHCC97H cells, further illustrating the induction of EMT by SOCS3 silencing.
In conclusion, the present study provides direct evidence that SOCS3 silencing is able to promote EMT in MHCC97H cells via changing the phenotypic characteristics and modulating the characteristic markers, suggesting that SOCS3 could play a potential role in EMT in MHCC97H cells. We also identified a novel relationship between SOCS3 silencing and EMT in MHCC97H cells, however, further in vitro and in vivo studies are necessary to find out the exact molecular mechanism associated with the effect of SOCS3 in EMT of HCC.
The main cause of death of patients with hepatocellular carcinoma (HCC) is intrahepatic metastasis, but the underlying mechanism is still not fully understood. Epithelial-mesenchymal transition (EMT) is being increasingly recognized as a crucial step that promotes HCC invasiveness and metastasis. However, the role of suppressor of cytokine signaling (SOCS) in EMT involved in HCC is not well documented.
This study demonstrated that depletion of SOCS3 resulted in the decrease of the epithelial marker E-cadherin, and the increase of the mesenchymal markers Vimentin and α-smooth muscle actin and the expression of transcription factor Snail in MHCC97H cells. In addition, SOCS3 small-interfering RNA evidently enhanced transforming growth factor-β1 secretion in MHCC97H cells. These results reveal that interference of SOCS3 is able to facilitate EMT involved in MHCC97H cells.
The present study provides direct evidence that SOCS3 silencing has the ability to promote EMT in MHCC97H cells via changing the phenotypic characteristics and modulating the characteristic markers, suggesting that SOCS3 could play a potential role in EMT in MHCC97H cells.
The authors found a novel relationship between SOCS3 silencing and EMT in HCC, thus understanding the influential factor of EMT could allow the development of novel therapies targeting at HCC invasion and metastasis.
EMT is the morphological and molecular changes that occur when epithelial cells lose their characteristics, gain mesenchymal properties and become motile, which is a key event in tumor invasion and metastasis; The SOCS proteins are a family of negative regulatory proteins related to wide cytokine and growth correlation factor, especially by the signal molecule in the pathway of Janus kinase/signal transducer and transcription activator.
This study presented results suggesting that SOCS3 silencing has the ability to promote EMT in MHCC97H cells. It will help to define the role of SOCS3 as a tumor suppressor in HCC. The manuscript is well presented and of interest and it can contribute to increase the knowledge of SOCS3 silencing on EMT involved in MHCC97H cell line.
P- Reviewers Grassi G, Zhou YM, Aghakhani A S- Editor Huang XZ L- Editor Ma JY E- Editor Li JY
| 1. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1568] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 2. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 669] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1482] [Cited by in RCA: 1597] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 4. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7747] [Article Influence: 484.2] [Reference Citation Analysis (0)] |
| 5. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4877] [Cited by in RCA: 5118] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 6. | Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1182] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 7. | Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2783] [Cited by in RCA: 2982] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 8. | Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 627] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 9. | Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, Sugimachi K, Tsuneyoshi M. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Gavert N, Ben-Ze’ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2790] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 12. | Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 502] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 15. | Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol. 1999;163:2640-2647. [PubMed] |
| 16. | Chu PY, Yeh CM, Hsu NC, Chang YS, Chang JG, Yeh KT. Epigenetic alteration of the SOCS1 gene in hepatocellular carcinoma. Swiss Med Wkly. 2010;140:w13065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 553] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 19. | Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Elliott J. SOCS3 in liver regeneration and hepatocarcinoma. Mol Interv. 2008;8:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Wang Z, Zhou J, Fan J, Tan CJ, Qiu SJ, Yu Y, Huang XW, Tang ZY. Sirolimus inhibits the growth and metastatic progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:715-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Ji Y, Wang Z, Li Z, Li K, Le X, Zhang T. Angiotensin II induces angiogenic factors production partly via AT1/JAK2/STAT3/SOCS3 signaling pathway in MHCC97H cells. Cell Physiol Biochem. 2012;29:863-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Baltayiannis G, Baltayiannis N, Tsianos EV. Suppressors of cytokine signaling as tumor repressors. Silencing of SOCS3 facilitates tumor formation and growth in lung and liver. J BUON. 2008;13:263-265. [PubMed] |
| 24. | Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider MJ. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011;122:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Bonnomet A, Brysse A, Tachsidis A, Waltham M, Thompson EW, Polette M, Gilles C. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia. 2010;15:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 28. | Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 29. | Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1448] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 30. | Moreno-Bueno G, Cubillo E, Sarrió D, Peinado H, Rodríguez-Pinilla SM, Villa S, Bolós V, Jordá M, Fabra A, Portillo F. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543-9556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 31. | Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 32. | Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer. 2002;86:98-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764-5774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1310] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 34. | Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 853] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 35. | Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2177] [Article Influence: 136.1] [Reference Citation Analysis (0)] |