Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9282
Revised: July 12, 2013
Accepted: July 23, 2013
Published online: December 28, 2013
Processing time: 263 Days and 3 Hours
AIM: To assess tumour regression grade (TRG) and lymph node downstaging to help define patients who benefit from neoadjuvant chemotherapy.
METHODS: Two hundred and eighteen consecutive patients with adenocarcinoma of the esophagus or gastro-esophageal junction treated with surgery alone or neoadjuvant chemotherapy and surgery between 2005 and 2011 at a single institution were reviewed. Triplet neoadjuvant chemotherapy consisting of platinum, fluoropyrimidine and anthracycline was considered for operable patients (World Health Organization performance status ≤ 2) with clinical stage T2-4 N0-1. Response to neoadjuvant chemotherapy (NAC) was assessed using TRG, as described by Mandard et al. In addition lymph node downstaging was also assessed. Lymph node downstaging was defined by cN1 at diagnosis: assessed radiologically (computed tomography, positron emission tomography, endoscopic ultrasonography), then pathologically recorded as N0 after surgery; ypN0 if NAC given prior to surgery, or pN0 if surgery alone. Patients were followed up for 5 years post surgery. Recurrence was defined radiologically, with or without pathological confirmation. An association was examined between t TRG and lymph node downstaging with disease free survival (DFS) and a comprehensive range of clinicopathological characteristics.
RESULTS: Two hundred and eighteen patients underwent esophageal resection during the study interval with a mean follow up of 3 years (median follow up: 2.552, 95%CI: 2.022-3.081). There was a 1.8% (n = 4) inpatient mortality rate. One hundred and thirty-six (62.4%) patients received NAC, with 74.3% (n = 101) of patients demonstrating some signs of pathological tumour regression (TRG 1-4) and 5.9% (n = 8) having a complete pathological response. Forty four point one percent (n = 60) had downstaging of their nodal disease (cN1 to ypN0), compared to only 15.9% (n = 13) that underwent surgery alone (pre-operatively overstaged: cN1 to pN0), (P < 0.0001). Response to NAC was associated with significantly increased DFS (mean DFS; TRG 1-2: 5.1 years, 95%CI: 4.6-5.6 vs TRG 3-5: 2.8 years, 95%CI: 2.2-3.3, P < 0.0001). Nodal down-staging conferred a significant DFS advantage for those patients with a poor primary tumour response to NAC (median DFS; TRG 3-5 and nodal down-staging: 5.533 years, 95%CI: 3.558-7.531 vs TRG 3-5 and no nodal down-staging: 1.114 years, 95%CI: 0.961-1.267, P < 0.0001).
CONCLUSION: Response to NAC in the primary tumour and in the lymph nodes are both independently associated with improved DFS.
Core tip: Predictive markers of benefit from neoadjuvant chemotherapy (NAC) in esophageal adenocarcinoma are urgently required to provide a “personalised medicine” approach: directing treatment to those most likely to benefit. Before prospective studies can be initiated, retrospective series need to be interrogated to identify likely candidate markers of a positive response. In defining a positive response attention needs to be given to both response in the primary tumour and in the lymph nodes, as a previously unidentified group of patients who appear to have a poor tumoural response to NAC (tumour regression grade 3-5) do benefit from combination therapy by nodal downstaging.
- Citation: Noble F, Nolan L, Bateman AC, Byrne JP, Kelly JJ, Bailey IS, Sharland DM, Rees CN, Iveson TJ, Underwood TJ, Bateman AR. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J Gastroenterol 2013; 19(48): 9282-9293
- URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9282.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9282
Neoadjuvant therapy followed by surgery is established as the gold standard in the management of patients with locally advanced adenocarcinoma of the esophagus/esophagogastric junction. In the United Kingdom neoadjuvant chemotherapy (NAC) in conjunction with transthoracic esophagogastrectomy is the current standard of care for these patients[1]. The potential benefits of neoadjuvant therapy include: downstaging of the primary tumour[2] and lymph nodes[3], an increase in the resectability of the tumour[4], elimination of micrometastases[5] and improved survival[6]. A recently suggested advantage of neoadjuvant therapy and early assessment of response is the potential for assessing in vivo the chemosensitivity of the tumour and so providing information to tailor multimodal therapy[7]. Both NAC and surgery are associated with considerable morbidity and mortality[8] and evidence remains inconsistent for the survival benefit for patients who undergo NAC[4,8,9]. The most recent meta-analysis to compare NAC vs surgery alone in 2062 patients suggests a 5.1% survival advantage at 2 years for patients treated with NAC for adenocarcinoma[6]. Patients who have a significant pathological response to neoadjuvant therapy have consistently been shown to have improved survival when compared to patients who have not had a significant response[10-13]. For those patients who do not have a significant pathological response, the consequences of delay to surgery and the benefits of neoadjuvant chemotherapy are not known. Furthermore, it is unclear which patients should be considered for tailored adjuvant systemic therapy or alternative neoadjuvant therapy.
The pathological response to chemotherapy is most widely assessed using Tumour Regression Grading (TRG)[1] as described by Mandard et al[14] although this has not gained universal acceptance[15]. This system is based on the amount of residual tumour and the degree of fibrosis at the primary tumour[14]. Other proposed pathological systems for measuring neoadjuvant treatment response include complete pathological response[16], size of residual tumour[17], number of residual tumour cells[15,18], response classification system[19], size based pathological response[17] and downstaging of cT and cN stage[10]. These grading systems have predominately been developed following chemoradiotherapy with heterogeneous histology with few studies assessing their utility following chemotherapy in patients with esophageal adenocarcinoma[2,20-23]. A number of clinically important questions could be addressed by a robust and universally accepted measure of response to neoadjuvant treatment including: the ability to accurately predict an individual patient’s tumour response to preoperative therapy leading to non-responders proceeding directly to surgery or being considered for alternative neoadjuvant regimes; assessment of new neoadjuvant regimes, and identification of patients who are likely to benefit from adjuvant therapy.
We have therefore assessed pathological response to neoadjuvant chemotherapy by assessing the tumour response as well as the response in the lymph nodes in a large contemporary cohort of patients with esophagogastric adenocarcinoma managed with neoadjuvant platinum based triplet chemotherapy, and describe their associations with short- and long-term outcomes. In addition we suggest combining both local tumour and nodal responses to NAC.
For this retrospective study, a prospectively collected database of consecutive patients undergoing esophagogastric resection treated at University Hospital Southampton National Health Service Foundation Trust (UHSFT) between January 2005 and December 2011 was reviewed. All patients were discussed at a specialist multidisciplinary team meeting (MDT). Standard staging investigations included endoscopic ultrasonography, high-resolution computed tomography, integrated fluorodeoxyglucose positron emission tomography/computed tomography (PET-CT) and staging laparoscopy, where indicated and were uniformly applied during the study interval. Patients considered suitable for potential surgical resection with tumours staged as T2N0M0 or above were considered for neoadjuvant chemotherapy.
Neoadjuvant chemotherapy consisted of three 21 d cycles of anthracycline, platinum and fluoropyrimidine: ECF (epirubicin 50 mg/m2, cisplatin 60 mg/m2, both intravenously on 1 d and protracted venous infusion 5-FU 200 mg/m2 per day) or ECX (epirubicin 50 mg/m2, cisplatin 60 mg/m2, both intravenously on 1 d and capecitabine 625 mg/m2 orally twice daily for 21 d) or EOX (epirubicin 50 mg/m2iv bolus and oxaliplatin 130 mg/m2iv infusion over 2 h on 1 d, capecitabine 625 mg/m2 orally twice daily for 21 d).
Surgery was performed at UHSFT after initial staging or 4-6 wk following neoadjuvant chemotherapy. A repeat CT scan was performed, prior to surgery, for those who received chemotherapy to assess their response to chemotherapy and disease operability. Types of esophagogastrectomies included Ivor Lewis, left thoracoabdominal with or without cervical anastomosis and transhiatal esophagogastrectomy or minimally invasive esophagogastrectomy (MIO) either 2 stage (MIO-2) or 3 stage (MIO-3) in accordance with recommendations arising from the consensus statement from the Association of Upper Gastrointestinal Surgeons and the Association of Laparoscopic Surgeons for introduction of MIO[24].
Data recorded included demographics, tumour characteristics, resection type, estimated blood loss (calculated from suction bottles and weighed swabs) and histopathological analysis of the surgical specimen. TNM-7 (International Union Against Cancer TNM Classification 7th Edition) was used to report tumour stage after analysis of pathology reports[25]. Pathological tumour clearance (“R”-status) was determined according the Royal College of Pathologists’ guidance.
Postoperative complications were graded according to the Clavien-Dindo (CD) classification[26]. An AL was defined as a leak sufficient to cause symptoms and confirmed by radiology (contrast enhanced multi-detector CT scan with on-table oral contrast or water soluble contrast studies), endoscopy or during surgical exploration.
All patients were cared for by a specialist esophagogastric team who applied a similar perioperative regime to all patients. Patients were routinely followed-up for 5 years post surgery according to the following protocol: 2-4 wk post-discharge, 3 monthly for 1 year, 6 monthly for 2 years and yearly thereafter. Patients were also seen on an “as required” basis if symptomatic. Recurrence of disease during follow-up was defined as the first site or sites of recurrence with radiological or pathological confirmation. For assessment of disease free survival (DFS), recurrence was defined as time from operation to development of local, nodal (regional) and distant metastasis (whichever occurred first).
Pathological response to chemotherapy was assessed using the TRG system developed by Mandard et al[14] who scored regression based on the degree of fibrosis and residual cancer cells (TRG 1-5)[14,27], see Table 1. All dissected lymph nodes were stained with hematoxylin and eosin and microscopically analysed for metastatic disease. TRG was scored by specialist gastrointestinal pathologists; initially by one pathologist (Bateman AC) prior to its introduction by all pathologists as part of routine pathological reporting.
Descriptive data are represented as median and range unless indicated with Kruskal-Wallis, Mann Whitney U, P and χ2 test, which were used as appropriate for comparison. Kaplan-Meier, univariate and multivariate cox logistic regression modelling were used to assess the relationship between pathological response grading systems with DFS. All factors that showed statistical significance on univariate analysis were entered to derive the final model. DFS curves of the patients were plotted by using the Kaplan-Meier method and analysed using the Log-rank test. Stratified analyses were performed based on receipt of neoadjuvant chemotherapy, nodal stage and response to chemotherapy. A P < 0.05 was considered statistically significant for all tests. Statistical analysis was performed with SPSS® version 19 (SPSS, Chicago, Illinois, United States).
A total of 218 patients underwent esophageal resection during the study interval with a mean follow up of 3 years (median follow up: 2.552, 95%CI: 2.022-3.081). There was a 1.8% (n = 4) inpatient mortality rate. Detailed patient characteristics and clinical and pathological outcomes are summarised in Table 2, grouped by treatment.
| Characteristic | Surgery only 82 (37.6) | Neoadjuvant chemotherapy and surgery 136 (62.4) | Pvalue | |
| Preoperative status | ||||
| Age (range)1 yr | 74.32 (42.08-85.41) | 63.76 (32.77-81.28) | < 0.0001 | |
| Sex ratio (M:F)1 | 68 (82.9):14 (17.1) | 118 (86.8):18 (13.2) | 0.439 | |
| cT stage | 1 | 17 (20.7) | 0 (0.0) | < 0.0001 |
| 2 | 30 (36.6) | 16 (16.0) | ||
| 3 | 34 (41.5) | 114 (84.0) | ||
| 4 | 1 (1.2) | 6 (4.4) | ||
| cN stage | 0 | 36 (43.9) | 19 (14.0) | < 0.0001 |
| 1 | 46 (56.1) | 117 (86.0) | ||
| cM stage | 0 | 80 (97.6) | 134 (98.5) | 0.613 |
| 1 | 1 (2.4) | 2 (1.4) | ||
| Performance status | 0 | 8 (11.6) | 35 (25.7) | 0.001 |
| 1 | 51 (73.9) | 96 (70.6) | ||
| 2 | 10 (14.5) | 5 (3.7) | ||
| ASA | 1 | 3 (3.7) | 11 (8.1) | 0.005 |
| 2 | 56 (68.3) | 106 (78.5) | ||
| 3 | 23 (28) | 18 (13.3) | ||
| O-POSSUM | 18 (12-30) | 16 (12-26) | < 0.0001 | |
| Tumour site | Middle 1/3 | 1 (1.2) | 1 (0.7) | 0.418 |
| Lower 1/3 | 32 (39) | 57 (41.9) | ||
| GEJ-S1 | 19 (23.2) | 23 (16.9) | ||
| GEJ-S2 | 18 (22.0) | 34 (25.0) | ||
| GEJ-S3 | 12 (14.6) | 20 (14.7) | ||
| Operative outcomes | ||||
| Length of operation (min)1 | 255 (120-480) | 261 (120-471) | 0.409 | |
| Blood loss (mL)1 | 300 (0-2200) | 318 (0-3000) | 0.429 | |
| Clavien Dindo Max | 0 | 26 (31.7) | 53 (39.3) | 0.59 |
| 1 | 5 (6.1) | 8 (5.9) | ||
| 2 | 35 (42.7) | 40 (29.6) | ||
| 3 | 6 (7.3) | 17 (12.6) | ||
| 4 | 6 (7.3) | 17 (12.6) | ||
| 5 | 4 (4.9) | 0 (0) | ||
| Anastomotic leaks | 8 (9.8) | 9 (6.7) | 0.413 | |
| Pathological outcomes | ||||
| pT or ypT | 0 | 3 (3.6) | 8 (5.9) | 0.692 |
| 1 | 23 (28) | 23 (16.9) | ||
| 2 | 17 (20.7) | 34 (25) | ||
| 3 | 34 (41.5) | 66 (48.5) | ||
| 4 | 5 (6.1) | 5 (3.7) | ||
| pN or ypN | 0 | 40 (48.8) | 73 (53.7) | 0.758 |
| 1 | 20 (24.4) | 21 (15.4) | ||
| 2 | 11 (13.4) | 25 (18.4) | ||
| 3 | 11 (13.4) | 17 (12.5) | ||
| pM or ypM | 0 | 82 (100) | 136 (100) | 1.00 |
| Tumour regression grade | 1 | - | 8 (5.8) | n/a |
| 2 | - | 28 (20.6) | ||
| 3 | - | 20 (14.7) | ||
| 4 | - | 45 (33.1) | ||
| 5 | - | 35 (25.7) | ||
| Nodal downstaged (cN1 to p or ypN1) | 13 (15.9) | 60 (44.1) | < 0.0001 | |
| Positive nodes1 | 1 (0-21) | 0 (0-24) | 0.789 | |
| Nodal yield1 | 18 (4-49) | 18 (3-53) | 0.242 | |
| Resection clearance | R0 | 65 (79.3) | 110 (80.9) | 0.772 |
| Vascular invasion | 24 (29.3) | 41 (30.1) | 0.891 | |
| Lymphatic invasion | 9 (11) | 22 (16.2) | 0.28 | |
| Perineural invasion | 8 (9.8) | 20 (14.7) | 0.291 | |
| Maximum tumour diameter (mm)1 | 25 (0-90) | 25 (0-155) | 0.998 | |
| Morphology | Ulcer | 48 (60) | 96 (74.4) | 0.029 |
| Polypoid | 22 (27.5) | 23 (17.8) | ||
| Fungating | 2 (2.5) | 3 (2.3) | ||
| Diffuse infiltrating | 8 (10) | 7 (5.4) | ||
| Grade | G1 | 6 (7.3) | 16 (11.8) | 0.669 |
| G2 | 30 (36.6) | 37 (27.2) | ||
| G3 | 46 (56.1) | 82 (60.3) | ||
| G4 | 0 (0) | 1 (0.7) | ||
| Sites of recurrence | Local | 3 (3.7) | 8 (5.9) | 0.461 |
| Nodal | 5 (6.1) | 14 (10.4) | 0.281 | |
| Distant | 18 (22.0) | 44 (32.6) | 0.093 |
Patients who underwent surgery alone (n = 82; 37.6%) were significantly older (P < 0.0001), had worse physiological status (ASA P = 0.005; performance status P = 0.001; O-POSSUM P < 0.0001) and lower preoperative staged disease (cT stage P < 0.0001; cN stage P < 0.0001) compared to patients that underwent multimodal therapy.
One hundred thirty-six (62.4%) patients received multimodal therapy, neoadjuvant chemotherapy and surgery, with 74.3% (n = 101) of patients demonstrating some signs of pathological tumour regression (TRG 1-4) with 5.9% (n = 8) having a complete pathological response. Forty four point one percent (n = 60) had downstaging of their nodal stage compared to only 15.9% (n = 13) whose lymph node status was cN1 on preoperative staging and pN0 following surgery alone (P < 0.0001).
There were no statistically significant differences in postoperative pathological tumour stage (yp or pT, P = 0.692); yp or pN P = 0.758), postoperative complications (CD maximum grade, P = 0.590) or completeness of resection (P = 0.772) in patients that underwent multimodal therapy vs surgery alone.
The relationship between patient and tumour characteristics and response to neoadjuvant chemotherapy, as defined by tumour regression grade, are presented in Table 3.
| TRG 1-2 36 (26.5) | TRG 3-5 100 (73.5) | Pvalue | ||
| Preoperative status | ||||
| Age (range) yr1 | 65.27 (26.99-76.04) | 63.51 (32.77-81.28) | 0.410 | |
| Sex ratio (M:F)1 | 32 (88.9):4 (11.1) | 86 (86):14 (14) | 0.662 | |
| cT stage | 1 | 0 (0) | 0 (0) | 0.396 |
| 2 | 2 (5.6) | 14 (14) | ||
| 3 | 33 (91.7) | 81 (81) | ||
| 4 | 1 (2.8) | 5 (5) | ||
| cN stage | 0 | 5 (13.9) | 14 (14) | 0.987 |
| 1 | 31 (86.1) | 86 (86) | ||
| cM stage | 0 | 35 (97.1) | 99 (99) | 0.456 |
| 1 | 1 (2.8) | 1 (1) | ||
| Performance status | 0 | 12 (33.3) | 23 (23) | 0.225 |
| 1 | 23 (63.9) | 73 (73) | ||
| 2 | 1 (2.8) | 4 (4.0) | ||
| ASA | 1 | 2 (5.6) | 9 (9.1) | 0.408 |
| 2 | 32 (88.9) | 74 (74.7) | ||
| 3 | 2 (5.6) | 16 (16.2) | ||
| O-POSSUM | 15 (12-23) | 16 (12-26) | 0.476 | |
| Tumour site | Middle 1/3 | 1 (2.8) | 0 (0) | 0.738 |
| Lower 1/3 | 15 (41.7) | 42 (42) | ||
| GEJ-S1 | 7 (19.4) | 16 (16) | ||
| GEJ-S2 | 9 (25) | 25 (25) | ||
| GEJ-S3 | 4 (11.1) | 16 (16) | ||
| Operative outcomes | ||||
| Length of operation (min)1 | 262 (163-427) | 260 (120-471) | 0.513 | |
| Blood loss (mL)1 | 300 (0-3000) | 325 (0-1700) | 0.673 | |
| Clavien Dindo Max | 0 | 14 (38.9) | 39 (39.4) | 0.531 |
| 1 | 2 (5.6) | 6 (6.1) | ||
| 2 | 14 (38.9) | 26 (26.3) | ||
| 3 | 4 (11.1) | 13 (13.1) | ||
| 4 | 2 (5.6) | 15 (15.2) | ||
| 5 | 0 (0) | 0 (0) | ||
| Anastomotic leaks | 1 (2.8) | 8 (8.1) | 0.276 | |
| Pathological outcomes | ||||
| yPT | 0 | 8 (22.2) | 0 (0) | < 0.0001 |
| 1 | 11 (30.6) | 12 (12) | ||
| 2 | 9 (25) | 25 (25) | ||
| 3 | 8 (22.2) | 58 (58) | ||
| 4 | 0 (0) | 5 (5) | ||
| yPN | 0 | 34 (94.4) | 39 (39) | < 0.0001 |
| 1 | 0 (0) | 21 (21) | ||
| 2 | 2 (5.6) | 23 (23) | ||
| 3 | 0 (0) | 17 (17) | ||
| yPM | 0 | 36 (100) | 100 (100) | 0.579 |
| Nodal downstaged (cN1 to ypN0) | 30 (83.3) | 30 (30) | < 0.0001 | |
| Positive nodes1 | 0 (0-5) | 1 (0-24) | < 0.0001 | |
| Nodal yield1 | 18 (4-25) | 18 (3-53) | 0.984 | |
| Resection clearance | R0 | 35 (97.2) | 75 (75) | 0.004 |
| Vascular invasion | 4 (11.1) | 37 (37) | 0.004 | |
| Lymphatic invasion | 4 (11.1) | 18 (18) | 0.338 | |
| Perineural invasion | 2 (5.6) | 18 (18) | 0.072 | |
| Maximum tumour diameter1 | (mm) | 15 (0-110) | 30 (0-155) | < 0.0001 |
| Morphology | Ulcer | 30 (93.8) | 66 (68) | 0.003 |
| Polypoid | 2 (6.3) | 21 (21.6) | ||
| Fungating | 0 (0) | 3 (3.1) | ||
| Diffuse infiltrating | 0 (0) | 7 (7.2) | ||
| Grade | G1 | 8 (22.2) | 8 (8) | 0.104 |
| G2 | 9 (25) | 28 (28) | ||
| G3 | 19 (52.8) | 63 (63) | ||
| G4 | 0 (0) | 1 (1) | ||
| Sites of recurrence | Local | 0 (0) | 8 (8.1) | 0.080 |
| Nodal | 1 (2.8) | 13 (13.1) | 0.082 | |
| Distant | 2 (5.6) | 42 (42.4) | < 0.0001 |
Of the 136 patients that underwent NAC, 36 (26.5%) patients had a significant pathological response (TRG 1-2; responders) compared to 100 (73.5%) patients with no significant pathological response (TRG 3-5; non-responders). Responders and non-responders had similar preoperative clinical features (age, sex and physiological status) and clinical stage of disease (cT stage, P = 0.396; cN stage, P = 0.987; cM stage, P = 0.456), yet responders had markedly reduced ypT stage (P < 0.0001), maximal pathological tumour diameter (P < 0.0001), and ypN stage (P < 0.0001) and were more likely to have their nodal stage downstaged (P < 0.0001) compared to non-responders (Table 3). In addition, responders had tumours that were more likely to be ulcers (P = 0.003), showing less vascular (P = 0.004), and perineural invasion (P = 0.072) compared to non-responders. Complete resection (R0) was achieved in 97.2% (n = 35) of responders compared with 75% (n = 75) of non-responders (P = 0.04). There was no significant difference in postoperative complications as classified by the Clavien Dindo system, nodal yield, blood loss or operative time between groups.
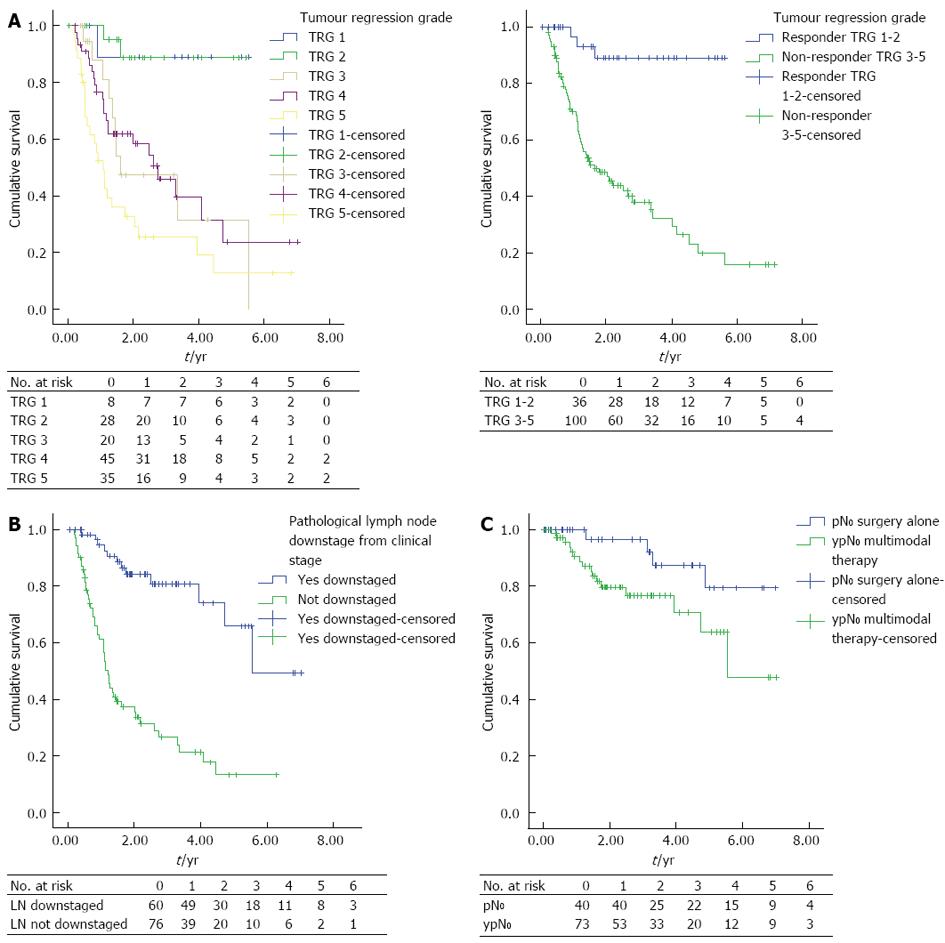
There was a significant difference in survival between responders compared to non-responders, shown in Figure 1A [mean DFS; TRG 1-2: 5.064 years, 95%CI: 4.560-5.569 (median DFS: not reached) vs TRG 3-5: 2.759 years, 95%CI: 2.193-3.325 (median DFS: 1.613, 95%CI: 0.834-2.39), P < 0.0001].
There was no statistically significant difference in survival between patients graded as TRG 1 compared to TRG 2 [mean DFS; TRG-1: 5.021, 95%CI: 4.069-5.973 vs TRG-2: 4.983, 95%CI: 4.069-5.973, P < 0.0001 (median DFS’s: not reached)].
Patients with lymph node downstaging following NAC had improved DFS vs patients without downstaging, Figure 1B [median DFS; lymph node (LN) downstaged: 5.316 years, 95%CI: 4.504-6.127 (median DFS: 5.544) vs LN not downstaged: 2.118 years, 95%CI: 1.594-2.643 (median DFS: 1.210, 95%CI: 1.026-1.394), P < 0.0001].
Univariate and multivariate analysis confirmed known predictors of DFS in esophageal adenocarcinoma (OAC) that are detailed in Table 4. Factors that retained significance for the prediction of worse DFS on multivariate analysis were: vascular invasion (HR = 1.929, 95%CI: 1.034-3.6, P = 0.039), perineural invasion (HR = 2.766, 95%CI: 1.444-5.3, P = 0.002), no significant response to NAC (HR = 6.315, 95%CI: 1.261-31.616, P = 0.025) and the absence of lymph node downstaging (HR = 6.161, 95%CI: 1.683-22.554, P = 0.006).
| Univariate | Multivariate | ||||||
| HR | 95%CI | Pvalue | HR | 95%CI | Pvalue | ||
| Patient factors | |||||||
| Age | 0.972 | (0.944-1.00) | 0.054 | ||||
| Sex | Female | 1.000 | Ref | ||||
| Male | 0.953 | (0.453-2.005) | 0.899 | ||||
| ASA | 1 | 1.000 | Ref | ||||
| 2 | 0.696 | (0.313-1.548) | 0.374 | ||||
| 3 | 0.947 | (0.352-2.546) | 0.914 | ||||
| Performance status | 0 | 1.000 | Ref | ||||
| 1 | 1.016 | (0.578-1.789) | 0.955 | ||||
| 2 | 0.950 | (0.218-4.129) | 0.945 | ||||
| O-POSSUM | |||||||
| Tumour response | |||||||
| TRG | 1 | 1.000 | Ref | ||||
| 2 | 1.099 | (0.099-12.148) | 0.939 | ||||
| 3 | 8.404 | (1.071-65.929) | 0.043 | ||||
| 4 | 7.829 | (1.054-58.163) | 0.044 | ||||
| 5 | 15.422 | (2.083-114.189) | 0.007 | ||||
| TRG grouped | 1-2 | 1.000 | Ref | 1.000 | Ref | ||
| 3-5 | 9.504 | (2.973-30.380) | < 0.0001 | 6.315 | (1.261-31.616) | 0.025 | |
| Lymph node response | |||||||
| Lymph nodes downstaged | Yes | 1.000 | Ref | 1.000 | Ref | ||
| No | 5.784 | (3.064-10.919) | < 0.0001 | 6.161 | (1.683-22.554) | 0.006 | |
| Tumour factors | |||||||
| ypT stage | 0 | 1.000 | Ref | 1.000 | Ref | ||
| 1 | 2.085 | (0.232-18.711) | 0.512 | 0.281 | (0.020-3.928) | 0.345 | |
| 2 | 5.214 | (0.687-39.549) | 0.110 | 0.286 | (0.022-3.705) | 0.338 | |
| 3 | 9.490 | (1.293-69.635) | 0.027 | 0.469 | (0.034-6.460) | 0.571 | |
| 4 | 52.907 | (6.008-465.873) | < 0.0001 | 1.519 | (0.087-26.389) | 0.774 | |
| ypN stage | 0 | 1.000 | Ref | 1.000 | Ref | ||
| 1 | 4.791 | (2.434-9.431) | < 0.0001 | 0.476 | (0.133-1.700) | 0.253 | |
| 2 | 4.102 | (2.005-8.392) | < 0.0001 | 0.254 | 0.070-0.927) | 0.038 | |
| 3 | 7.449 | (3.522-15.756) | < 0.0001 | 0.476 | (0.129-1.755) | 0.265 | |
| ypM stage | 0 | 1.000 | Ref | 1.000 | Ref | ||
| 1 | 3.172 | (1.253-8.031) | 0.015 | 2.693 | (0.924-7.847) | 0.069 | |
| Vascular invasion | No | 1.000 | Ref | 1.000 | Ref | ||
| Yes | 3.444 | (2.080-5.702) | < 0.0001 | 1.929 | (1.034-3.600) | 0.039 | |
| Lymphatic invasion | No | 1.000 | Ref | 1.000 | Ref | ||
| Yes | 2.201 | (1.268-3.821) | 0.005 | 1.253 | (0.637-2.462) | 0.514 | |
| Perineural invasion | No | 1.000 | Ref | 1.000 | Ref | ||
| Yes | 5.073 | (2.896-8.886) | < 0.0001 | 2.766 | (1.444-5.300) | 0.002 | |
| Resection clearance | R0 | 1.000 | Ref | 1.000 | Ref | ||
| R1 | 3.869 | (2.272-6.588) | < 0.0001 | 1.805 | (0.940-3.468) | 0.076 | |
Patients with no pathological lymph node involvement were compared (pN0vs ypN0), grouped as those who had surgery alone (pN0) vs multimodal therapy (ypN0), with detailed clinical and pathological characteristics presented in Table 5 and DFS shown in Figure 1C.
| pN0 Surgery alone 40 (35.4) | ypN0 Neoadjuvant chemotherapy and surgery 73 (64.6) | Pvalue | ||
| Preoperative status | ||||
| Age (range) yr1 | 73.62 (56.73-85.41) | 65.59 (32.77-78.43) | < 0.0001 | |
| Sex ratio (M:F)1 | 31 (77.5):9 (22.5) | 66 (90.4):7 (9.8) | 0.061 | |
| cT stage | 1 | 13 (32.5) | 0 (0) | < 0.0001 |
| 2 | 17 (42.5) | 9 (12.3) | ||
| 3 | 10 (25) | 61 (83.6) | ||
| 4 | 0 (0) | 3 (4.1) | ||
| cN stage | 0 | 27 (67.5) | 13 (17.8) | < 0.0001 |
| 1 | 13 (32.5) | 60 (82.2) | ||
| cM stage | 0 | 40 (100) | 71 (97.3) | 0.293 |
| 1 | 0 (0) | 2 (2.8) | ||
| Performance status | 0 | 3 (9.4) | 16 (21.9) | 0.045 |
| 1 | 25 (78.1) | 54 (74) | ||
| 2 | 4 (12.5) | 3 (4.1) | ||
| ASA | 1 | 2 (5) | 6 (8.2) | 0.268 |
| 2 | 31 (77.5) | 59 (80.8) | ||
| 3 | 7 (17.5) | 8 (11) | ||
| O-POSSUM1 | 17 (14-29) | 16 (12-26) | 0.015 | |
| Tumour site | Middle 1/3 | 0 (0) | 1 (1.4) | 0.190 |
| Lower 1/3 | 15 (37.5) | 35 (47.9) | ||
| OGJ-S1 | 11 (27.5) | 12 (16.4) | ||
| OGJ-S2 | 8 (20) | 16 (21.9) | ||
| OGJ-S3 | 6 (15) | 9 (12.3) | ||
| Operative outcomes | ||||
| Length of operation (min)1 | 240 (120-360) | 278 (120-471) | 0.082 | |
| Blood loss (mL)1 | 200 (0-2200) | 350 (0-3000) | 0.167 | |
| Clavien Dindo Max | 0 | 14 (35) | 24 (32.9) | 0.709 |
| 1 | 1 (2.5) | 3 (4.1) | ||
| 2 | 17 (42.5) | 27 (37) | ||
| 3 | 4 (10) | 10 (13.7) | ||
| 4 | 2 (5) | 9 (12.3) | ||
| 5 | 2 (5) | 0 (0) | ||
| Anastomotic leaks | 4 (10) | 7 (9.6) | 0.944 | |
| Pathological outcomes | ||||
| TRG 1-2 | - | 34 (46.6) | NA | |
| TRG 3-5 | - | 39 (53.4) | ||
| pT or ypT | 0 | 2 (5) | 11 (15.1) | 0.224 |
| 1 | 22 (55) | 20 (27.4) | ||
| 2 | 5 (12.5) | 20 (27.4) | ||
| 3 | 10 (25) | 24 (32.9) | ||
| 4 | 0 (0) | 1 (1.4) | ||
| Nodal Downstaged (cN1 to p or ypN0) | 15 (37.5) | 61 (83.6) | < 0.0001 | |
| Nodal yield1 | 16 (4-49) | 18 (3-52) | 0.150 | |
| Resection clearance | R0 | 35 (87.5) | 69 (94.5) | 0.189 |
| Vascular invasion | 7 (17.5) | 10 (13.7) | 0.590 | |
| Lymphatic invasion | 2 (5) | 6 (8.2) | 0.525 | |
| Perineural invasion | 2 (5) | 5 (6.8) | 0.698 | |
| Maximum tumour diameter (mm)1 | 24 (0-50) | 24 (0-110) | 0.324 | |
| Morphology | Ulcer | 25 (65.8) | 53 (79.1) | 0.135 |
| Polypoid | 10 (26.3) | 11 (16.4) | ||
| Fungating | 1 (2.6) | 1 (1.5) | ||
| Diffuse infiltrating | 2 (5.3) | 2 (3) | ||
| Grade | G1 | 4 (10) | 13 (17.8) | 0.811 |
| G2 | 17 (42.5) | 20 (27.4) | ||
| G3 | 19 (47.5) | 40 (54.8) | ||
| G4 | 0 (0) | 0 (0) | ||
| Site of recurrence | Local | 0 (0) | 2 (2.7) | 0.293 |
| Nodal | 1 (2.5) | 4 (5.5) | 0.463 | |
| Distant | 3 (7.5) | 12 (16.4) | 0.182 |
For patients with no evidence of pathological lymph node involvement increased pre-operative clinical stage (cT stage, P < 0.0001; cN stage, P < 0.0001) of disease and increased nodal downstaging (NAC 83.6% vs surgery alone 37.5%, P < 0.0001) was observed in patients who received multimodal therapy vs surgery alone despite pathological stage being similar (yp or pT stage, P = 0.224; yp or pN stage, P = 1.00).
Patients who underwent surgery alone (pN0) had increased DFS compared to patients who underwent NAC and surgery (ypN0) (mean DFS; pN0: 6.285 years, 95%CI: 5.647-6.923 vs ypN0: 5.102 years, 95%CI: 4.314-5.891 (median DFS’s: not reached, P = 0.042).
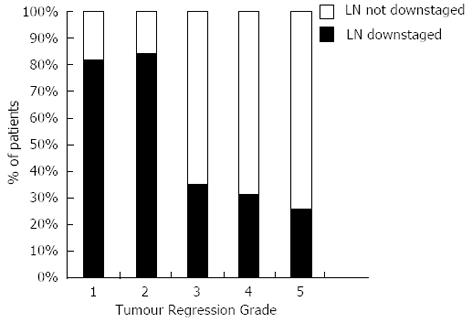
Eighty-three point three percent of responders’ additionally demonstrated downstaging of their regional lymph nodes compared to only 30% of non-responders, spread across TRG 3-5, Figure 2.
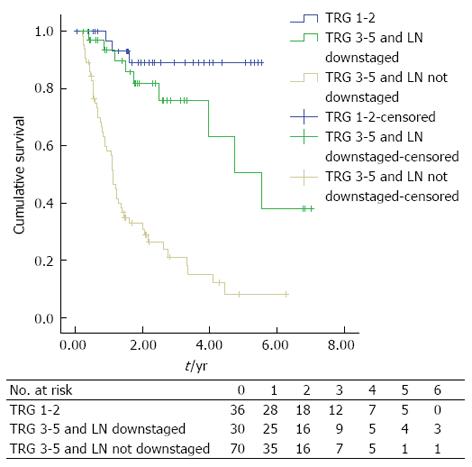
The presence of lymph node downstaging in apparent non-responders was associated with significantly improved DFS (median DFS; TRG 3-5 and nodal down-staging: 5.544, 95%CI: 3.558-7.531 vs TRG 3-5 and LN not downstaged: 1.114, 95%CI: 0.961-1.267, P < 0.0001), Figure 3.
Neoadjuvant treatment for esophageal cancer is associated with increased survival. However, it is clear that not all patients (and their tumours) respond to neoadjuvant therapy in the same way. It is likely that improved outcomes will be observed by the tailoring of neoadjuvant and adjuvant therapy based on patient stratification according to tumour response.
In this study we have analysed a consecutive cohort of patients with esophageal adenocarcinoma (OAC) undergoing treatment with curative intent to assess the primary tumour and regional lymph node response to NAC. We have described three main findings: firstly, we have confirmed that a significant pathological response as described by Mandard et al[14] is associated with improved DFS; Secondly we have confirmed that lymph node downstaging leads to improved DFS[10]; Thirdly, and most importantly, we describe that when tumour and nodal response are combined, a group of patients who previously would have been classified as non-responders to NAC actually have significantly increased DFS.
There is considerable debate regarding the role of tumour regression in OAC. Conflicting opinions are evident, for what represents a significant tumour response, even within the TRG grading system. In our study TRG-3 tumours, despite representing tumours whose fibrosis outgrows the residual tumour, clearly grouped with TRG-4 and TRG-5 and not TRG-1 and TRG-2 tumours in terms of DFS. This is in keeping with previous studies that have observed a significant increase in survival and/or metabolic response on serial PET imaging for TRG groups 1 and 2 compared to TRG groups 3 to 5[14,18,19,22,28,29]. In addition, we found there to be no significant difference in DFS between complete pathological responders (TRG-1) vs major responders (TRG-2) consistent with other studies[14,22]. As has been previously suggested this may reflect a type II error due to insufficient sample size or the intensity of pathological sampling[22]. The observed increase in DFS in patients with a significant tumour response to NAC in this study may also reflect the significantly increased resectability (R0 rate) of the primary tumour. It may also reflect the selection of tumours that are biologically more favourable as suggested by reduced vascular invasion (P = 0.004), tumour morphology (P = 0.003) and increased lymph node downstaging (P < 0.0001).
In this study we confirmed the association between lymph node downstaging after NAC and improved DFS[10]. Bollschweiler et al[3] showed regression in lymph nodes, such as central fibrosis, to predict improved survival and response to chemoradiotherapy[3]. This would require additional pathological time and expertise whereas downstaging can be more simply assessed from the data available to the multidisciplinary team (MDT) after surgery, to assess a patient’s prognosis and potential for adjuvant therapies. The number of positive lymph nodes is consistently the most important prognostic factor associated with survival[30]. However, the clinical significance of downstaging is controversial due to the difficulties in evaluating preoperative status. This study has the advantage of using contemporary and uniformly implemented clinical staging based on current United Kingdom practice. The comparison of nodal stage based on pre-operative staging assessment (cN) and post-operative pathology (pN) is open to the criticism that any downstaging simply reflects overdiagnosis of lymph node metastases on preoperative staging. To address this point we assessed the survival of patients with no positive lymph nodes in the pathological specimen, comparing NAC with surgery alone (ypN0vs pN0). We found that patients receiving NAC with ypN0 disease had reduced DFS across all sites of recurrence compared to patients treated by surgery alone with pN0 disease. This reached statistical significance when overall DFS was assessed (P = 0.042). Whilst the patients that underwent multimodal or surgery only had comparable pathological staged disease they are different based on their clinical stage and survival. It is therefore unlikely that our clinical staging was inadequate and suggests that the majority of patients with ypN0 disease in fact had lymph node metastases prior to treatment.
The increased survival observed with lymph node downstaging has important implications for the staging of OAC as neoadjuvant therapy is increasingly used. Although the final pathological stage of disease may be similar between patients treated with either multimodal therapy or surgery alone we have demonstrated that the long-term DFS of these patients are different. This would suggest revisions for the staging system for OAC to take into account the differences in outcomes for patients who have similar pathologically staged disease after multimodal therapy compared to those treated by surgery alone. This hypothesis is further supported by the results of our multivariate analysis of factors independently related to outcome in neoadjuvant chemotherapy for OAC. This showed that nodal downstaging and TRG were independent predictors of DFS but that the classical markers of disease burden, PT stage and PN stage, were only statistically significant on univariate analysis. Similar observations and suggestions have been made for patients who have undergone neoadjuvant chemoradiotherapy followed by surgery when compared to patients who underwent surgery alone[31].
There are several advantages of our study compared to other published series. This study consists of a large number of consecutive patients (n = 218) of uniform histological type, with consistent clinical and pathological staging and treatment provided over a contemporary time period. The retrospective nature of this study and the use of multiple pathologists assessing TRG on an individual basis are potential limitations. However, the data was vigorously collected prospectively and the use of multiple pathologists reflects the usefulness of TRG in clinical practice and is pragmatic. A debate also remains as to what system to use to assess a local tumour response to neoadjuvant therapy[10,11,14,15,17-19]. The use of TRG is not without controversy as significant tumour regression has been reported in patients who underwent surgery alone, in up to 13.7% of cases. It has been suggested that this reflects tumour growth within abundant stroma and/or lymphocytic infiltration leading to partial tumour regression[21]. While the association of lymphocytic infiltration and stromal features with survival in cancer is not new their association with survival in OAC is yet to be fully understood and the clinical impact is unknown[32].
Although a good pathological response of the primary tumour might be expected to represent a prognostic predictor after NAC, the low response rate observed following NAC remains problematic. In this study we observed a significant response rate of 26.5% (n = 36) as assessed by TRG. However when lymph node downstaging is also considered this proportion increases to 48.5% (n = 66). It can be hypothesised that patients who have a partial response to NAC reflected by downstaging of lymph nodes with modest or no response in the primary tumour (TRG 3-5) may be the most appropriate to be considered for trials of adjuvant treatment; as there is limited data from other disease sites to suggest only patients responding to neoadjuvant treatment benefit from further treatment[33]. This is relevant as the role of adjuvant therapy in esophageal cancer is controversial due to concerns over the additional benefit of post operative treatment over neoadjuvant alone[8] and toxicity[34], and has resulted in the lack of adoption in the United Kingdom[1]. What is clear is that the group of patients with no significant downstaging and ypN1 post neoadjuvant treatment have a particularly poor outlook. This group urgently requires identification at diagnosis and new trial treatments. This requires the ongoing studies of prognostic and predictive biomarkers from this cohort and others to yield meaningful and validated results.
One can now begin to consider an evolving algorithm for perioperative treatment of OAC that may involve induction chemotherapy followed by an early assessment of response and the curtailment of, or a change of, neoadjuvant therapy for non-responders. Further analysis of the primary tumour and lymph nodes after surgery would direct patients with modest or no tumour response (TRG 3-5) to NAC, but with nodal downstaging, to adjuvant therapy. This kind of stratified therapy will be supported by ongoing studies of biomarkers and molecular imaging. The contribution of the tumour microenvironment is also likely to offer new targets for therapy and may be the place to look to explain the different responses to therapy observed between otherwise similar tumours.
In summary, this study has shown that a response to NAC in the primary tumour and in the lymph nodes is associated with improved outcomes after surgery for adenocarcinoma of the esophageal and gastro-esophageal. A previously unidentified group of patients who appear to have a poor tumoural response to NAC (TRG 3-5) do benefit from NAC with nodal downstaging and increased DFS.
We propose that methods to assess the pathological response to NAC are refined so that both the response in the primary tumour and the regional lymph nodes is used to guide selection of tailored post operative treatment strategies, identify biomarkers of response to chemotherapy, provide prognostic information and assess multimodal therapies.
Adenocarcinoma of the esophagus and esophageal adenocarcinoma (OAC) is a significant and increasing health problem in many countries; linked to rates of obesity, smoking, gastro-esophageal reflux disease and Barrett’s oesophagus. At presentation, even in operable cases, tumours are often locally advanced (T3N1) with multi-institutional randomised studies of surgery alone giving 5 years survival rates in the order of 15%-24%. So as well as a focus on earlier detection and screening of at risk groups, clinical research has focused on adjuvant and specifically neo-adjuvant treatments prior to resection.
Neoadjuvant chemotherapy can be considered one standard of care, with a modest improvement in outcome over surgery alone; detailed in a recent meta-analysis as HR = 0.83 (95%CI: 0.71-0.95), or an absolute benefit of 5%-10% at 2 years. A key focus now is on identifying optimum neoadjuvant approaches (which chemotherapy regimens, chemoradiotherapy, small molecule inhibitors, biologic agents etc) and which patients should receive them e.g., patients with human epidermal growth factor receptor (HER)-2 over expressing tumours receiving a Trastuzumab containing regimen.
To date prognostic information for OAC has been from standard clinicopathological data, and bar HER-2 expression predictive markers of response to treatment are lacking. The authors cannot predict at diagnosis who is going to gain from neoadjuvant treatment. Globally collaborative groups have been set up to generate large clinical datasets to link patient outcomes to molecular features: groups such as the oesophageal cancer clinical and molecular stratification study group in the United Kingdom, which are beginning to highlight important molecular determinants of OAC behaviour and identify attractive targets for therapy. The expectation is this will lead to valuable prognostic information and also identify who should, and should not proceed to a particular neoadjuvant strategy.
The identification here that both T and N downstaging post neoadjuvant treatment need to be accounted for will help refine clinical datasets and provide prognostic information, as well as inform decisions concerning adjuvant treatment.
When reporting the anatomical extent of cancer after preoperative treatment has been given pathologists include the prefix “y” to the PTNM.
This study is an excellent clinical research as it confirms the association between regression grade and prognosis in a large and histologically homogenous group of patients treated with platinum based triplet chemotherapy and staged uniformly. It contains novel findings that are clinically relevant to physicians treating oesophageal cancer and assessment of both T and N responses to neoadjuvant therapy may be of relevance and interest to specialists treating other solid tumours.
P- Reviewers: Deng B, Ma JY, Shi CJ S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
| 1. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Bollschweiler E, Hölscher AH, Metzger R, Besch S, Mönig SP, Baldus SE, Drebber U. Prognostic significance of a new grading system of lymph node morphology after neoadjuvant radiochemotherapy for esophageal cancer. Ann Thorac Surg. 2011;92:2020-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 950] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 5. | Matsuyama J, Doki Y, Yasuda T, Miyata H, Fujiwara Y, Takiguchi S, Yamasaki M, Makari Y, Matsuura N, Mano M. The effect of neoadjuvant chemotherapy on lymph node micrometastases in squamous cell carcinomas of the thoracic esophagus. Surgery. 2007;141:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 7. | Ott K, Herrmann K, Krause BJ, Lordick F. The Value of PET Imaging in Patients with Localized Gastroesophageal Cancer. Gastrointest Cancer Res. 2008;2:287-294. [PubMed] |
| 8. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 9. | Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1084] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 10. | Korst RJ, Kansler AL, Port JL, Lee PC, Kerem Y, Altorki NK. Downstaging of T or N predicts long-term survival after preoperative chemotherapy and radical resection for esophageal carcinoma. Ann Thorac Surg. 2006;82:480-484; discussion 484-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, Zaninotto G, Bonavina L, Peracchia A. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Donington JS, Miller DL, Allen MS, Deschamps C, Nichols FC, Pairolero PC. Tumor response to induction chemoradiation: influence on survival after esophagectomy. Eur J Cardiothorac Surg. 2003;24:631-636; discussion 636-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, Ajani JA, Kocha W, Minsky BD, Roth JA. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719-3725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 401] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 15. | Hermann RM, Horstmann O, Haller F, Perske C, Christiansen H, Hille A, Schmidberger H, Füzesi L. Histomorphological tumor regression grading of esophageal carcinoma after neoadjuvant radiochemotherapy: which score to use? Dis Esophagus. 2006;19:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Meredith KL, Weber JM, Turaga KK, Siegel EM, McLoughlin J, Hoffe S, Marcovalerio M, Shah N, Kelley S, Karl R. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Verlato G, Zanoni A, Tomezzoli A, Minicozzi A, Giacopuzzi S, Di Cosmo M, Franceschetti I, de Manzoni G. Response to induction therapy in oesophageal and cardia carcinoma using Mandard tumour regression grade or size of residual foci. Br J Surg. 2010;97:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Brücher BL, Becker K, Lordick F, Fink U, Sarbia M, Stein H, Busch R, Zimmermann F, Molls M, Höfler H. The clinical impact of histopathologic response assessment by residual tumor cell quantification in esophageal squamous cell carcinomas. Cancer. 2006;106:2119-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Schneider PM, Baldus SE, Metzger R, Kocher M, Bongartz R, Bollschweiler E, Schaefer H, Thiele J, Dienes HP, Mueller RP. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg. 2005;242:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Fareed KR, Al-Attar A, Soomro IN, Kaye PV, Patel J, Lobo DN, Parsons SL, Madhusudan S. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer. 2010;102:1600-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Fareed KR, Ilyas M, Kaye PV, Soomro IN, Lobo DN, Parsons SL, Madhusudan S. Tumour regression grade (TRG) analyses in patients with resectable gastro-oesophageal adenocarcinomas treated with platinum-based neoadjuvant chemotherapy. Histopathology. 2009;55:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Barbour AP, Jones M, Gonen M, Gotley DC, Thomas J, Thomson DB, Burmeister B, Smithers BM. Refining esophageal cancer staging after neoadjuvant therapy: importance of treatment response. Ann Surg Oncol. 2008;15:2894-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Akita H, Doki Y, Yano M, Miyata H, Miyashiro I, Ohigashi H, Ishikawa O, Nishiyama A, Imaoka S. Effects of neoadjuvant chemotherapy on primary tumor and lymph node metastasis in esophageal squamous cell carcinoma: additive association with prognosis. Dis Esophagus. 2009;22:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Hardwick RH. A Consensus View and Recommendations on the Development and Practice of Minimally Invasive Oesophagectomy AUGIS/ALSGBI 2008. Available from: http://www.augis.org/news_guidelines/augis_reports.htm. |
| 25. | Hardwick RH; UICC. TNM Classification of malignant tumours Seventh edition. America: Wiley-Blackwell 2009; . |
| 26. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8635] [Article Influence: 539.7] [Reference Citation Analysis (0)] |
| 27. | Bateman AC, Jaynes E, Bateman AR. Rectal cancer staging post neoadjuvant therapy--how should the changes be assessed? Histopathology. 2009;54:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Swisher SG, Erasmus J, Maish M, Correa AM, Macapinlac H, Ajani JA, Cox JD, Komaki RR, Hong D, Lee HK. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschläger G, Busch R, Siewert JR, Schwaiger M. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058-3065. [PubMed] |
| 30. | Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Leers JM, Ayazi S, Hagen JA, Terterov S, Klipfel N, Oezcelik A, Abate E, Lipham JC, DeMeester SR, Banki F. Survival in lymph node negative adenocarcinoma of the esophagus after R0 resection with and without neoadjuvant therapy: evidence for downstaging of N status. J Am Coll Surg. 2009;208:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Saadi A, Shannon NB, Lao-Sirieix P, O’Donovan M, Walker E, Clemons NJ, Hardwick JS, Zhang C, Das M, Save V. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci USA. 2010;107:2177-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 34. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2437] [Article Influence: 101.5] [Reference Citation Analysis (0)] |