Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.802
Revised: December 3, 2012
Accepted: December 15, 2012
Published online: February 14, 2013
Non-alcoholic fatty liver disease (NAFLD) is recognized as the most common type of chronic liver disease in Western countries. Insulin resistance is a key factor in the pathogenesis of NAFLD, the latter being considered as the hepatic component of insulin resistance or obesity. Adiponectin is the most abundant adipose-specific adipokine. There is evidence that adiponectin decreases hepatic and systematic insulin resistance, and attenuates liver inflammation and fibrosis. Adiponectin generally predicts steatosis grade and the severity of NAFLD; however, to what extent this is a direct effect or related to the presence of more severe insulin resistance or obesity remains to be addressed. Although there is no proven pharmacotherapy for the treatment of NAFLD, recent therapeutic strategies have focused on the indirect upregulation of adiponectin through the administration of various therapeutic agents and/or lifestyle modifications. In this adiponectin-focused review, the pathogenetic role and the potential therapeutic benefits of adiponectin in NAFLD are analyzed systematically.
- Citation: Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroenterol 2013; 19(6): 802-812
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.802
Non-alcoholic fatty liver disease (NAFLD) is the most common type of chronic liver injury in many countries[1,2]. NAFLD includes a spectrum of syndromes, ranging from simple steatosis, non-alcoholic steatohepatitis (NASH) to fibrosis, cirrhosis and hepatocellular carcinoma[3]. The overall prevalence of NAFLD is 15%-40% in Western countries and 9%-40% in the Asian population[4]. NAFLD has dramatically increased over the past 15 years, mainly as a consequence of its close association with two major worldwide epidemics, obesity and type 2 diabetes mellitus (T2DM)[5]. Mortality in patients with NAFLD is significantly higher than in the age and gender-matched general population[6]. Disease progression to NASH and cirrhosis appears to be very slow, and only a few patients develop life-threatening advanced liver disease.
In many cases of NAFLD, the risks of developing metabolic and cardiovascular morbidities are much higher than the risks of developing hepatic diseases[7,8]. In fact, NAFLD is considered as the hepatic manifestation of the metabolic syndrome, which refers to a cluster of cardiovascular risk factors associated with insulin resistance, including central obesity, hypertension, dyslipidemia and T2DM[9]. The association between NAFLD and metabolic syndrome has been established in many cross-sectional and prospective studies[8]. NAFLD significantly increases the risk of diabetes and is a better predictor of the development of metabolic disorders than obesity itself[10]. Some studies have reported an association of NAFLD with multiple classical and non-classical risk factors for cardiovascular diseases[7]. NAFLD predicts future cardiovascular events independently of other prognostic factors, including the component of metabolic syndrome. In summary, NAFLD is associated with a future high incidence of cardiovascular and metabolic complications and should be considered beyond a liver disease confined to classical boundaries. Understanding the disease and its management is a vital issue in current clinical practice.
Although the pathogenesis of NAFLD remains largely unknown, insulin resistance, oxidative stress and inflammation play important roles in the development and progression of NAFLD[11,12]. Fatty liver itself is a status of insulin resistance. Hepatic fat accumulation can lead to hepatic insulin resistance, which may occur before the alterations in peripheral insulin actions and may induce peripheral insulin resistance[13,14]. Insulin regulates the uptake, oxidation and storage of fuel within insulin-sensitive tissues including the liver, skeletal muscle and fat. Peripheral insulin resistance impairs glucose uptake from blood into skeletal muscle and adipose tissue; serum non-esterified fatty acid (NEFA) levels may also be elevated because of the failure of insulin to suppress lipolysis[15,16]. In the liver, insulin resistance is associated with increased cellular contents of fatty acids and their metabolites (fatty acyl-CoAs, diacylglycerides and ceramides)[17-19]. Hyperinsulinemia caused by insulin resistance, in the presence of increased circulating levels of NEFA, enhances the hepatic uptake of fatty acid and promotes lipogenesis[1,20]. In addition, defects in mitochondrial β-oxidation, enhanced fatty acid synthesis and impaired secretion of triacylglyceride-rich very low density lipoproteins also contribute to hepatic steatosis[21-23]. A growing body of evidence from animal models suggests a “two-hit” hypothesis as being responsible for the development of NAFLD[24-26]. According to this theory, the first hit is the occurrence of fatty liver (steatosis), followed by a second event leading to the development of NASH. The potential secondary hits include endotoxin exposure, alcohol consumption and virus infections, which expand hepatic lipid stores, cause hepatocellular injury, and promote oxidative stress and inflammation in the liver. Lipotoxicity, and the release of cytokines and other pro-inflammatory mediators, play important roles during this process. Moreover, inflammation in the development of NASH can further impede insulin signaling[27]. Histologically, NASH is manifested by hepatocyte nuclear ballooning, hepatocyte apoptosis, Mallory’s hyaline and inflammation foci[28]. NAFLD patients have a high circulating free fatty acids (FFAs) level that correlates with the severity of liver disease. Overloaded FFAs may exhibit lipotoxicity by inducing the expression of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α)[29].
Obesity, especially visceral obesity, is frequently associated with NAFLD and their coexistence in the same individual increases the likelihood of having more advanced forms of liver disease[30,31]. NAFLD occurs in 60%-95% of people with obesity[32]. Visceral fat is a key mediator of NASH and is strongly associated with alanine aminotransferase (ALT) levels in the nondiabetic obese population[31,33,34]. The importance of visceral fat in the pathogenesis of NAFLD has also been shown in many animal models, including fa/fa obese rats. In these animals, surgical resection of intra abdominal fat depots reverses hepatic insulin resistance and steatosis[35].
Recent evidence suggests that visceral adipose tissue is a metabolic and inflammatory organ that signals and modulates the action and metabolism of the brain, liver, muscle and cardiovascular system[36,37]. The imbalanced production of pro- and anti-inflammatory adipokines secreted from fat contributes to the pathogenesis of NAFLD[38]. Modulation of endocrine/immune/inflammatory interactions of adipose tissue may provide novel therapeutic (pharmacological) targets for the treatment of NAFLD. For example, in patients with severe lipodystrophy, injection with leptin reverses nonalcoholic fatty liver diseases[39,40]. However, in cases of NAFLD associated with obesity, serum levels of leptin are increased, and the liver becomes refractory to the “anti-steatotic” effects of leptin[41-43]. Leptin infusion is therefore unlikely to be of therapeutic value for patients with NAFLD. TNF-α, a pro-inflammatory adipokine that interferes with insulin signaling and favors steatosis, may play a casual role in the pathogenesis of NASH[38]. Circulating levels of TNF-α and hepatic expression of its type 1 receptor are increased in NASH, but could not discriminate steatohepatitis from steatosis[44-46]. Neutralization of TNF-α activity improves fatty liver disease in animals[47]. Conversely, nutritional steatohepatitis can still be produced experimentally in both TNF-α and TNF-α type 1 receptor knockout mice, suggesting that this adipokine might not be an essential mediator of NAFLD[48,49]. In contrast to leptin and TNF-α, adiponectin is more closely implicated in the pathogenesis of NAFLD/NASH. Unlike other adipokines, serum levels of adiponectin are decreased in obesity and its associated medical complications[50]. A negative association between serum levels of adiponectin and liver enzyme levels has been shown in healthy subjects[51]. Numerous epidemiological investigations in diverse ethnic groups have identified lower adiponectin level as an independent risk factor for NAFLDs and liver dysfunctions[37]. Compared with healthy controls, adiponectin levels are lower by more than 50% in NASH patients[52]. Adiponectin expression is decreased by 20%-40% during the development of NAFLD, from simple steatosis to NASH[52,53]. Moreover, NASH patients with lower levels of adiponectin show higher grades of inflammation, suggesting that adiponectin deficiency is an important risk factor for the development of fatty liver, steatohepatitis and other forms of liver injuries[52-55]. In patients with T2DM, plasma adiponectin concentrations are inversely related to hepatic fat content[56]. There is a direct relationship between hypoadiponectinemia and NASH, independent of insulin resistance[52]. Animal-based studies have demonstrated that adiponectin possesses potent protective activities against various forms of liver injury, including those induced by carbon tetrachloride, lipopolysaccharide (LPS)/D-galactosamine, pharmacological compounds, bile duct ligations and methionine-deficient diet[57-61]. In animal models of both alcoholic and nonalcoholic steatohepatitis, exogenous adiponectin reduces hepatomegaly, depletes lipid accumulation, quenches hepatic inflammation and decreases hepatic expression and plasma concentrations of TNF-α[62]. Adiponectin knockout mice exhibit an enhanced pattern of hepatic fibrosis induced by carbon tetrachloride[58]. The lack of adiponectin expression could accelerate hepatic tumor formation in a NASH model in mice[63]. Among the known adipokines, adiponectin stands out for its insulin-sensitizing and anti-inflammatory roles, and may be used as a promising drug candidate for the treatment of liver diseases.
Four independent groups originally identified adiponectin, also termed Acrp30, AdipoQ, apM1 or GBP28, in both mice and humans[64-67]. This adipokine has attracted much attention because of its multiple beneficial effects on a cluster of obesity-related metabolic and cardiovascular dysfunctions. Hypoadiponectinemia is a key etiological factor contributing to almost all the major pathological conditions associated with obesity[68]. The physiological functions and clinical relevance of adiponectin in obesity-related medical complications have been extensively reviewed elsewhere[50,69-72]. In the following sections, we will discuss recent advances on the structural regulations of adiponectin as well as the molecular evidence supporting the role of adiponectin as a major protective agent against obesity-related NAFLD.
A unique feature of the structure of adiponectin is its ability to assemble into several characteristic oligomeric isoforms, including trimers [low molecular weight (LMW)], hexamers [middle molecular weight (MMW)] and the oligomeric complexes comprising 18 protomers or above [high molecular weight (HMW)][73]. Adiponectin presents predominantly in the circulation as these three oligomeric complexes[74-79]. Trimeric adiponectin is the basic building block of adiponectin. The subunits in the trimer are associated via hydrophobic interactions. Two LMW adiponectin molecules linked by disulfide bonds form hexameric adiponectin. The structural properties of the HMW adiponectin remain poorly characterized because of the heterogeneous nature of this isoform. Analysis of adiponectin oligomers by non-denaturing and non-heating gel electrophoresis shows that the human HMW adiponectin composes of a mixture of 18-30 mers, or even larger molecular weight species[73,78,80,81]. Dynamic light scattering and transmission electron microscopy shows that the bovine HMW adiponectin forms a bouquet-like architecture resembling that of complement C1q[82]. Six globular objects can be seen atop a thin stalk, which presumably correspond to the six LMW adiponectins. The stalks bunch together in a manner that is consistent with the requirement for NH2-terminal disulfide bonding. The side views of HMW adiponectin suggest a conical structure of the oligomer with the COOH-terminal portion forming the base. Interestingly, these globular domains are arranged in a tight ring. This circular arrangement might enable polyvalent interactions of the globular domains with a single receptor. Recently, the HMW oligomeric structures formed by multiples of adiponectin trimers have been determined by single-particle analysis of electron micrographs[83]. Pleiomorphic ensembles of collagen-like stretches of the trimers lead to a highly dynamic structure of HMW adiponectin, which can be classified into two major classes: the fan-shaped (Class I) and bouquet-shaped (Class II). In both of these conformations, the globular domains assume a variety of arrangements, covering an area of up to 4.9 × 105 Å2 and up to 320 Å apart. The conformational flexibility of the HMW oligomer can allow it to access and cluster disparate target ligands or receptors, which may be necessary to activate cellular signaling leading to the remarkable functional diversity of adiponectin.
Obese individuals have different distribution of adiponectin oligomers compared with lean controls. Relatively lower content of HMW adiponectin is closely associated with obesity-related metabolic complications[81]. The increases in the ratio of HMW vs total adiponectin, but not total adiponectin level per se, correlate well with improved insulin sensitivity during treatment with the insulin-sensitizing drug thiazolidinediones, in both diabetic mice and patients with T2DM. On the other hand, weight reduction by either calorie restriction or gastric bypass surgery results in a selective elevation of the HMW adiponectin, but not the trimeric and hexameric complexes[84-86]. An independent inverse association exists between ALT and HMW adiponectin[87]. Taken together, these epidemiological and genetic data suggest that the beneficial effects of adiponectin in humans might be mediated primarily by its HMW isoform, and the deficiency of this oligomer is an important etiological factor that links obesity with its medical complications.
Evidence from both in vitro and animal-based studies also supports the role of the HMW oligomer as the major active form in mediating the multiple actions of adiponectin in liver tissue. Recombinant adiponectin produced from mammalian cells, which can form the HMW oligomers, potently decreases hyperglycemia in diabetic mice through inhibition of hepatic glucose production[88]. However, bacterially generated full-length adiponectin, which lacks the capacity to form the HMW adiponectin, is almost inactive. Intravenous injection of the HMW adiponectin, but not the hexameric adiponectin, leads to a dose-dependent decrease in serum glucose levels[81]. The formation of the HMW oligomers is obligatory to mediate the insulin sensitizing effects of adiponectin on suppression of hepatic gluconeogenesis in primary rat hepatocytes[80]. Acute injection of recombinant adiponectin enriched with the HMW oligomers results in a marked activation of AMP-activated kinase (AMPK) in the liver, while chronic infusion with this protein leads to prolonged alleviation of hyperglycemia and insulin resistance in db/db diabetic mice[89]. This animal-based evidence is consistent with the clinical observations showing that the ratio of HMW/total adiponectin correlates closely with hepatic insulin sensitivity[81]. The role of the HMW oligomer as a predominant active form of adiponectin mediating its hepatic actions is also supported by two recent independent reports demonstrating that the insulin-sensitizing effects of the peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists thiazolidinediones were diminished in ob/ob obese mice with the targeted mutation of the adiponectin gene[90,91]. Notably, treatment with thiazolidinediones causes a selective elevation of the HMW oligomeric adiponectin[79,81]. In addition to the hepatic insulin-sensitizing activity, the HMW adiponectin has also been suggested to be the most potent isoform for alleviation of fatty liver disease in high fat diet-induced obese mice[92], and inhibition of apolipoprotein B and E release from human hepatocytes[93]. HMW adiponectin dose-dependently suppressed growth factor-induced hepatic stellate cell proliferation[94]. Taken together, these data suggest that the HMW form predominantly mediates the beneficial effects of adiponectin in hepatic tissue.
Two adiponectin receptors (adipoR1 and adipoR2) have been identified and found to be expressed in various tissues[95]. AdipoR1 is abundantly expressed in skeletal muscles, whereas adipoR2 is present predominantly in the liver, suggesting a role of adipoR2 in hepatic adiponectin signaling[68,96]. Recently, several laboratories have investigated the physiological roles of adipoR1 and adipoR2 in adipoR1/2 knockout mice. Both adipoR1 and adipoR2 knockout mice exhibit mild insulin resistance[97]. In adipoR1/R2 double knockout mice, the binding and actions of adiponectin are abolished, resulting in increased tissue triglyceride content, inflammation and oxidative stress[97]. AdipoR2 knockout mice reported by Liu et al[98] displayed reduced diet-induced insulin resistance, but promoted T2DM. These data support the physiological roles of adipoR1 and adipoR2 as the predominant receptors for adiponectin in the regulation of glucose and lipid metabolism. Despite this information, the detailed roles and expression of adipoRs in NAFLD are not conclusive[38,99-102].
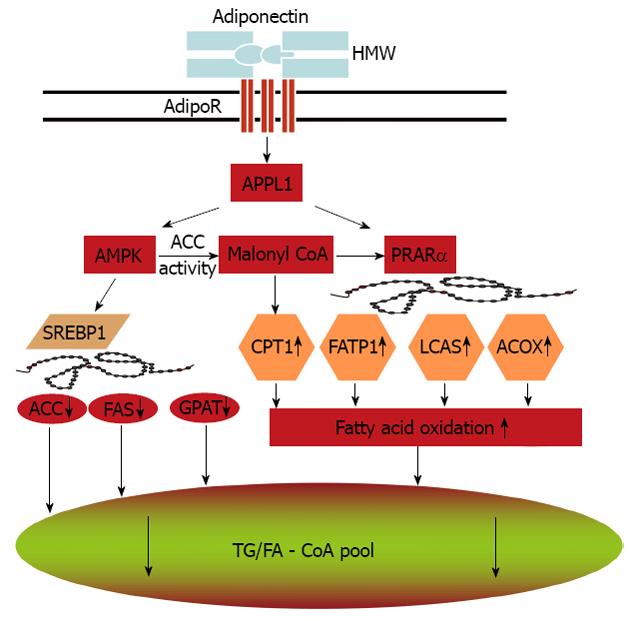
Adiponectin stimulates AMPK in almost all its major target tissues, including skeletal muscle, liver, heart, endothelium, adipocytes and brain[75,89,103-106]. Notably, most biological effects of adiponectin in these target tissues are abrogated by expression of a dominant negative version of AMPK, supporting its obligatory role in mediating adiponectin’s multiple actions. The precise mechanisms whereby adiponectin activates AMPK through its receptors remain to be determined. APPL1, an adaptor protein containing a pleckstrin homology domain, a phosphotyrosine binding domain and a leucine zipper motif, appears to be a key signaling molecule that couples adiponectin receptors and its downstream AMPK activation[103,107]. Adiponectin enhances the binding of APPL1 to both adipoR1 and adipoR2, and these interactions are essential for subsequent phosphorylation and activation of AMPK. Studies also indicate the important role of APPL1 in the metabolic syndrome[108,109]. AMPK activation in turn phosphorylates acetyl Coenzyme A carboxylase (ACC) and attenuates ACC activity. Inhibition of ACC reduces lipid synthesis and enhances fatty acid oxidation by blocking the production of malonyl-CoA, an allosteric inhibitor of carnitine palmitoyl transferase 1, the rate-limiting enzyme in fatty acid oxidation. In addition, activation of AMPK downregulates the expression of sterol regulatory element-binding protein 1c (SREBP1c), a transcription factor that regulates cholesterol and lipid synthesis. Reduction of SREBP1c results in downregulation of genes involved in lipogenesis, including ACC, fatty acid synthase, and glycerol-3-phosphate acyltransferase[104,110,111].
PPARα is a transcription factor controlling the transcription of a panel of genes encoding fatty acid oxidation enzymes, such as FATP, acyl-CoA oxidase and long chain acyl-CoA synthetase. Adiponectin stimulates PPARα activity possibly through PPARγ coactivator-1α[112]. These adiponectin-mediated signaling pathways lead to enhanced fat oxidation, reduced lipid synthesis and prevention of hepatic steatosis (Figure 1).
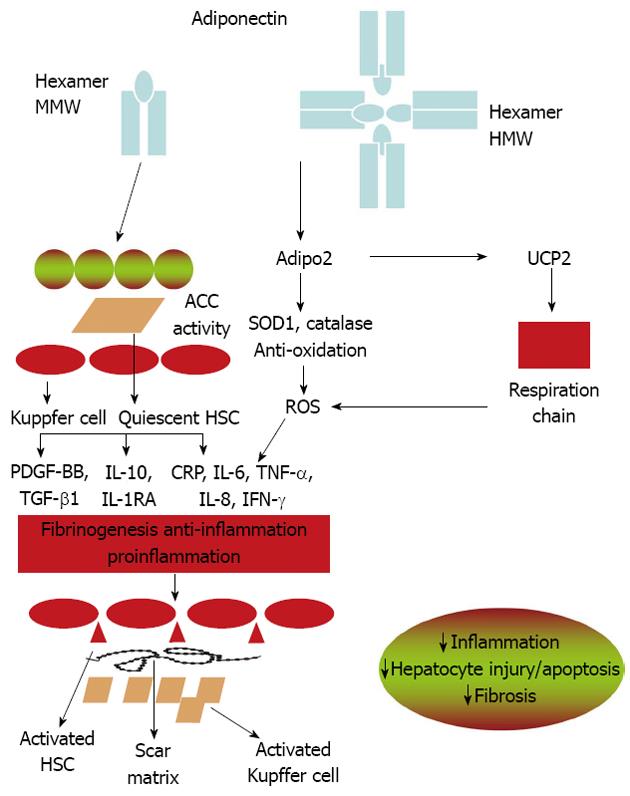
Inflammatory cytokines are key mediators of hepatic inflammation, cell death, and fibrosis, as well as regeneration after massive or focal liver injury[38,113]. Adiponectin levels are negatively associated with mediators of inflammation, including interleukin-6 (IL-6) and C-reactive protein; but positively related to anti-inflammatory cytokine IL-10[114,115]. It suppresses TNF-α functions by inhibiting its expression and antagonizing its activities[61,62,116,117]. In the liver, cytokines such as IL-6 and TNF-α, are mainly produced from Kupffer cells and hepatic stellate cells (HSC), and partly from inflamed hepatocytes[52,118,119]. Adiponectin ameliorates NASH and liver fibrosis by suppressing the activation of Kupffer cells and HSC (Figure 2). In porcine blood-derived macrophages, adiponectin suppresses both TNF-α and IL-6 production stimulated by LPS and induces IL10 expression. The attenuation of proinflammatory cytokine production by adiponectin is mediated in part by attenuating the translocation of nuclear factor kappa B (NF-κB) to the nucleus[120]. Adiponectin can also induce the expression of the anti-inflammation cytokine interleukin-1-receptor antagonist[121,122]. The anti-inflammatory effects of adiponectin in macrophages may involve the toll-like receptor-4 (TLR-4) signaling pathway. However, the mechanisms by which adiponectin suppresses TLR-4 mediated responses are not well understood[123].
The transformation of HSC into myofibroblasts is the key step that initiates the fibrotic process during liver injury[124,125]. The activated hepatic stellate cells increase the accumulation of extracellular matrix. Both adiponectin receptors, adipoR1 and adipoR2, are expressed in HSC. Adiponectin treatment maintains HSC quiescence, inhibits platelet-derived growth factor-stimulated proliferation and migration of human HSCs, and reduces the secretion and of monocyte chemoattractant protein-1 through AMPK-dependent mechanisms[94,125,126]. Additionally, adiponectin also regulates hepatic expression of TGFβ1, a pro-fibrotic factor involved in HSC activation[58,127] that plays an important role in neofibrogenesis of NAFLD[128].
Inhibition of adipoR2 expression by short hairpin RNAi-expressing adenovirus can induce TGFβ1 expression, and overexpression of adipoR2 diminishes TGFβ1 mRNA level.
Mitochondrial dysfunction represents a central mechanism linking obesity with associated metabolic complications[129]. In patients with NASH, the hepatic mitochondria exhibit ultrastructural lesions and decreased activity of the respiratory chain complexes[130,131]. In this condition, the decreased activity of the respiratory chain results in accumulation of reactive oxygen species (ROS) that oxidize fat deposits to form lipid peroxidation products, which in turn, cause steatohepatitis, necrosis, inflammation and fibrosis. The increased mitochondrial ROS formation in steatohepatitis could directly damage mitochondria DNA and respiratory chain polypeptides, induce NF-κB activation and the hepatic synthesis of TNFα[132]. Oxidative phosphorylation reactions mediated by mitochondria respiratory chain (MRC) complexes are directly involved in regulating intracellular ROS activities and preventing accumulation of lipids and lipid peroxidation products in the liver.
Mice without adiponectin show an increased lipid accumulation even under normal chow feeding[117]. This pre-existing hepatic steatotic condition might be the direct consequence of dysregulated mitochondria functions[117]. Adiponectin treatment restores the MRC activities, decreases the levels of mitochondrial lipid peroxidation products through regulating hepatic mitochondrial functions, which might represent a common mechanism underlying the multiple beneficial activities of this hormone in various obesity-related pathologies. Moreover, we have provided evidence supporting an essential role of uncoupling protein 2 (UCP2), a mitochondria inner membrane transporter, in mediating the beneficial effects of adiponectin on MRC activities. The protein and mRNA levels of UCP2 are decreased in the liver tissues of adiponectin knockout mice and can be significantly upregulated by adiponectin treatment. Overexpression of adipoR2 upregulates mRNA levels of UCP2, catalase, and superoxide dismutase 1 in the liver[97]. Furthermore, the effects of adiponectin on MRC activities are dramatically attenuated in Ucp2-deficient mice, suggesting that the increased UCP2 expression might be obligatory for adiponectin to elicit its activities on mitochondria functions (Figure 2). UCP2 possesses anti-oxidant activities through inhibition of ROS production from mitochondria[133]. It can also inhibit the production of pro-inflammatory cytokines in both macrophage and Kupffer cells[134]. A growing body of evidence suggests that UCP2 may play a beneficial role in various stages of fatty liver diseases[134,135]. These results suggest the existence of a reciprocal relationship between uncoupling proteins and adiponectin. However, the detailed signaling mechanisms underlying adiponectin-induced UCP2 expression are not clear and warrant further investigation.
To date, there have been very few effective drug treatments for NAFLD and NASH. Early diagnosis and management of the underlying condition remains the mainstay of treatment. The present “gold standard” for treatment of NAFLD is weight reduction or a reduction of central obesity[4]. These “life-style adjustment” or anti-obesity measures (including bariatric surgery) impressively reduce liver cell injury, inflammation and hepatic fibrosis, as well as steatosis[136,137]. The potential for correcting steatosis by dietary or pharmacological approaches should provide a sound therapeutic approach for the treatment of steatosis and steatohepatitis. Strategies to block oxidative stress are of great interest, with some evidence that ALT normalization or histological improvement occurs with vitamin E (alone or with vitamin C or pioglitazone) and betaine[138].
Adiponectin and its agonists might represent emerging therapeutic agents for the treatment and/or prevention of liver dysfunctions[139-141]. Adiponectin replacement therapy is not yet available as a treatment option. Pharmacological intervention aimed at elevating adiponectin production might hold promise for the treatment and/or prevention of NAFLD.
Based on our data, polymorphic UCP1 (AG + GG) obese patients with low adiponectin levels appear to be high-risk subjects for worsening of liver steatosis, an NAFLD, possibly requiring a second-step evaluation by liver biopsy[142].
The role of adiponectin in systemic inflammation and critical illness is not well defined. Early data suggest that plasma levels of adiponectin are decreased in critical illness[143]. Whether this is a result of the disease process itself or whether patients with lower levels of this hormone are more susceptible to developing a critical illness is not known. This observation of lower adiponectin levels then raises the possibility of therapeutic options to increase circulating adiponectin levels[143]. The various options for modulation of serum adiponectin (recombinant adiponectin, thiazolidinediones) are discussed.
Nevertheless, adiponectin-based therapeutics for NAFLD represent a promising area for further investigation.
Adiponectin is an abundant adipocyte-derived hormone with well established anti-inflammatory and insulin sensitizing properties. The significance of adiponectin in protecting obesity-related NAFLD has been increasingly recognized. Despite the advances made in recent years, the detailed molecular and cellular mechanisms underlying its hepato-protective functions remain largely uncharacterized.
P- Reviewers Bala S, van der Velde A S- Editor Gou SX L- Editor Stewart GJ E- Editor Li JY
| 1. | Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Tajiri K, Shimizu Y. Role of NKT Cells in the Pathogenesis of NAFLD. Int J Hepatol. 2012;2012:850836. [PubMed] |
| 4. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 5. | Hamaguchi M, Takeda N, Kojima T, Ohbora A, Kato T, Sarui H, Fukui M, Nagata C, Takeda J. Identification of individuals with non-alcoholic fatty liver disease by the diagnostic criteria for the metabolic syndrome. World J Gastroenterol. 2012;18:1508-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 7. | Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev. 2009;10:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Tsochatzis EA, Papatheodoridis GV. Is there any progress in the treatment of non-alcoholic fatty liver disease? World J Gastrointest Pharmacol Ther. 2011;2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Lee J, Chung DS, Kang JH, Yu BY. Comparison of visceral fat and liver fat as risk factors of metabolic syndrome. J Korean Med Sci. 2012;27:184-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [PubMed] |
| 12. | Tarantino G, Colao A, Capone D, Conca P, Tarantino M, Grimaldi E, Chianese D, Finelli C, Contaldo F, Scopacasa F. Circulating levels of cytochrome C, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in overweight/obese patients with non-alcoholic fatty liver disease. J Biol Regul Homeost Agents. 2011;25:47-56. [PubMed] |
| 13. | Tarantino G, Finelli C, Colao A, Capone D, Tarantino M, Grimaldi E, Chianese D, Gioia S, Pasanisi F, Contaldo F. Are hepatic steatosis and carotid intima media thickness associated in obese patients with normal or slightly elevated gamma-glutamyl-transferase? J Transl Med. 2012;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Thorn SR, Rozance PJ, Brown LD, Hay WW. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med. 2011;29:225-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1111-R1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Joseph AM, Joanisse DR, Baillot RG, Hood DA. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res. 2012;2012:642038. [PubMed] |
| 17. | Rocha PM, Barata JT, Minderico CS, Silva AM, Teixeira PJ, Sardinha LB. Visceral abdominal and subfascial femoral adipose tissue have opposite associations with liver fat in overweight and obese premenopausal caucasian women. J Lipids. 2011;2011:154672. [PubMed] |
| 18. | Longato L, Ripp K, Setshedi M, Dostalek M, Akhlaghi F, Branda M, Wands JR, de la Monte SM. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid Med Cell Longev. 2012;2012:479348. [PubMed] |
| 19. | Yoshimura E, Kumahara H, Tobina T, Matono S, Kiyonaga A, Kimura M, Tsukikawa H, Kono S, Etou T, Irie S. Relationships between fat deposition in the liver and skeletal muscle and insulin sensitivity in Japanese individuals: a pilot study. Diabetes Metab Syndr Obes. 2011;4:35-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Gathercole LL, Morgan SA, Bujalska IJ, Hauton D, Stewart PM, Tomlinson JW. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS One. 2011;6:e26223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22:353-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 22. | Capeau J. Insulin resistance and steatosis in humans. Diabetes Metab. 2008;34:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Nagarajan P, Mahesh Kumar MJ, Venkatesan R, Majundar SS, Juyal RC. Genetically modified mouse models for the study of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:1141-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007;32:453-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Mirza MS. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN Gastroenterol. 2011;2011:592404. [PubMed] |
| 26. | Charlton M. Noninvasive indices of fibrosis in NAFLD: starting to think about a three-hit (at least) phenomenon. Am J Gastroenterol. 2007;102:409-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 805] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 28. | Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010;9:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, Peduto T, Chisholm DJ, George J. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 31. | El-Koofy NM, Anwar GM, El-Raziky MS, El-Hennawy AM, El-Mougy FM, El-Karaksy HM, Hassanin FM, Helmy HM. The association of metabolic syndrome, insulin resistance and non-alcoholic fatty liver disease in overweight/obese children. Saudi J Gastroenterol. 2012;18:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Jang S, Lee CH, Choi KM, Lee J, Choi JW, Kim KA, Park CM. Correlation of fatty liver and abdominal fat distribution using a simple fat computed tomography protocol. World J Gastroenterol. 2011;17:3335-3341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (7)] |
| 33. | Verrijken A, Francque S, Mertens I, Talloen M, Peiffer F, Van Gaal L. Visceral adipose tissue and inflammation correlate with elevated liver tests in a cohort of overweight and obese patients. Int J Obes (Lond). 2010;34:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol. 2007;42:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Foster MT, Shi H, Seeley RJ, Woods SC. Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav. 2010;101:282-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 37. | Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 38. | Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773-4783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 40. | Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Huang XD, Fan Y, Zhang H, Wang P, Yuan JP, Li MJ, Zhan XY. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World J Gastroenterol. 2008;14:2888-2893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Kim IK, Kim J, Kang JH, Song J. Serum leptin as a predictor of fatty liver in 7-year-old Korean children. Ann Nutr Metab. 2008;53:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Kukla M, Mazur W, Bułdak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol Med. 2011;17:1397-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 282] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 45. | Tarantino G, Scopacasa F, Colao A, Capone D, Tarantino M, Grimaldi E, Savastano S. Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:5280-5288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Chakraborty JB, Oakley F, Walsh MJ. Mechanisms and biomarkers of apoptosis in liver disease and fibrosis. Int J Hepatol. 2012;2012:648915. [PubMed] |
| 47. | Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver. 2012;6:149-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 48. | Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Di Minno MN, Iervolino S, Peluso R, Russolillo A, Lupoli R, Scarpa R, Di Minno G, Tarantino G. Hepatic steatosis and disease activity in subjects with psoriatic arthritis receiving tumor necrosis factor-α blockers. J Rheumatol. 2012;39:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Chu SH, Lee MK, Ahn KY, Im JA, Park MS, Lee DC, Jeon JY, Lee JW. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS One. 2012;7:e34710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801-2811. [PubMed] |
| 52. | Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, Bastard JP, Poupon R, Housset C, Capeau J. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 54. | Musso G, Gambino R, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Cassader M, Durazzo M, Rizzetto M. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 55. | Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 56. | Van Wagner LB, Rinella ME. The role of insulin-sensitizing agents in the treatment of nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2011;4:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 737] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 58. | Yang Z, Wang X, Wen J, Ye Z, Li Q, He M, Lu B, Ling C, Wu S, Hu R. Prevalence of non-alcoholic fatty liver disease and its relation to hypoadiponectinaemia in the middle-aged and elderly Chinese population. Arch Med Sci. 2011;7:665-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300-2308. [PubMed] |
| 60. | Matsumoto H, Tamura S, Kamada Y, Kiso S, Fukushima J, Wada A, Maeda N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin deficiency exacerbates lipopolysaccharide/D-galactosamine-induced liver injury in mice. World J Gastroenterol. 2006;12:3352-3358. [PubMed] |
| 61. | Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Fukushima J, Kamada Y, Matsumoto H, Yoshida Y, Ezaki H, Takemura T, Saji Y, Igura T, Tsutsui S, Kihara S. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol Res. 2009;39:724-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 751] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 65. | Fukuhara Y, Suda T, Kobayashi M, Tamura Y, Igarashi M, Waguri N, Kawai H, Aoyagi Y. Identification of cellular genes showing differential expression associated with hepatitis B virus infection. World J Hepatol. 2012;4:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Daniele A, Cammarata R, Pasanisi F, Finelli C, Salvatori G, Calcagno G, Bracale R, Labruna G, Nardelli C, Buono P. Molecular analysis of the adiponectin gene in severely obese patients from southern Italy. Ann Nutr Metab. 2008;53:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Bracale R, Labruna G, Finelli C, Daniele A, Sacchetti L, Oriani G, Contaldo F, Pasanisi F. The absence of polymorphisms in ADRB3, UCP1, PPARγ, and ADIPOQ genes protects morbid obese patients toward insulin resistance. J Endocrinol Invest. 2012;35:2-4. [PubMed] |
| 68. | Woo YC, Tso AW, Xu A, Law LS, Fong CH, Lam TH, Lo SV, Wat NM, Cheung BM, Lam KS. Combined use of serum adiponectin and tumor necrosis factor-alpha receptor 2 levels was comparable to 2-hour post-load glucose in diabetes prediction. PLoS One. 2012;7:e36868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr. 2009;4:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 70. | Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1-E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 384] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 71. | Li R, Lau WB, Ma XL. Adiponectin resistance and vascular dysfunction in the hyperlipidemic state. Acta Pharmacol Sin. 2010;31:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 73. | Simpson F, Whitehead JP. Adiponectin--it’s all about the modifications. Int J Biochem Cell Biol. 2010;42:785-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Briggs DB, Giron RM, Malinowski PR, Nuñez M, Tsao TS. Role of redox environment on the oligomerization of higher molecular weight adiponectin. BMC Biochem. 2011;12:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Jungtrakoon P, Plengvidhya N, Tangjittipokin W, Chimnaronk S, Salaemae W, Chongjaroen N, Chanprasert K, Sujjitjoon J, Srisawat C, Yenchitsomanus PT. Novel adiponectin variants identified in type 2 diabetic patients reveal multimerization and secretion defects. PLoS One. 2011;6:e26792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Misu H, Ishikura K, Kurita S, Takeshita Y, Ota T, Saito Y, Takahashi K, Kaneko S, Takamura T. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One. 2012;7:e34952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 77. | Mohammadzadeh G, Zarghami N. Hypoadiponectinemia in obese subjects with type II diabetes: A close association with central obesity indices. J Res Med Sci. 2011;16:713-723. [PubMed] |
| 78. | Richards AA, Stephens T, Charlton HK, Jones A, Macdonald GA, Prins JB, Whitehead JP. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 79. | Rothan HA, Teh SH, Haron K, Mohamed Z. A Comparative Study on the Expression, Purification and Functional Characterization of Human Adiponectin in Pichia pastoris and Escherichia coli. Int J Mol Sci. 2012;13:3549-3562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne). 2011;2:62. [PubMed] |
| 81. | Vrachnis N, Belitsos P, Sifakis S, Dafopoulos K, Siristatidis C, Pappa KI, Iliodromiti Z. Role of adipokines and other inflammatory mediators in gestational diabetes mellitus and previous gestational diabetes mellitus. Int J Endocrinol. 2012;2012:549748. [PubMed] |
| 82. | Suzuki S, Wilson-Kubalek EM, Wert D, Tsao TS, Lee DH. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32 Suppl 7:S13-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 84. | Polak J, Kovacova Z, Holst C, Verdich C, Astrup A, Blaak E, Patel K, Oppert JM, Langin D, Martinez JA. Total adiponectin and adiponectin multimeric complexes in relation to weight loss-induced improvements in insulin sensitivity in obese women: the NUGENOB study. Eur J Endocrinol. 2008;158:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Salani B, Briatore L, Andraghetti G, Adami GF, Maggi D, Cordera R. High-molecular weight adiponectin isoforms increase after biliopancreatic diversion in obese subjects. Obesity (Silver Spring). 2006;14:1511-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Hage MP, Safadi B, Salti I, Nasrallah M. Role of Gut-Related Peptides and Other Hormones in the Amelioration of Type 2 Diabetes after Roux-en-Y Gastric Bypass Surgery. ISRN Endocrinol. 2012;2012:504756. [PubMed] |
| 87. | Liu Y, Retnakaran R, Hanley A, Tungtrongchitr R, Shaw C, Sweeney G. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab. 2007;92:4313-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 88. | Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1868] [Cited by in RCA: 1832] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 89. | Xie L, Boyle D, Sanford D, Scherer PE, Pessin JE, Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem. 2006;281:7253-7259. [PubMed] |
| 90. | Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 91. | Cao J, Puri N, Sodhi K, Bellner L, Abraham NG, Kappas A. Apo A1 Mimetic Rescues the Diabetic Phenotype of HO-2 Knockout Mice via an Increase in HO-1 Adiponectin and LKBI Signaling Pathway. Int J Hypertens. 2012;2012:628147. [PubMed] |
| 92. | Hendricks GL, Hadley JA, Krzysik-Walker SM, Prabhu KS, Vasilatos-Younken R, Ramachandran R. Unique profile of chicken adiponectin, a predominantly heavy molecular weight multimer, and relationship to visceral adiposity. Endocrinology. 2009;150:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Neumeier M, Sigruener A, Eggenhofer E, Weigert J, Weiss TS, Schaeffler A, Schlitt HJ, Aslanidis C, Piso P, Langmann T. High molecular weight adiponectin reduces apolipoprotein B and E release in human hepatocytes. Biochem Biophys Res Commun. 2007;352:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 95. | Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1817] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 96. | Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 97. | Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1057] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 98. | Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Ma H, Gomez V, Lu L, Yang X, Wu X, Xiao SY. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Nannipieri M, Cecchetti F, Anselmino M, Mancini E, Marchetti G, Bonotti A, Baldi S, Solito B, Giannetti M, Pinchera A. Pattern of expression of adiponectin receptors in human liver and its relation to nonalcoholic steatohepatitis. Obes Surg. 2009;19:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur J Gastroenterol Hepatol. 2010;22:643-650. [PubMed] |
| 102. | Vuppalanchi R, Marri S, Kolwankar D, Considine RV, Chalasani N. Is adiponectin involved in the pathogenesis of nonalcoholic steatohepatitis? A preliminary human study. J Clin Gastroenterol. 2005;39:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22-E36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 104. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3050] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 105. | Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, Walsh K. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 106. | Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 601] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 107. | Holmes RM, Yi Z, De Filippis E, Berria R, Shahani S, Sathyanarayana P, Sherman V, Fujiwara K, Meyer C, Christ-Roberts C. Increased abundance of the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif (APPL1) in patients with obesity and type 2 diabetes: evidence for altered adiponectin signalling. Diabetologia. 2011;54:2122-2131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Gu W, Li Y. The therapeutic potential of the adiponectin pathway. BioDrugs. 2012;26:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Cleasby ME, Lau Q, Polkinghorne E, Patel SA, Leslie SJ, Turner N, Cooney GJ, Xu A, Kraegen EW. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J Endocrinol. 2011;210:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferré P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704-6711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 325] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 111. | Polakof S, Panserat S, Craig PM, Martyres DJ, Plagnes-Juan E, Savari S, Aris-Brosou S, Moon TW. The metabolic consequences of hepatic AMP-kinase phosphorylation in rainbow trout. PLoS One. 2011;6:e20228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood). 2009;234:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 113. | Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 114. | Garg MK, Dutta MK, Mahalle N. Adipokines (adiponectin and plasminogen activator inhhibitor-1) in metabolic syndrome. Indian J Endocrinol Metab. 2012;16:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Savastano S, Valentino R, Di Somma C, Orio F, Pivonello C, Passaretti F, Brancato V, Formisano P, Colao A, Beguinot F. Serum 25-Hydroxyvitamin D Levels, phosphoprotein enriched in diabetes gene product (PED/PEA-15) and leptin-to-adiponectin ratio in women with PCOS. Nutr Metab (Lond). 2011;8:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23 Suppl 1:S50-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 117. | Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol. 2011;54:773-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 387] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 118. | Adler M, Taylor S, Okebugwu K, Yee H, Fielding C, Fielding G, Poles M. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol. 2011;17:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 120. | Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 121. | Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 122. | Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 601] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 123. | Wang SN, Wang ST, Lee KT. The potential interplay of adipokines with toll-like receptors in the development of hepatocellular carcinoma. Gastroenterol Res Pract. 2011;2011:215986. [PubMed] |
| 124. | Bertolani C, Marra F. Role of adipocytokines in hepatic fibrosis. Curr Pharm Des. 2010;16:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 125. | Handy JA, Fu PP, Kumar P, Mells JE, Sharma S, Saxena NK, Anania FA. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem J. 2011;440:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 126. | Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, Trappoliere M, Vizzutti F, Gelmini S, Laffi G, Pinzani M. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 127. | Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, Mizutani A, Yokoyama H, Irie R, Sumimoto H. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 128. | Tarantino G, Conca P, Riccio A, Tarantino M, Di Minno MN, Chianese D, Pasanisi F, Contaldo F, Scopacasa F, Capone D. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: is it really benign? J Transl Med. 2008;6:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Ruiz JR, Lasa A, Simon E, Larrarte E, Labayen I. Lower plasma NAMPT/visfatin levels are associated with impaired hepatic mitochondrial function in non-diabetic obese women: a potential link between obesity and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Ren LP, Chan SM, Zeng XY, Laybutt DR, Iseli TJ, Sun RQ, Kraegen EW, Cooney GJ, Turner N, Ye JM. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLoS One. 2012;7:e30816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 131. | Hsieh PS, Hsieh YJ. Impact of liver diseases on the development of type 2 diabetes mellitus. World J Gastroenterol. 2011;17:5240-5245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22 Suppl 1:S20-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 133. | Deng S, Yang Y, Han Y, Li X, Wang X, Li X, Zhang Z, Wang Y. UCP2 inhibits ROS-mediated apoptosis in A549 under hypoxic conditions. PLoS One. 2012;7:e30714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW. Persistent nuclear factor-kappa B activation in Ucp2-/- mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem. 2005;280:19062-19069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 135. | Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 136. | Petersen KF, Dufour S, Morino K, Yoo PS, Cline GW, Shulman GI. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc Natl Acad Sci USA. 2012;109:8236-8240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 137. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 138. | Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 139. | Massip-Salcedo M, Zaouali MA, Padrissa-Altés S, Casillas-Ramirez A, Rodés J, Roselló-Catafau J, Peralta C. Activation of peroxisome proliferator-activated receptor-alpha inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia-reperfusion. Hepatology. 2008;47:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 140. | Walter R, Wanninger J, Bauer S, Eisinger K, Neumeier M, Weiss TS, Amann T, Hellerbrand C, Schäffler A, Schölmerich J. Adiponectin reduces connective tissue growth factor in human hepatocytes which is already induced in non-fibrotic non-alcoholic steatohepatitis. Exp Mol Pathol. 2011;91:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Elias-Miró M, Jiménez-Castro MB, Mendes-Braz M, Casillas-Ramírez A, Peralta C. The Current Knowledge of the Role of PPAR in Hepatic Ischemia-Reperfusion Injury. PPAR Res. 2012;2012:802384. [PubMed] |
| 142. | Labruna G, Pasanisi F, Nardelli C, Tarantino G, Vitale DF, Bracale R, Finelli C, Genua MP, Contaldo F, Sacchetti L. UCP1 -3826 AG+GG genotypes, adiponectin, and leptin/adiponectin ratio in severe obesity. J Endocrinol Invest. 2009;32:525-529. [PubMed] |
| 143. | Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |