Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9425
Revised: October 15, 2013
Accepted: November 1, 2013
Published online: December 28, 2013
Processing time: 149 Days and 7.4 Hours
AIM: To analyze the expression of kallikrein gene 10 (KLK10) in gastric cancer and to determine whether KLK10 has independent prognostic value in gastric cancer.
METHODS: We studied KLK10 expression in 80 histologically confirmed gastric cancer samples using real-time quantitative reverse transcription-PCR and hK10 expression using immunohistochemistry. Correlations with clinicopathological variables (lymph node metastasis, depth of invasion and histology) and with outcomes (disease-free survival and overall survival) during a median follow-up period of 31 mo were assessed. Gastric cancer tissues were then classified as KLK10 positive or negative.
RESULTS: KLK10 was found to be highly expressed in 57/80 (70%) of gastric cancer samples, while its expression was very low in normal gastric tissues. Positive relationships between KLK10 expression and lymph node metastasis (P = 0.048), depth of invasion (P = 0.034) and histology (P = 0.015) were observed. Univariate survival analysis revealed that gastric cancer patients with positive KLK10 expression had an increased risk for relapse/metastasis and death (P = 0.005 and 0.002, respectively). Cox multivariate analysis indicated that KLK10 was an independent prognostic indicator of disease-free survival and overall survival in patients with gastric cancer.
CONCLUSION: KLK10 expression is an independent biomarker of unfavorable prognosis in patients with gastric cancer.
Core tip: The study examined the clinicopathologic and prognostic significance of kallikrein gene 10 (KLK10) expression in gastric cancer. Based on collective findings, we hypothesize that KLK10 expression in gastric cancer tissues may have prognostic/predictive value in patients with gastric cancer. KLK10 expression is an independent biomarker for predicting unfavorable prognosis in patients with gastric cancer.
- Citation: Jiao X, Lu HJ, Zhai MM, Tan ZJ, Zhi HN, Liu XM, Liu CH, Zhang DP. Overexpression of kallikrein gene 10 is a biomarker for predicting poor prognosis in gastric cancer. World J Gastroenterol 2013; 19(48): 9425-9431
- URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9425
Gastric cancer is the fourth most common cancer, and the second leading cause of cancer death worldwide[1]. Mortality due to gastric cancer has risen in China over the past 20 years, especially in rural areas and in aging populations[2,3]. Although the increased use of screening for early disease diagnosis and the widespread administration of systemic adjuvant therapies have led to a decline in mortality rates, the incidence and mortality of gastric cancer are still second only to lung cancer[4,5].
The kallikrein gene family of secreted serine proteases, consisting of 15 genes, is localized in tandem on chromosome 19q13.4 and shows significant homologies at both the nucleotide and the protein levels[6,7]. Kallikrein-related peptidase 10 is a member of the kallikrein family and has been shown in numerous reports to be upregulated in ovarian cancer[8,9]. The human kallikrein (KLK) gene 10 encodes human kallikrein gene 10 (KLK10) protein. Recent studies have shown that human KLKs are involved in human carcinogenesis and that several KLKs are promising biomarkers of prostate, ovarian, testicular and breast cancer[10,11]. For instance, prostate-specific antigen (PSA/hK3) which is encoded by the KLK3 gene is used as a cancer-specific marker for male population screening, early diagnosis and monitoring of prostate cancer[12]. In addition, quantification of KLK5 expression is critical for both the discovery of early cellular and molecular alterations in breast cancer, as well as the identification of novel diagnostic and prognostic biomarkers[13]. Many other KLKs are also expected to act as tumor biomarkers[14-17]. More recent evidence also implicates the KLKs in many cancer-related processes, including cell growth regulation, angiogenesis, invasion and metastasis[7]. Several authors have reported that KLK10 mRNA was highly expressed in ovarian cancer tissue and that hK10 may be a useful serum biomarker for the diagnosis and management of ovarian cancer[18]. However, few studies have focused on KLK10 expression in human gastric cancer.
In the present study, we examined the clinicopathologic and prognostic significance of KLK10 expression in gastric cancer. Based on collective findings, we hypothesize that KLK10 expression in gastric cancer tissues may have prognostic/predictive value in patients with this malignancy.
Tumor specimens from 80 consecutive patients undergoing surgical treatment for primary gastric cancer at the Department of General Surgery, Tianjin First Central Hospital (Tianjin, China) were analyzed in this study. Patient age ranged from 35 to 74 years, with a median of 51 years (Table 1). All tumor specimens and matched control samples taken from normal tissues at the incision edge were snap-frozen in liquid nitrogen and stored at -80 °C for subsequent RNA extraction. Investigations were carried out in accordance with the ethical standards of the 1975 Helsinki Declaration, as revised in Tokyo 2004. The patients had not received hormonal therapy or chemotherapy prior to surgery. After surgery, all patients were treated with oxaliplatin-based chemotherapy regimens based on a platinum compound, alone or in combination with other drugs; grade 1 and stage I patients received no further treatment. Follow-up information (median follow-up period of 31 mo) was available for 80 patients (Table 1). Two time-to-event outcomes after surgery were recorded: disease-free survival (DFS) and overall survival (OS). DFS in each case was defined as the time interval between the date of primary cancer removal and the date of the first documented evidence of relapse. OS was defined as the time interval between the date of surgery and the date of death, or the date of last follow-up for those who were alive at the end of the study.
Clinical and pathological information documented at the time of surgery included clinical stage, histology, depth of invasion and lymph node metastasis (Table 2). All pathological factors were established as described by the 2010 National Comprehensive Cancer Network Guideline.
The study protocol was approved by the Ethics Committee of the hospital and written informed consent was obtained from each patient.
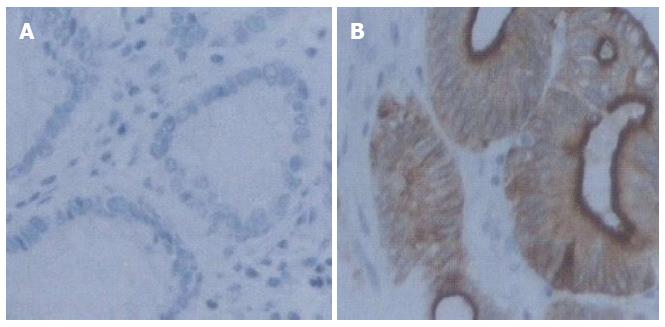
Immunohistochemical studies of hK10 were carried out using the avidin-biotin-peroxidase method (LSAB2 kit, Dako, Kyoto, Japan) on formalin-fixed, paraffin-embedded surgical specimens from patients with gastric cancer. All sections were counterstained with hematoxylin. Primary goat polyclonal antibodies against hK10 (Santa Cruz, United States) were used at dilutions of 1:700.
All sections were independently examined by two researchers (Xin Jiao, Mi-Mi Zhai). The expression of hK10 was scored as positive when the carcinoma cell cytoplasm was stained brown. We examined hK10 protein expression in tumor tissues and corresponding normal tissues from 80 gastric cancer cases.
Tumor tissues of 100 mg were minced on dry ice using a scalpel and immediately transferred to 2 mL polypropylene tubes. Total RNA was isolated from these samples using TRI-reagent (Ambion Inc., Austin, TX, United States) following the manufacturer’s instructions. Total RNA concentration and quality were determined spectrophotometrically at 260 and 280 nm, and RNA integrity was evaluated using agarose gel electrophoresis. Reverse transcription of the mRNA molecules into first-strand cDNA was carried out using 1 μg of total RNA from each tissue specimen, M-MuLV Reverse Transcriptase RNase H (Finnzymes Oy, Espoo, Finland) and an oligo(dT) oligonucleotide as a reverse transcription primer, according to the manufacturer’s instructions. Confirmation of the integrity of total RNA is shown in Figure 1.
Based on the mRNA sequences from the NCBI Sequence database, gene specific primers were designed and synthesized for the target KLK10 gene (NCBI Reference Sequence: NM_002776) and HPRT1 (hypoxanthine phosphoribosyltransferase-1) endogenous reference gene (NCBI Reference Sequence: NM_000194.2) using the Primer Express software (Applied Biosystems, CA, United States). A KLK10 fragment was amplified using the primers: forward, 5’-CTCTGGCGAAGCTGCTG-3’ and reverse, 5’-ATAGGCTTCGGGGTCCAA-3’, whereas the primers for HPRT1 were: forward, 5’-TGGAAAGGGTGTTTATTCCTCAT-3’ and reverse, 5’-ATGTAATCCAGCAGGTCAGCAA-3’.
Real-time PCR assays of KLK10 mRNA expression levels were performed using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, United States), and the reagents TaqMan Fast Universal PCR Master Mix (29) (Applied Biosystems, United States) according to the manufacturer’s instructions. With an initial polymerase activation step at 95 °C for 10 min, the amplification conditions of the 40 cycles consisted of denaturation at 95 °C for 15 s, annealing at 59 °C for 30 s, and elongation at 72 °C for 30 s. The products were then subjected to a temperature gradient from 55 °C to 95 °C at 0.1 °C/s with continuous fluorescence monitoring to produce a melting curve of the products. KLK10 mRNA expression was calculated from the standard curve, and quantitative normalization of cDNA in each sample was performed using the expression of GAPDH mRNA as an internal control[19]. We classified the 80 cases into two groups using the mean expression level of KLK10 mRNA in tumor tissues (0.03): i.e., a positive-expression group (≥ 0.03, n = 57) and a negative-expression group (< 0.03, n = 23).
Statistical analysis were performed using SPSS for Windows version 19.0 (SPSS, Chicago, IL, United States). Associations between clinicopathological parameters, such as depth of invasion, lymph node metastasis, histology and KLK10 expression were analyzed by the Chi-square test or Fisher’s exact test, where appropriate. Survival analysis were performed by constructing Kaplan-Meier DFS and OS curves and differences between curves were evaluated by the log-rank test (Mantel, 1966), and by estimating the relative risks for relapse and death using the Cox proportional hazards regression model (Cox, 1972). Cox analysis was conducted at both univariate and multivariate levels. Only the patients with known status of all variables were included in the multivariate regression models, which incorporated KLK10 and all other variables, for which the patients were characterized.
Of the 80 patients included in this study, 57 (70 °C) were positive for KLK10 expression in gastric cancer tissues. In normal gastric tissues, the level of KLK10 was undetectable or low. Table 2 shows the distribution of KLK10 expression (positive or negative) in gastric cancer tissues in relation to age, sex, lymph node metastasis, depth of invasion and histology. Patients with KLK10-positive gastric cancer more frequently had more lymph node metastasis (P = 0.048), greater depth of invasion (P = 0.034) and poorer histology (P = 0.015). No significant associations between KLK10 expression and age (P = 0.581) and sex (P = 0.258) were found.
In 55 of the 57 patients who were positive for KLK10 mRNA expression, specific expression of hK10 protein was only found in cancer tissues, but not in the corresponding normal tissues (Figure 2). In 23 cases with negative expression of KLK10 mRNA, 20 exhibited negative or weak expression of hK10 in cancer tissues. In contrast, 2 cases with high KLK10 mRNA expression exhibited negative or poor hK10 protein expression in cancer tissues.
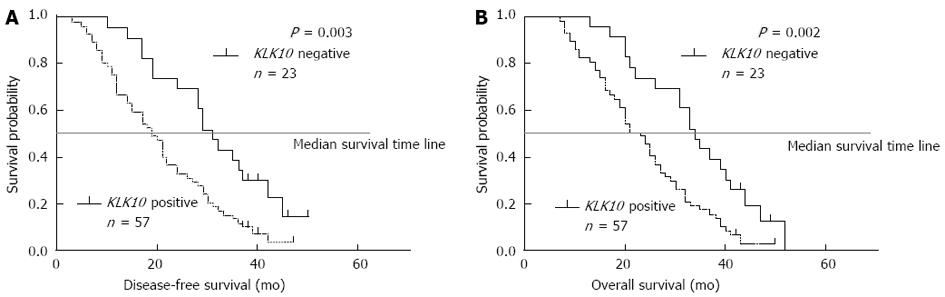
The clinicopathologic factors analyzed in relation to KLK10 mRNA expression in tumor tissues are shown in Table 2. The level of lymphatic invasion was significantly higher (P = 0.048) in the positive-expression group than in the negative-expression group. The depth of gastric wall invasion was greater (P = 0.034) in the positive-expression group than in the negative-expression group. The histotype also correlated with these groups (P = 0.015). In contrast, no significant difference was observed regarding age and sex. The 3-year actuarial OS rates in patients with gastric cancer and positive KLK10 mRNA expression and in patients with negative KLK10 mRNA expression were 20% and 42%, respectively (Figure 3). The survival difference between these two groups was statistically significant (P = 0.002; log-rank test).
The degree of association between each clinicopathological variable and DFS and OS is shown in Table 3. In univariate analysis, patients with KLK10-positive gastric cancer had a significantly increased risk of relapse (decreased DFS) and death (decreased OS) (hazards ratios of 0.46 and 0.43; P = 0.005 and 0.002, respectively). Nevertheless, positive KLK10 expression was weakly associated with an increased risk of death (decreased OS) (hazards ratio of 0.55; P = 0.03) in multivariate analysis compared with univariate analysis, while no significant association was found between the positive KLK10 expression and relapse (decreased DFS) in patients with gastric cancer in multivariate analysis (P = 0.06).
| Survival variable | Disease-free survival | Overall survival | ||||
| 95%CI2 | P value | HR1 | 95%CI2 | P value | HR1 | |
| Univariate analysis | ||||||
| KLK10 | ||||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 0.25-0.74 | 0.002 | 0.46 | 0.26-0.79 | 0.005 | 0.43 |
| Age | 0.97-1.01 | 0.46 | 0.99 | 0.97-1.01 | 0.49 | 0.99 |
| Sex | 0.55-1.53 | 0.75 | 0.90 | 0.54-1.51 | 0.69 | 0.92 |
| Depth of invasion | 1.55-2.81 | < 0.001 | 2.13 | 1.57-2.89 | < 0.001 | 2.08 |
| Lymph node metastasis | 0.97-1.97 | 0.07 | 1.42 | 0.99-2.02 | 0.051 | 1.38 |
| Histology | 6.42-22.46 | < 0.001 | 10.25 | 5.73-18.32 | < 0.001 | 12.01 |
| Multivariate analysis | ||||||
| KLK10 | ||||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 0.31-0.96 | 0.03 | 0.58 | 0.33-1.02 | 0.06 | 0.55 |
| Age | 0.95-1.00 | 0.02 | 0.97 | 0.95-0.99 | 0.04 | 0.97 |
| Sex | 0.77-2.22 | 0.31 | 1.33 | 0.77-2.28 | 0.30 | 1.31 |
| Depth of invasion | 0.99-1.90 | 0.06 | 1.42 | 1.02-1.98 | 0.03 | 1.37 |
| Lymph node metastasis | 0.84-1.59 | 0.35 | 1.21 | 0.87-1.67 | 0.24 | 1.16 |
| Histology | 6.55-25.41 | 0.03 | 11.11 | 5.87-21.02 | < 0.001 | 12.90 |
Depth of invasion and histotype were the strongest independent indicators of poor prognosis (P < 0.05, except for depth of invasion for OS in multivariate analysis). As expected, Kaplan-Meier survival curves (Figure 3) indicated that patients with KLK10-positive gastric cancer had shorter DFS (P = 0.003) and OS (P = 0.002) compared with KLK10-negative patients.
Gastric cancer is a common malignant tumor of the gastrointestinal tract. The optimal management of patients with gastric cancer involves a multidisciplinary approach: diagnosis, surgery, and chemotherapy, including the use of biological markers. Several authors have reported that KLK10 mRNA is highly expressed in human cancer tissues[20-23]. In the current study, we found a significant relationship between KLK10 mRNA expression and lymph node metastasis, depth of invasion and histology in patients with gastric cancer, as shown in Table 1. These findings indicate that the overexpression of KLK10 was significantly associated with both an increased incidence of lymphatic invasion and poor histology in patients with gastric cancer. These results suggest that enhanced expression of KLK10 may play an important role in various pathologic processes of gastric cancer. The results obtained in this study are in agreement with previous studies which examined the association between KLK10 expression status and the clinicopathological features of patients with gastric cancer[24].
Some members of the KLK family have been identified as potential biological markers of prognosis, including KLK5, KLK14 and KLK7[25]. For example, KLK5 expression is an indicator of poor prognosis in ovarian cancer[26]. Furthermore, stratifying patients based on the presence or absence of such markers may result in a different prognosis in individuals. For example, both KLK8 mRNA and KLK6 mRNA are highly expressed in human breast cancer tissues, however, it is unknown whether a breast cancer patient with high expression of KLK8 has a good/poor prognosis compared to a breast cancer patient with high expression of KLK6.
In this study, we identified KLK10 as a new biomarker of poor prognosis in gastric cancer. Patients with KLK10-positive tumors were more likely to have poor histology and advanced stage disease. Our findings demonstrate that KLK10 expression can reduce DFS in patients with gastric cancer in univariate, but not in multivariate analysis (Table 2). In addition, when assessing KLK10 expression to predict survival outcomes, we found an increased risk of death in patients with KLK10-positive tumors in both univariate and multivariate analysis (Table 2). This indicates that KLK10-positivity may be an independent prognostic factor in patients with gastric cancer. That is, KLK10 may induce gastric cancer cell growth and proliferation. However, the function of the KLK10 signaling pathway is unclear. Some reports indicate that serine proteinases (the KLK gene family of secreted serine proteases includes 15 genes) participate in tumor growth and invasion by cleaving and activating proteinase-activated receptors (PARs: PAR-1 and PAR-2)[27-29]. A recent study demonstrated that KLK4 is aberrantly expressed in colon cancer and capable of inducing PAR-1 signaling in cancer cells[30]. KLK4 is a tumorigenic factor. Another report showed that KLK14 induced significant extracellular signal-regulated kinases 1 and 2 (ERK1/2) phosphorylation and HT29 cell proliferation, presumably by activating PAR-2. A PAR-2 cleavage and activation-blocking antibody markedly reduced KLK14-induced ERK1/2 signaling[31]. Our lack of knowledge of KLK10 function and regulation in gastric cancer tissues does not allow us to formulate reasonable hypothesis to explain these observations. More studies with a larger group of patients are necessary to substantiate these findings.
The current study indicates that KLK10 mRNA was significantly overexpressed in gastric cancer tissues and high KLK10 expression levels were associated with lymphatic invasion, tumor invasion and poor patient prognosis. hK3 has been well documented to be an excellent tumor marker for prostate cancer. Moreover, hK10 is a promising serum biomarker for ovarian cancer. Therefore, studies are now underway to investigate whether hK10 may also be a useful biomarker for gastric cancer using serum samples from the patients treated at our hospital.
Gastric carcinoma is one of the most common tumors worldwide. The expression of kallikreins is involved in cancer cell formation. Abnormal expression of kallikrein gene 10 (KLK10) is associated with carcinogenesis, and it is a promising serum biomarker for cancers.
Several authors have reported that KLK10 mRNA is highly expressed in ovarian cancer tissue and that hK10 could be a useful serum biomarker for the diagnosis and management of ovarian cancer. However, there is little information on KLK10 expression in human gastric cancer.
This study assessed the clinicopathologic and prognostic significance of KLK10 expression in gastric cancer. Furthermore, based on the collective findings, this study investigated whether KLK10 expression in gastric cancer tissues may have prognostic/predictive value in patients with gastric cancer.
By exploring the relation between the expression of KLK10 and clinicopathology in gastric cancer, this study may provide a strategy for predicting the prognosis of gastric cancer patients.
It is a well written paper. The authors investigated the clinicopathologic and prognostic significance of KLK10 expression in gastric cancer, which helps understand the pathogenesis and predict the prognosis of gastric cancer. The experimental procedure is quite well performed.
P- Reviewers: Ji JF, Li W, TongQS S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 2. | Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991-2005. Br J Cancer. 2004;90:2157-2166. [PubMed] |
| 3. | Yin XD, Huang WB, Lü CY, Zhang L, Wang LW, Xie GH. A preliminary study on correlations of triple-phase multi-slice CT scan with histological differentiation and intratumoral microvascular/lymphatic invasion in gastric cancer. Chin Med J (Engl). 2011;124:347-351. [PubMed] |
| 4. | Zhang SW, Lei ZL, Li GL, Zou XN, Zhao P, Chen WQ. A report of cancer incidence and mortality from 34 cancer registries in China. Zhongguo Aizheng. 2010;19:356-365. [DOI] [Full Text] |
| 5. | Liu J, Chen L. Current status and progress in gastric cancer with liver metastasis. Chin Med J (Engl). 2011;124:445-456. [PubMed] |
| 6. | Diamandis EP, Yousef GM. Human tissue kallikreins: a family of new cancer biomarkers. Clin Chem. 2002;48:1198-1205. [PubMed] |
| 7. | Borgoño CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4:876-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 485] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Bayani J, Paliouras M, Planque C, Shan SJ, Graham C, Squire JA, Diamandis EP. Impact of cytogenetic and genomic aberrations of the kallikrein locus in ovarian cancer. Mol Oncol. 2008;2:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Luo LY, Katsaros D, Scorilas A, Fracchioli S, Bellino R, van Gramberen M, de Bruijn H, Henrik A, Stenman UH, Massobrio M. The serum concentration of human kallikrein 10 represents a novel biomarker for ovarian cancer diagnosis and prognosis. Cancer Res. 2003;63:807-811. [PubMed] |
| 10. | Shan SJ, Scorilas A, Katsaros D, Diamandis EP. Transcriptional upregulation of human tissue kallikrein 6 in ovarian cancer: clinical and mechanistic aspects. Br J Cancer. 2007;96:362-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Yousef GM, Borgoño CA, Scorilas A, Ponzone R, Biglia N, Iskander L, Polymeris ME, Roagna R, Sismondi P, Diamandis EP. Quantitative analysis of human kallikrein gene 14 expression in breast tumours indicates association with poor prognosis. Br J Cancer. 2002;87:1287-1293. [PubMed] |
| 12. | McCormack RT, Rittenhouse HG, Finlay JA, Sokoloff RL, Wang TJ, Wolfert RL, Lilja H, Oesterling JE. Molecular forms of prostate-specific antigen and the human kallikrein gene family: a new era. Urology. 1995;45:729-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Avgeris M, Papachristopoulou G, Polychronis A, Scorilas A. Down-regulation of kallikrein-related peptidase 5 (KLK5) expression in breast cancer patients: a biomarker for the differential diagnosis of breast lesions. Clin Proteomics. 2011;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Kishi T, Grass L, Soosaipillai A, Scorilas A, Harbeck N, Schmalfeldt B, Dorn J, Mysliwiec M, Schmitt M, Diamandis EP. Human kallikrein 8, a novel biomarker for ovarian carcinoma. Cancer Res. 2003;63:2771-2774. [PubMed] |
| 15. | Borgoño CA, Grass L, Soosaipillai A, Yousef GM, Petraki CD, Howarth DH, Fracchioli S, Katsaros D, Diamandis EP. Human kallikrein 14: a new potential biomarker for ovarian and breast cancer. Cancer Res. 2003;63:9032-9041. [PubMed] |
| 16. | Talieri M, Diamandis EP, Gourgiotis D, Mathioudaki K, Scorilas A. Expression analysis of the human kallikrein 7 (KLK7) in breast tumors: a new potential biomarker for prognosis of breast carcinoma. Thromb Haemost. 2004;91:180-186. [PubMed] |
| 17. | Talieri M, Li L, Zheng Y, Alexopoulou DK, Soosaipillai A, Scorilas A, Xynopoulos D, Diamandis EP. The use of kallikrein-related peptidases as adjuvant prognostic markers in colorectal cancer. Br J Cancer. 2009;100:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | White NM, Chow TF, Mejia-Guerrero S, Diamandis M, Rofael Y, Faragalla H, Mankaruous M, Gabril M, Girgis A, Yousef GM. Three dysregulated miRNAs control kallikrein 10 expression and cell proliferation in ovarian cancer. Br J Cancer. 2010;102:1244-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Ogawa K, Utsunomiya T, Mimori K, Tanaka Y, Tanaka F, Inoue H, Murayama S, Mori M. Clinical significance of elongation factor-1 delta mRNA expression in oesophageal carcinoma. Br J Cancer. 2004;91:282-286. [PubMed] |
| 20. | Dasgupta S, Tripathi PK, Qin H, Bhattacharya-Chatterjee M, Valentino J, Chatterjee SK. Identification of molecular targets for immunotherapy of patients with head and neck squamous cell carcinoma. Oral Oncol. 2006;42:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Luo LY, Diamandis EP, Look MP, Soosaipillai AP, Foekens JA. Higher expression of human kallikrein 10 in breast cancer tissue predicts tamoxifen resistance. Br J Cancer. 2002;86:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Olkhov-Mitsel E, Van der Kwast T, Kron KJ, Ozcelik H, Briollais L, Massey C, Recker F, Kwiatkowski M, Fleshner NE, Diamandis EP. Quantitative DNA methylation analysis of genes coding for kallikrein-related peptidases 6 and 10 as biomarkers for prostate cancer. Epigenetics. 2012;7:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Luo LY, Bunting P, Scorilas A, Diamandis EP. Human kallikrein 10: a novel tumor marker for ovarian carcinoma? Clin Chim Acta. 2001;306:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Feng B, Xu WB, Zheng MH, Ma JJ, Cai Q, Zhang Y, Ji J, Lu AG, Qu Y, Li JW. Clinical significance of human kallikrein 10 gene expression in colorectal cancer and gastric cancer. J Gastroenterol Hepatol. 2006;21:1596-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Inoue Y, Yokobori T, Yokoe T, Toiyama Y, Miki C, Mimori K, Mori M, Kusunoki M. Clinical significance of human kallikrein7 gene expression in colorectal cancer. Ann Surg Oncol. 2010;17:3037-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Kim H, Scorilas A, Katsaros D, Yousef GM, Massobrio M, Fracchioli S, Piccinno R, Gordini G, Diamandis EP. Human kallikrein gene 5 (KLK5) expression is an indicator of poor prognosis in ovarian cancer. Br J Cancer. 2001;84:643-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Ramachandran R, Eissa A, Mihara K, Oikonomopoulou K, Saifeddine M, Renaux B, Diamandis E, Hollenberg MD. Proteinase-activated receptors (PARs): differential signalling by kallikrein-related peptidases KLK8 and KLK14. Biol Chem. 2012;393:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Hollenberg MD, Oikonomopoulou K, Hansen KK, Saifeddine M, Ramachandran R, Diamandis EP. Kallikreins and proteinase-mediated signaling: proteinase-activated receptors (PARs) and the pathophysiology of inflammatory diseases and cancer. Biol Chem. 2008;389:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Ramsay AJ, Reid JC, Adams MN, Samaratunga H, Dong Y, Clements JA, Hooper JD. Prostatic trypsin-like kallikrein-related peptidases (KLKs) and other prostate-expressed tryptic proteinases as regulators of signalling via proteinase-activated receptors (PARs). Biol Chem. 2008;389:653-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Gratio V, Beaufort N, Seiz L, Maier J, Virca GD, Debela M, Grebenchtchikov N, Magdolen V, Darmoul D. Kallikrein-related peptidase 4: a new activator of the aberrantly expressed protease-activated receptor 1 in colon cancer cells. Am J Pathol. 2010;176:1452-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Gratio V, Loriot C, Virca GD, Oikonomopoulou K, Walker F, Diamandis EP, Hollenberg MD, Darmoul D. Kallikrein-related peptidase 14 acts on proteinase-activated receptor 2 to induce signaling pathway in colon cancer cells. Am J Pathol. 2011;179:2625-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |