Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9392
Revised: October 7, 2013
Accepted: November 3, 2013
Published online: December 28, 2013
Processing time: 166 Days and 3.9 Hours
AIM: To evaluate the relationship between Helicobacter pylori (H. pylori)-induced gastritis and white gastric mucosal crypt openings (COs) in the gastric corpus.
METHODS: A total of 175 consecutive patients (including 69 patients with gastric cancer) were enrolled in this study. We used magnifying endoscopy (ME) to observe the mucosa microsurface of the lesser and greater curvature of the gastric corpus (350 areas in all). We focused on areas with a round pit microstructure (primarily observed in non-atrophied areas) and evaluated the white openings of these gastric pits. We classified the whiteness of the COs as the “white-edged dark spot” type (consisting of a dark spot bordered by white); the “white” type (pure white with no dark spot); and the “dense white pit (DWP)” type (dense white, resembling a snowball). Gastritis was also histologically evaluated according to the updated Sydney System.
RESULTS: We detected round COs using ME in 246 of the 350 areas examined. The histological examination showed significantly more mononuclear cells and neutrophil infiltration in the “white” and “DWP” types than the “white-edged dark spot” type (P < 0.001). Furthermore, significantly high-grade inflammation and evidence of active H. pylori-induced gastritis was observed in the “DWP” type (P < 0.001). Significant differences were observed in the whiteness of COs between H. pylori-positive (n = 139) and negative (n = 36) patients (P < 0.001). The sensitivity and specificity of the “white” and “DWP” types for predicting H. pylori infection were 78.5% and 81.7%, respectively. Of the patients with gastric cancer, 22.5% (18/80) had “white-edged dark spots”, 51.3% (41/80) had “white” COs, and 26.3% (21/80) had “DWP”-type COs. “DWPs” were frequently observed among patients with undifferentiated gastric cancer [45.7% (16/35)].
CONCLUSION: CO whiteness detected via ME was associated with histological evidence of gastritis and helps to predict the severity of inflammation and H. pylori-induced activity.
Core tip: Recent studies have reported that advances in magnifying endoscopy (ME) have led to better correlations between histopathological findings and the ME features of Helicobacter pylori (H. pylori)-induced gastritis. However, the ME findings regarding H. pylori-induced severe inflammation are insufficient. Therefore, we evaluated the relationship between H. pylori-induced gastritis and the whiteness of gastric mucosal crypt openings (COs) in the gastric corpus using ME. Our results showed that mononuclear cell and neutrophil infiltration differed significantly among the CO subtypes. CO whiteness detected via ME was associated with histological evidence of gastritis and helps to predict the severity of inflammation or activity induced by H. pylori in the gastric corpus.
-
Citation: Kawamura M, Sekine H, Abe S, Shibuya D, Kato K, Masuda T. Clinical significance of white gastric crypt openings observed
via magnifying endoscopy. World J Gastroenterol 2013; 19(48): 9392-9398 - URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9392.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9392
Helicobacter pylori (H. pylori) infection causes acute and chronic inflammation accompanied by neutrophil or lymphocyte infiltration. This type of persistent inflammation can result in gastric mucosal changes such as glandular atrophy, intestinal metaplasia, dysplasia, and eventually carcinoma[1-5]. Previous studies using conventional standard endoscopy have reported correlations between H. pylori-induced gastritis and endoscopic findings. Endoscopic atrophy is correlated with the grade of glandular atrophy and intestinal metaplasia[6]. With regard to severe grades of H. pylori-induced inflammation, nodular gastritis in the antral area is an endoscopic marker for the early phase of H. pylori infection and an exaggerated immune response[7-10].
Advances in magnifying endoscopy (ME) and narrow-band imaging have enabled the real-time observation of the microsurface structure and microvascular architecture of the gastric mucosa. Recent studies have reported that these advances have led to stronger correlations between histopathological findings and the ME features of H. pylori-induced gastritis compared with data obtained using standard endoscopy[11-17]. Using ME, one can observe the microsurface structure of the gastric mucosa change from a round pit pattern to vertical long pits, tubular and granular patterns after the start of H. pylori-induced gastritis.
However, many investigators have reported that the morphological changes identified using ME are closely associated with histological glandular atrophy or intestinal metaplasia. The data regarding the characteristics of the ME findings in H. pylori-induced severe inflammation, such as nodular gastritis in the antral area, are insufficient. Therefore, we used high-resolution ME to investigate the characteristics of H. pylori-induced inflammation of the gastric corpus in H. pylori-negative and H. pylori-positive patients.
This observational study was performed in the endoscopy unit of a city hospital (JR Sendai Hospital, Sendai, Mi-, Japan). Between September 2007 and November 2010, 175 consecutive patients who had undergone ME in our hospital as part of their annual health checks to investigate digestive symptoms (or as an additional pretreatment examination) were enrolled. Patients were excluded if they had severe systemic disease; had a history of upper digestive tract surgery; had been treated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants within 7 d of endoscopy; or had active bleeding, advanced gastric cancer, or other non-gastric malignancies. Patients who received H. pylori eradication therapy were also excluded. H. pylori infection was diagnosed using a rapid urease test, which examined the histology of biopsy specimens obtained from the greater curvature of the gastric antrum and body, and the urea breath test. Patients who tested positive on any of these tests were considered positive for H. pylori infection. Written informed consent was obtained from each patient, and the institutional review board of JR Sendai Hospital approved the study protocol.
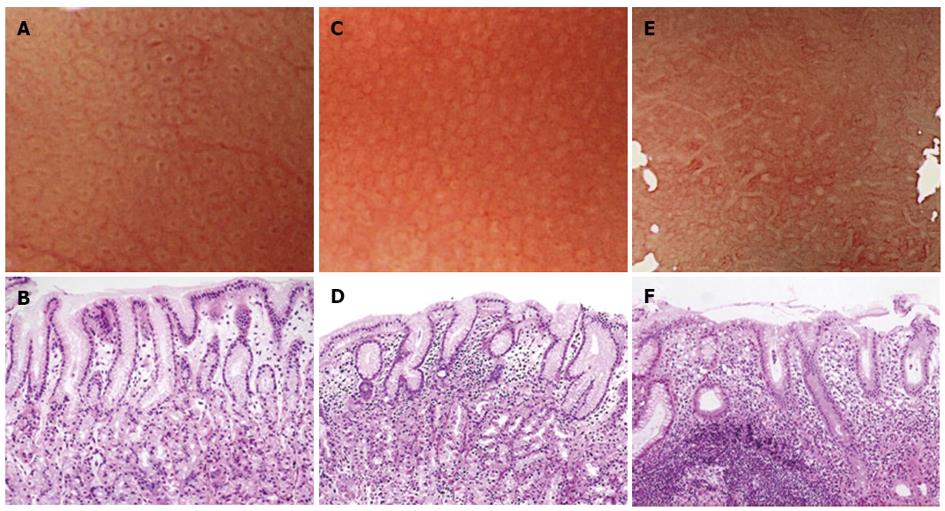
Magnifying endoscopy was performed using either a CV-240 or CV-260 video system (Olympus Optical, Tokyo, Japan) and a magnifying endoscope (Model Q240Z or H260Z, Olympus). To obtain a clear view using ME, a black rubber attachment (MB-46 or MB-162, Olympus) was fitted to the tip of the videoendoscope to ensure an appropriate distance between the lens and the mucosal surface. A single experienced endoscopist (Masashi Kawamura) performed all procedures. A videoendoscope was inserted into the patient’s stomach, and the diseased and uninvolved gastric mucosa were visualized using standard and magnified views. The microsurface structures of the non-cancerous areas in the greater and lesser curvatures of the upper gastric corpus were evaluated for the presence of patterns such as round pit, long pit, tubular, or granular. If areas with round pit microstructures were observed under maximum magnification, then we evaluated the whiteness of the gastric pit crypt openings (COs). Then, the specific area that had just been magnified was biopsied under magnification. The whiteness of each round CO was classified into one of the following three categories: A round dark spot bordered by white was classified as a “white-edged dark spot” CO; pure white COs without a dark spot were classified as “white” COs; and densely white COs resembling snowballs were classified as “dense white pit” (“DWP”) COs (Figure 1).
Biopsy specimens were fixed with buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. An expert pathologist who was unaware of the endoscopic findings assessed the grade of histological gastritis in each biopsy sample. The degree of mononuclear cell and neutrophil infiltration, atrophy, and intestinal metaplasia was assessed and graded as normal, mild, moderate, or marked using a visual analog scale included in the updated Sydney System[18]. Gastric carcinomas were classified as differentiated or undifferentiated based on the degree of glandular structure formation among the tumor cells (the Japanese Classification was proposed by the Japanese Research Society for Gastric Cancer)[19]. These types match the intestinal and diffuse types of gastric carcinoma, respectively, described in the Lauren classification[20].
The results are presented as the mean ± SD. Kruskal-Wallis and Mann-Whitney U tests were used to evaluate the relationship between the whiteness of the COs and the histological findings. The differences between the H. pylori-negative and H. pylori-positive groups with regard to CO type were compared using the Mann-Whitney U test. Data analyses were performed using R Version 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria); P values < 0.05 were considered significant.
Of the 175 enrolled patients, 116 were men and 59 women; their mean age was 63.9 years. The diagnoses included 46 patients with differentiated-type gastric cancer, 23 with undifferentiated-type gastric cancer, 22 with active duodenal ulcers, and eight with active gastric ulcers. A total of 76 patients had only gastritis or normal findings. The ME observations of the lesser and greater curvatures revealed round pit patterns in 246 of the 350 areas examined (Table 1).
| Diff-GC | Undiff-GC | GU | DU | Gastritis and normal | |
| Number of patients | 46 | 23 | 8 | 22 | 76 |
| Age (yr, mean ± SD) | 70.6 ± 9.2 | 65.5 ± 8.2 | 62.6 ± 10.6 | 49.2 ± 12.4 | 63.7 ± 12.5 |
| Sex, male/ female | 36/10 | 7/16 | 6/2 | 17/5 | 50/26 |
| Current Helicobacter pylori- infection | 40 (87.0) | 21 (91.3) | 8 (100) | 22 (100) | 48 (63.2) |
| Endoscopic degree of atrophy (mild/ moderate/ severe) | 3/14/29 | 9/12/2 | 2/2/4 | 20/2/0 | 41/15/20 |
| Round pits in LC | 12 (35.3) | 12 (52.2) | 3 (37.5) | 22 (100) | 48 (63.2) |
| Round pits in GC | 33 (71.7) | 23 (100) | 8 (100) | 22 (100) | 63 (82.9) |
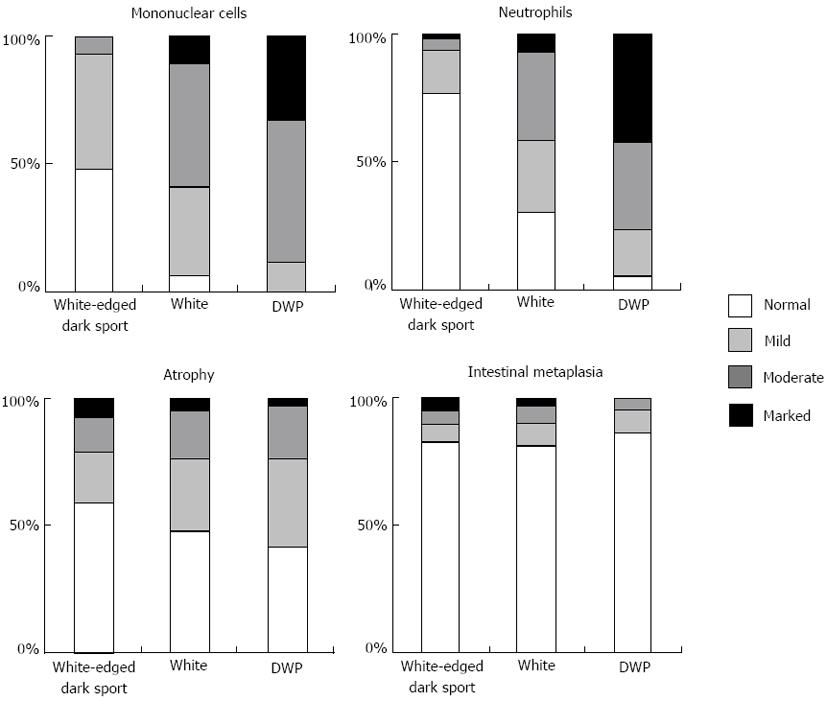
Regarding the whiteness of the round COs, 89 had the “white-edged dark spot”, 114 were “white”, and 43 were “DWP” COs. Figure 2 shows the relationship between CO whiteness and the severity of gastritis diagnosed histologically based on the updated Sydney System. In both “white” and “DWP” type COs, the histological examination tended to show moderate or marked mononuclear cell and neutrophil infiltration accompanied by normal-to-mild glandular atrophy and intestinal metaplasia. These variables were mostly classified as normal to mild among “white-edged dark spot” COs. We evaluated the histological findings according to the updated Sydney system, which uses 4 classes (none, mild, moderate and marked) for each parameter [inflammation (mononuclear cell infiltration), activity (neutrophil infiltration], atrophy (glandular atrophy), and intestinal metaplasia). Significant differences (P < 0.001) were found between the three CO types in the parameters of inflammation and activity; however, the degree of glandular atrophy and intestinal metaplasia did not differ significantly across CO type. The grades of inflammation and activity were higher among “DWP” COs compared with “white” COs (P < 0.001).
In this study, 139 patients were positive and 36 were negative for current H. pylori infection. Of the 186 round pit areas found in H. pylori-positive patients, 21.5% (40/186) were “white-edged dark spot” COs, 55.4% (103/186) were “white”, and 23.1% (43/186) were “DWP” COs. Of the 60 round pit areas found among H. pylori-negative patients, 81.7% (49/60) were “white-edged dark spot” COs, 18.3% (11/60) were “white”, and none (0/60) were “DWP” COs. Significant differences were found between the “white” and “DWP” COs in H. pylori-positive patients, and “white-edged dark spot” COs were found among H. pylori-negative patients (P < 0.001). The sensitivity and specificity of the “white” and “DWP” COs used to predict H. pylori infection were 78.5% and 81.7%, respectively.
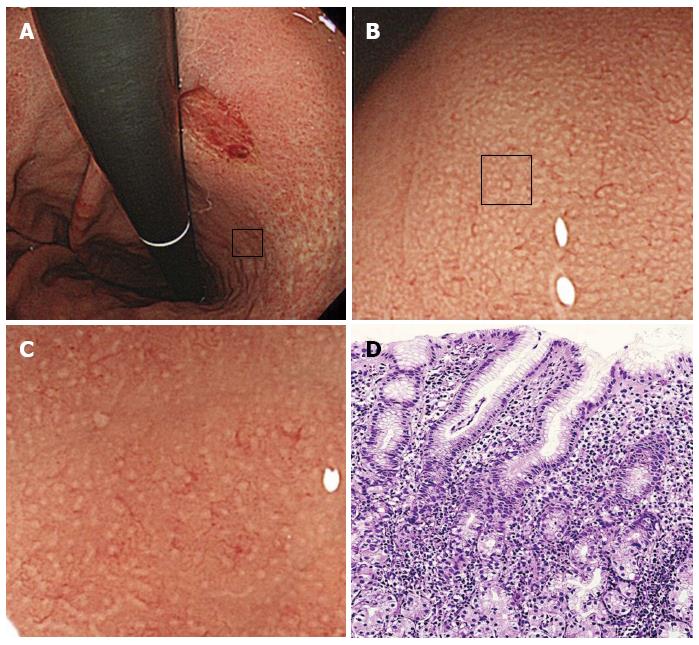
In total, 22.5% (18/80) of the COs among patients with H. pylori-related disease and gastric cancer were “white-edged dark spot” COs, 51.3% (41/80) were “white”, and 26.3% (21/80) were “DWP” COs (Table 2). The prevalence of “DWP” COs was higher [45.7% (16/35)] among patients with undifferentiated-type gastric cancer (Figure 3).
| Whiteness of COs | White-edged dark spot | White | DWP |
| Diff-GC | 16 (35.6) | 24 (53.3) | 5 (11.1) |
| Undiff-GC | 2 (5.7) | 17 (48.6) | 16 (45.7) |
| GU | 2 (18.2) | 6 (54.5) | 3 (27.3) |
| DU | 13 (29.5) | 26 (59.1) | 5 (11.4) |
| Gastritis and normal | 56 (50.5) | 41 (36.9) | 14 (12.6) |
This study is the first to investigate the relationship between CO whiteness type and H. pylori-induced gastritis. We categorized CO color in gastric corpus areas with round pit patterns, and we observed less inflammation and activity in “white-edged dark spot” COs than in other types of COs. The round dark spots observed in the ME images might correspond to tangential views of the foveolar gland COs, whereas the white portions that surround the dark spots might be tangential views of the epithelial cells that surround the ductal lumen. This CO type is the typical microsurface mucosal pattern of the gastric corpus among patients without H. pylori infection. A histological examination of the areas with “white” type COs revealed degenerated and hypertrophic surface epithelial cells accompanied by lymphocyte and neutrophil infiltration into the lamina propria. These histological changes might cause the dark spots to disappear and be replaced by a white color. The “DWP” COs were accompanied by a high degree of lymphocyte and neutrophil infiltration.
Several studies have evaluated the relationship between H. pylori-induced gastritis and the microsurface or microvascular structure of the gastric mucosa via ME. In 2007, Yagi et al[21] modified their former Z classification and created the A-B classification based on the combination of microsurface and microvascular patterns (type B-0 consists of pinhole pits, the network of true capillaries, and the regular arrangement of collecting venules; type B-1 consists of round pits and a network of capillaries; type B-2 consists of white pits and sulci; and type B-3 consists of dilated white pits with surrounding microvessels). Although this classification was considered useful for predicting the grade of H. pylori-induced gastritis, we often observed other combinations of microstructure and microvessel changes than those described in the A-B classification (e.g., pinhole pits without capillaries). The current study classified our observations into three types of ME findings based on the color of the gastric pits and found strong correlations with histological H. pylori-induced inflammation and activity. Our classification is advantageous because it is simple and easy to understand and does not include variations in the combination of microstructure and microvessels.
Several reports have described that the prevalence, distribution, and grade of H. pylori-induced gastritis varies among individuals[22,23]. We previously reported that the ME findings of the gastric mucosa in H. pylori-infected patients are also heterogeneous in the stomach[15]. The present study investigated each case at two sites (the greater and lesser curvature of the upper corpus) because a multipoint evaluation of gastritis is important for assessing its status. Our results indicated that most round pit areas existed in the endoscopic non-atrophied area; furthermore, more were found in the greater curvature of the corpus. These results are in agreement with those of a previous report showing that gastric atrophy starts at the lower portion of the lesser curvature in the corpus, then extends to the upper portion and laterally involves the greater curvature[24]. The diagnosis for the CO whiteness grade seemed to be homogenous under magnified observations (see the figures). We diagnosed using maximum magnification at all sites; therefore, the CO whiteness types were recognized as a homogenous pattern using these narrow fields of view (approximately 2-mm squares).
The present study showed that the CO whiteness type had higher sensitivity and specificity than that reported for conventional endoscopy[25,26] but lower sensitivity and specificity for detecting H. pylori infections than have been previously reported for ME assessment[13,14]. This discrepancy might have been caused by the differences in the H. pylori strain or immune responses. Our CO whiteness classification system was advantageous because it helped to predict the severity of inflammation and the H. pylori-induced activity in the gastric corpus.
Many reports have suggested that a relationship exists between differentiated-type gastric carcinoma and the severe atrophic gastritis caused by persistent H. pylori infection[2,23]. Conversely, undifferentiated-type gastric cancer is associated with H. pylori-induced active gastritis[23,27]. The present report showed that “DWP”-type COs that were accompanied by a histologically high grade of inflammation and activity were frequently observed in the gastric corpus of patients with undifferentiated-type gastric cancer; however, additional investigations are needed to clarify the relationship between CO whiteness and gastric cancer in a large population study.
The current study did not detect an area with round pits in 104/350 of the areas examined. As noted in earlier reports[12,21], the microsurface pattern changes a round pit pattern to vertical long pits, tubular and granular patterns with continuous H. pylori inflammation. Thus, in the case of severe endoscopic atrophy, tubular and granular patterns (but not pit patterns) were often observed via ME. Given the difficulty of assessing histological inflammation (which differs from histological glandular atrophy) under endoscopic observations, our results suggest that ME is a useful method for predicting H. pylori inflammation in detail. However, our ME classification might not be acceptable in cases with severe atrophy.
Another limitation of this study is its small number of patients. An analysis of patients with other H. pylori-related diseases is needed. In addition, assessments of the inter- and intra-observer variability with regard to the classification of CO whiteness are required to generalize the diagnostic ability of our findings. Another limitation is that we did not investigate the nature of the white substance in “DWP”-type COs. As described in the updated Sydney System[11], the marked neutrophil infiltration of foveolar lumens induced by H. pylori-infection might cause the formation of “pit abscesses”. We speculate that the appearance of “DWP”-type COs is attributable to the severe degenerative and hypertrophic changes in surface epithelial cells that are accompanied by lymphocyte and neutrophil infiltration; additional analyses are needed to investigate this possibility.
In conclusion, we found that CO whiteness in ME images of the gastric corpus was correlated with histological findings of inflammation and activity. ME observation of CO whiteness might facilitate the histological diagnosis of the inflammation and activity induced by H. pylori.
We are grateful to the staff members of the Cancer Detection Center, Miyagi Cancer Society for their technical assistance.
Magnifying endoscopy (ME) can be used for approximately x 80 magnified observation. Advances in ME have enabled the real-time observation of the microsurface structure and microvascular architecture of the gastric mucosa.
Helicobacter pylori (H. pylori) infection causes chronic inflammation of the gastric mucosa. This persistent inflammation leads to morphological changes in gastric mucosa as observed via ME.
ME provides a detailed observation of H. pylori-induced gastritis, and the relationship between ME findings and histological gastritis was reported. Although many investigators have reported that the morphological changes identified via ME are closely associated with histological glandular atrophy and intestinal metaplasia, the data regarding the characteristics of these ME findings in H. pylori-induced gastritis are insufficient in cases of severe inflammation and activity. The current results indicate that the white gastric mucosa crypt openings observed via ME are useful for assessing histological inflammation and activity.
H. pylori infection causes chronic gastritis, gastric or duodenal ulcer, and carcinoma. Conventional standard endoscopy has shown a poor relationship between H. pylori infection and active H. pylori gastritis. The results enable the assessment of histological activity and inflammation without a biopsy.
This article is well-written and well-designed for publication. It gives very new information for gastroenterologist.
P- Reviewers: Goral V, Nishida T S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
| 1. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] |
| 2. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 3. | NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65-69. [PubMed] |
| 4. | Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720-727. [PubMed] |
| 5. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 496] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 6. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:1274-1285. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (3)] |
| 7. | Eastham EJ, Elliott TS, Berkeley D, Jones DM. Campylobacter pylori infection in children. J Infect. 1988;16:77-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Sbeih F, Abdullah A, Sullivan S, Merenkov Z. Antral nodularity, gastric lymphoid hyperplasia, and Helicobacter pylori in adults. J Clin Gastroenterol. 1996;22:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Miyamoto M, Haruma K, Yoshihara M, Sumioka M, Nishisaka T, Tanaka S, Inoue K, Chayama K. Five cases of nodular gastritis and gastric cancer: a possible association between nodular gastritis and gastric cancer. Dig Liver Dis. 2002;34:819-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Tanabe J, Kawai N, Abe T, Ueshima N, Mizutani S, Tsujimoto M, Meren H, Kawano S, Kamada T, Haruma K. A case of diffuse-type early gastric cancer with nodular gastritis. Dig Endosc. 2006;18:67-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Sakaki N, Iida Y, Okazaki Y, Kawamura S, Takemoto T. Magnifying endoscopic observation of the gastric mucosa, particularly in patients with atrophic gastritis. Endoscopy. 1978;10:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Sakaki N, Iida Y, Saito M, Tada M, Odawara M, Okazaki Y, Kawamura S, Takemoto T. New magnifying endoscopic classification of the fine gastric mucosal pattern. Gastroenterol Endosc. 1980;22:377-383 [In Japanese with English abstract]. |
| 13. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Kawamura M, Abe S, Oikawa K, Terai S, Saito M, Shibuya D, Kato K, Shimada T, Uedo N, Masuda T. Topographic differences in gastric micromucosal patterns observed by magnifying endoscopy with narrow band imaging. J Gastroenterol Hepatol. 2011;26:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Kanzaki H, Uedo N, Ishihara R, Nagai K, Matsui F, Ohta T, Hanafusa M, Hanaoka N, Takeuchi Y, Higashino K. Comprehensive investigation of areae gastricae pattern in gastric corpus using magnifying narrow band imaging endoscopy in patients with chronic atrophic fundic gastritis. Helicobacter. 2012;17:224-231. [PubMed] |
| 18. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 19. | Japanese Research Society for Gastric Cancer. The General Rules for the Gastric Cancer Study, 13th edition. Tokyo: Kanehara 1999; 26. |
| 20. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 21. | Yagi K, Watanabe J, Nakamura A, Sekine A. Magnifying views of Gastritis-AB classification [in Japanese with English abstract]. Stomach Intestine. 2007;42:697-704. |
| 22. | Meining A, Stolte M, Hatz R, Lehn N, Miehlke S, Morgner A, Bayerdörffer E. Differing degree and distribution of gastritis in Helicobacter pylori-associated diseases. Virchows Arch. 1997;431:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] |
| 24. | Satoh K, Kimura K, Taniguchi Y, Yoshida Y, Kihira K, Takimoto T, Kawata H, Saifuku K, Ido K, Takemoto T. Distribution of inflammation and atrophy in the stomach of Helicobacter pylori-positive and -negative patients with chronic gastritis. Am J Gastroenterol. 1996;91:963-969. [PubMed] |
| 25. | Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995;42:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. [PubMed] |
| 27. | Sipponen P, Kosunen TU, Valle J, Riihelä M, Seppälä K. Helicobacter pylori infection and chronic gastritis in gastric cancer. J Clin Pathol. 1992;45:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 5.1] [Reference Citation Analysis (0)] |