Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.769
Revised: December 18, 2012
Accepted: December 22, 2012
Published online: February 7, 2013
Processing time: 158 Days and 16.6 Hours
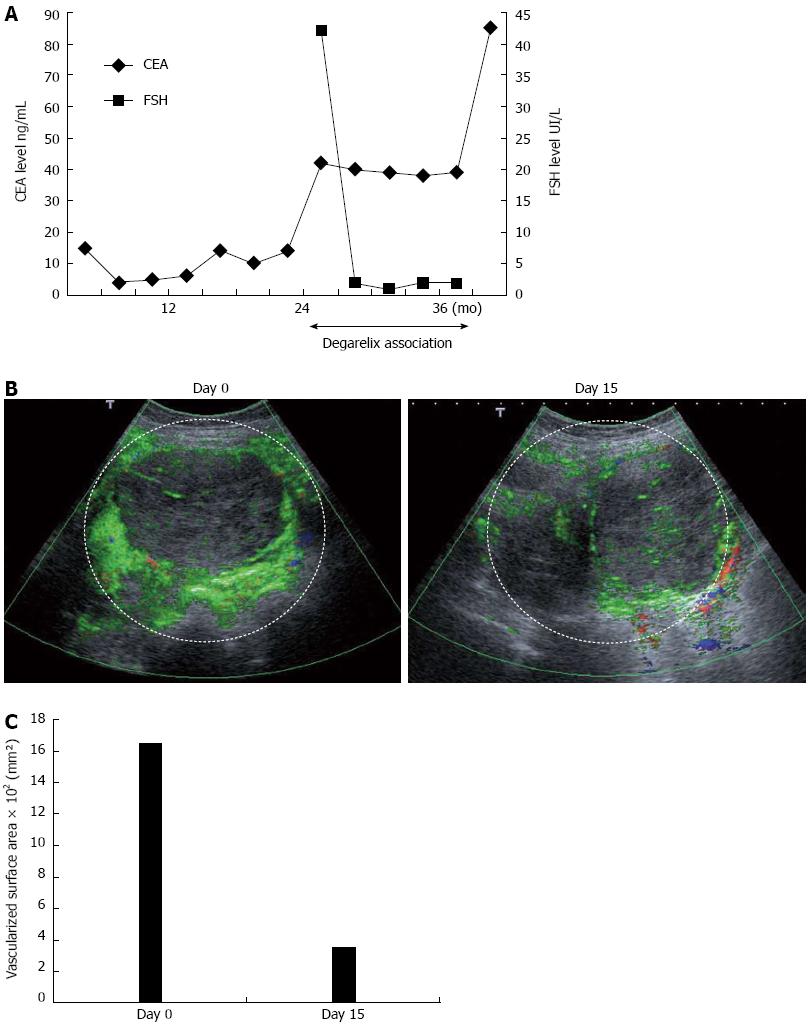
Recently, follicle stimulating hormone receptor was found to be selectively expressed by endothelial cells on tumor-associated blood vessels in a wide range of human cancers. In this context, we hypothesized that degarelix, a new gonadotropin-releasing hormone receptor antagonist developed for patients with prostate cancer, may have antiangiogenic effects via its capacity to block follicle stimulating hormone (FSH) production. We report the case of a patient with metastatic colon cancer exhibiting tumor progression after failure of all conventional chemotherapeutic regimens. The addition of degarelix to the last chemotherapeutic regimen was proposed as compassionate treatment. Degarelix induced a rapid decrease in FSH level. This treatment induced radiological stabilization and carcinoembryonic antigen stabilization during 1 year. Contrast-enhanced ultrasonography demonstrated reduction of tumor vasclature. This case represents the first report of an antitumoral effect of degarelix in metastatic colon cancer and suggests an antiangiogenic property of this drug.
- Citation: Ghiringhelli F, Isambert N, Ladoire S. Degarelix as a new antiangiogenic agent for metastatic colon cancer? World J Gastroenterol 2013; 19(5): 769-772
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.769
In adult humans, the follicle stimulating hormone receptor (FSHR) is expressed only in the testicular sertoli cells and the ovarian granulosa cells. Recently, FSHR was also found to be selectively expressed by endothelial cells on tumor-associated blood vessels in a wide range of human cancers, compared to blood vessels from normal tissue[1]. This leads to the hypothesis that follicle stimulating hormone (FSH) and FSHR could be involved in endothelial cell survival or proliferation, especially during tumor-associated angiogenesis. Thus, the FSH pathway could be a new target for cancer therapy, notably in cancer types in which tumor angiogenesis is critical for cancer progression. Degarelix is a new gonadotropin-releasing hormone (GnRH) receptor antagonist, developed for patients with prostate cancer requiring androgen-deprivation therapy. Degarelix blocks the GnRH receptors in the anterior pituitary gland, resulting in a rapid decrease in the secretion of both luteinising hormone (LH) and FSH[2]. In this context, we hypothesized that degarelix may have antiangiogenic effects via its capacity to block FSH production, thereby interrupting the FSH-FSHR pathway on tumor-associated blood vessels.
We report here on a patient with metastatic colon cancer exhibiting tumor progression after failure of all conventional chemotherapeutic regimens, and who experienced long term disease stabilization associated with tumor devascularization under degarelix treatment.
A 69 year-old woman was referred to our center for sideropenic anemia in March 2008. A tumor of the cecum was discovered and the patient underwent right colectomy. The tumor was staged T3 N2, and non-resectable peritoneal carcinomatosis was discovered. The tumor had a K-RAS mutation status. The patient was initially treated starting in July 2008 by FOLFIRI + bevacizumab, and subsequently by LV5FU2 + bevacizumab because of hematological toxicity and grade 3 diarrhea. During treatment, the tumor marker carcinoembryonic antigen (CEA) decreased from 15 ng/mL, to 6 ng/mL in July 2009. Disease progression occurred in December 2009, with a CEA level of 14 ng/mL. The patient received FOLFOX + bevacizumab from December 2009 to September 2010, at which time CEA level increased to 42 ng/mL, and the patient presented an occlusive syndrome with abdominal pain requiring morphine treatment. On compassionate grounds, we proposed the addition of degarelix to LV5FU2 + bevacizumab. Degarelix was initiated subcutaneously at 240 mg for 1 mo, followed by monthly maintenance doses of 80 mg. FSH level was 42.5 IU/L before the first injection. A peritoneal nodule of 135 mm was selected as a target lesion for radiological evaluation by computed tomography (CT) scan and contrast-enhanced ultrasonography. Fifteen days after the first injection, the FSH serum level was < 2 IU/L (Figure 1A), and remained at this level during the entire treatment period. Contrast-enhanced ultrasonography was performed on day 0 and day 15. Radiological evaluation was performed by the same 2 independent radiologists, who were blinded to the patient’s clinical, biological and treatment data. A single target tumor was studied and selected on the basis of size (> 2 cm), and site (tumors with a good acoustic window that enabled data acquisition for longer than 3 min). A dynamic study was conducted after a single intravenous bolus injection of 4.8 mL of a contrast agent consisting of sulfur hexafluoride-filled microbubbles (SonoVue; Bracco, Milan, Italy). The investigation recordings and timing were triggered as soon as the contrast agent was injected. A total of 720 images were acquired during each 3-min investigative examination. Three semi-quantitative perfusion parameters were extracted from the time-intensity curves, namely peak intensity, time to peak intensity, total area under the time-intensity curve (AUC)[3]. Before degarelix initiation, AUC of the peritoneal nodule was 1650 mm². Fifteen days after treatment initiation, a dramatic reduction of almost 80% in the vascularized surface was observed, at 351 mm² (Figure 1B, C) (The area of interest is marked by a dotted white line). Moreover, resolution of the occlusive syndrome was achieved 5 wk after degarelix initiation, and morphine treatment was stopped without residual abdominal pain. Tolerance was excellent, without side effects using NCI-CTCAE v3 grade. Patient was previously treated by amlodipine for bevacizumab related and blood pressure was not modified by degarelix. The patient was menopause at the beginning of the treatment with degarelix so no side affect related to gonad suppression were observed. CT scans performed 2 mo after the beginning of the treatment showed stable disease according to the RECIST criteria with a stable target lesion and a regression of small none target lesion of peritoneal carcinomatosis. Successive CT scans performed between September 2010 and August 2011 confirmed stable disease according to the RECIST criteria (version 1.1) (target lesion evaluated between 137 and 138 mm). CEA levels remained stable during this period (Figure 1A). Unfortunately, occlusive syndrome re-appeared in October 2011. CT scan showed peritoneal progression, and only best supportive care was maintained. The patient died 6 wk later.
To the best of our knowledge, this case represents the first report of an antitumoral effect of degarelix in metastatic colon cancer.
The FSH receptor was recently recognized as a specific marker of endothelial cells in blood vessels from different tumor types[1]. Biological data have shown that the binding of FSH to FSH receptor in granulosa cells induces increased production of the hypoxia-inducible factor 1α[4]. This transcriptional factor is commonly induced by hypoxic conditions and drives the up-regulation of vascular endothelial growth factor (VEGF), one of the most important pro-angiogenic factors secreted during cancer growth. Thus, we hypothesized that triggering the FSH-receptor could induce VEGF production and thereby promote tumor angiogenesis. FSH signaling is also known to generate activated Gq protein[5]. Gq protein has been shown to induce VEGFR-2 signaling in human endothelial cells, even in the absence of VEGF[6]. This effect may enhance the proliferation and migration of endothelial cells in cancer, independently of VEGF availability. Thus, blocking FSH-receptor signaling may represent a new antiangiogenic strategy. In this report, we present the first clinical observation that degarelix, a GnRH antagonist used for the treatment of castrate-sensitive prostate cancer, could considerably decrease FSH production in a post-menopausal cancer-bearing woman. Contrast-enhanced ultrasonography seemed to indicate that this period of 12 mo with disease stabilization in a previously chemorefractory patient could be related to an antiangiogenic effect of degarelix, suggesting that this treatment could overcome resistance to anti-VEGF therapies, such as bevacizumab. However, we cannot provide specific immunohistochemical analysis of FSHR expression on tumor-associated blood vessels from the patient’s peritoneal tumors. This constitutes a limitation of this report, but it should be noted that in the initial report by Radu et al[1], consistent expression of FSHR by endothelial cells was detected in all the colorectal cancers analyzed. As we do not test the efficacy of degarelix monotherapy was could not determine if only combination of 5 FU/leucovorin bevacizumab and degarelix or degarelix monotherapy alone could be proposed in further clinical trial. Nonetheless, further clinical trials guided by FSHR expression on tumor vasculature are warranted to investigate the antiangiogenic effect of degarelix in cancer patients.
P- Reviewers Kozono D, Takahashi M S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, Fromont G, Hai MT, Ghinea N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schröder F, Shore N, Moul JW, Jensen JK, Olesen TK, Persson BE. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol. 2011;186:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Schirin-Sokhan R, Winograd R, Roderburg C, Bubenzer J, do Ó NC, Guggenberger D, Hecker H, Trautwein C, Tischendorf JJ. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol. 2012;18:541-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology. 2009;150:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Castro-Fernández C, Maya-Núñez G, Méndez JP. Regulation of follicle-stimulating and luteinizing hormone receptor signaling by. Endocrine. 2004;25:49-54. [PubMed] |
| 6. | Zeng H, Zhao D, Yang S, Datta K, Mukhopadhyay D. Heterotrimeric G alpha q/G alpha 11 proteins function upstream of vascular endothelial growth factor (VEGF) receptor-2 (KDR) phosphorylation in vascular permeability factor/VEGF signaling. J Biol Chem. 2003;278:20738-20745. [PubMed] |