Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.778
Revised: November 14, 2012
Accepted: December 20, 2012
Published online: February 7, 2013
Processing time: 116 Days and 15.6 Hours
Ectopic adrenal cortical neoplasms are extremely rare. Ectopic adrenocortical tissue can be found in locations such as the celiac axis, the broad ligament, the adnexa of the testes, and the spermatic cord; however, they rarely involve the stomach. We report an unusual case of a patient with an ectopic adrenal cortical adenoma in the gastric wall. The patient was a 72-year old female admitted to our hospital with upper abdominal discomfort. Physical examination revealed tenderness below the xiphoid process. Both computed tomography and fibergastroscopy revealed a mass on the lesser curvature side of the gastric antrum; it was initially diagnosed as a gastric stromal tumor. After adequate preparation, the patient underwent surgery. During the procedure, we found a 30 mm × 30 mm mass with medium density in the lesser curvature near the gastric antrum within the serosa. Following immunohistochemistry examination, we corrected the diagnosis to an ectopic adrenal cortical adenoma; the tumor was nonfunctional.
- Citation: Ren PT, Fu H, He XW. Ectopic adrenal cortical adenoma in the gastric wall: Case report. World J Gastroenterol 2013; 19(5): 778-780
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.778
The adrenal gland arises from primordial mesenchyme in the wall of the dorsal coelom adjacent to the dorsal mesentery and urogenital structures. Therefore, most ectopic adrenocortical tissue is found along the path of embryonic migration within the urogenital tract. Ectopic adrenocortical tissue is found in such locations as the celiac axis, the broad ligament, the adnexa of the testes, and the spermatic cord[1]. However, an ectopic adrenal cortical adenoma rarely involves the stomach. We report an unusual case of a patient with an ectopic adrenal cortical adenoma in the gastric wall and review the literature.
The patient was a 72-year old female. She was admitted on 17th November, 2011 with upper abdominal discomfort for 4 d, accompanied by postprandial nausea and vomiting. She denied chills, fever, or diarrhea. Her medical and family history were noncontributory.
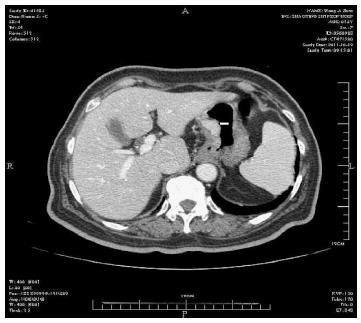
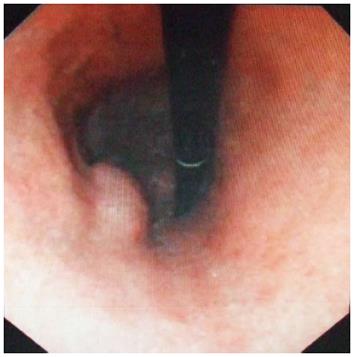
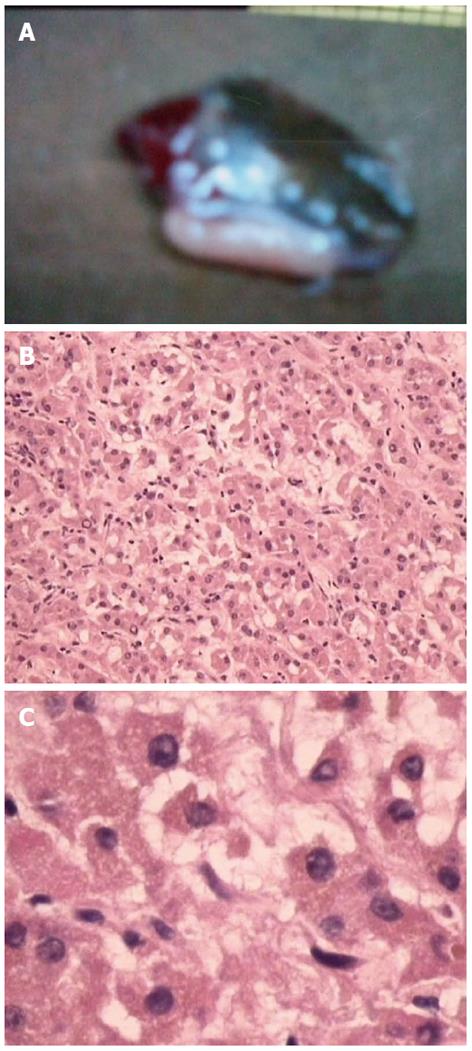
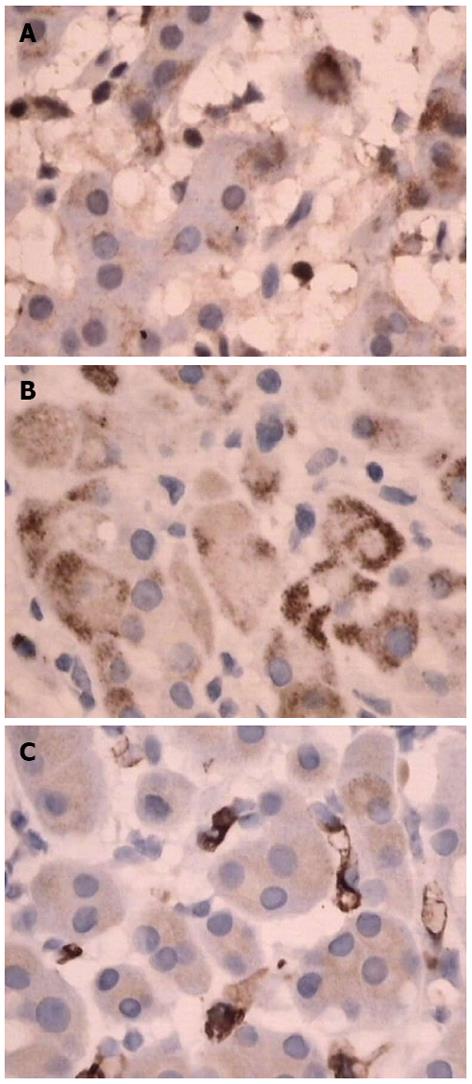
Physical examination: temperature 37.5 °C, pulse 90 bpm, respiration 20 bpm, blood pressure 172/96 mmHg. The heart and lungs were normal. The abdomen was flat and soft, with tenderness below the xiphoid process. No enlarged liver, spleen, or mass was palpable. Murphy’s sign was negative. Laboratory and radiology findings: Routine blood work showed white blood cell 12.7 × 109/L, neutrophils 85.3%. Liver function tests were in normal range. Fasting blood glucose was 8.88 mmol/L. Adrenocorticotropic hormone was in normal range. On B-ul-trasonography, the gallbladder was 64 mm × 37 mm. The gallbladder wall was thickened, with a stone incarcerated in the neck. Computed tomography (CT) scan showed a 15 mm × 25 mm abnormal enhanced nodule located in the submucosa of the lesser curvature of the stomach with a CT value of 150 HU. The size, morphology, and location of the kidneys and adrenal glands were normal bilaterally, and there were multiple cysts in both kidneys. The radiological diagnoses were multiple renal cysts and a gastric stromal tumor (Figure 1). Fibergastroscopy showed no hyperemia in the gastric mucosa. A raised nodule about 30 mm × 25 mm in size was found on the lesser curvature side of the gastric antrum; it was firm with soft mucosa. The endoscopic diagnosis was also a gastric stromal tumor (Figure 2). After adequate preparation, the patient underwent surgery. During the operation, we found a distended gallbladder with a stone inside. There was a 30 mm × 30 mm mass with medium density in the lesser curvature near the gastric antrum and the mass located in the serosa layer. The liver, spleen, pancreas, kidney, and adrenal glands were normal. We resected the gallbladder, ligated the vessels of lesser gastric curvature, and opened the gastric wall 3 cm from the margin of the mass. We found that the gastric mucosa above the mass was integrated, and we performed a simple resection of the mass. On gross examination, the mass was purplish-red in color. It was a 20 mm × 30 mm ellipse with soft margins and a medium texture (Figure 3A). On microscopic exam, the tumor cells were rich in cytoplasm and eosinophilic, and numerous sinusoid capillaries could be seen. The cytoplasm contained melanin, which was confirmed by decolorization. The tumor cells were arranged in cords or gobbets with a low ratio of nucleus to cytoplasm, and rare mitotic figures (Figure 3B, C). Immunohistochemistry: S-100 basement cell negative (Figure 4A), melan-A positive (Figure 4B), P63 basement cell negative, sinusoidal endothelial CD34 positive (Figure 4C). The pathologic diagnosis was of an ectopic adrenal cortical adenoma in the gastric wall.
Ectopic adrenal tissue is mostly found in children. According to Anderson et al[2], accessory adrenal tissue is found in 50% of post-mortem examinations in neonates and children. Usually, maturity leads to atrophy of the ectopic adrenal tissue, so that such tissue is found in only 1% of adults. The adrenal cells have a double embryological origin. The cortex arises from the coelomic mesothelium and the medulla from neural crest ectoderm. At approximately 7 ± 8 wk of pregnancy, the medulla components start moving towards the cortical elements, forming the adrenal gland. During migration of the medulla, fragments of tissue, most frequently the cortex, can be separated, forming accessory adrenal glands. Most ectopic adrenals remain in the vicinity of the adrenal gland, but they are also found to be closely related to the sex organs because of the spatial relationship between the adrenal primordium and the genital ridge in early embryogenesis. Accessory adrenal tissue can also be incorporated into adjacent organs due to incomplete separation of cortical adrenal cells from the coelomic mesothelium. Therefore, most ectopic adrenocortical tissue is found along the path of embryonic migration within the urogenital tract. The most common sites include the fat tissue of the posterior peritoneum near the adrenal glands, the celiac axis, the broad ligament, the adnexa of the testis, and the spermatic cord. There are also rare reported cases of ectopic adrenal cortical adenoma in the lung, spinal region, and brain[3-8].
However, there is no previous report of ectopic adrenal cortical adenoma of the gastric wall in the literature. In the case reported here, the patient presented with abdominal discomfort and the mass was found by CT. It was initially diagnosed as a gastric stromal tumor, which was corrected to ectopic adrenal cortical adenoma by pathology; the tumor was nonfunctional. The patient had no history of operation on adrenal tissue, so we believe the ectopic adrenal cortical adenoma in the gastric wall was due to the malposition or self-differentiation of mesothelial cells during the embryonic period.
P- Reviewers Zubarik R, Yen HH S- Editor Song XX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Harrison LA, McMillan JH, Batnitzky S, Kepes JJ. MR appearance of an ectopic intraspinal adrenal cortical adenoma. AJNR Am J Neuroradiol. 1990;11:1185-1187. [PubMed] |
| 2. | Anderson JR, Ross AH. Ectopic adrenal tissue in adults. Postgrad Med J. 1980;56:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Barwick TD, Malhotra A, Webb JA, Savage MO, Reznek RH. Embryology of the adrenal glands and its relevance to diagnostic imaging. Clin Radiol. 2005;60:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Dahl EV, Bahn RC. Aberrant adrenal contical tissue near the testis in human infants. Am J Pathol. 1962;40:587-598. [PubMed] |
| 6. | Mitchell A, Scheithauer BW, Sasano H, Hubbard EW, Ebersold MJ. Symptomatic intradural adrenal adenoma of the spinal nerve root: report of two cases. Neurosurgery. 1993;32:658-661; discussion 661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Vestfrid MA. Ectopic adrenal cortex in neonatal liver. Histopathology. 1980;4:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Cassarino DS, Santi M, Arruda A, Patrocinio R, Tsokos M, Ghatak N, Quezado M. Spinal adrenal cortical adenoma with oncocytic features: report of the first intramedullary case and review of the literature. Int J Surg Pathol. 2004;12:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |