Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8192
Revised: October 17, 2013
Accepted: November 3, 2013
Published online: December 7, 2013
Processing time: 95 Days and 18.2 Hours
Helicobacter pylori (H. pylori) is a major etiological factor in the development of gastric cancer. Large-scale epidemiological studies have confirmed the strong association between H. pylori infection and both cancer development and progression. Interleukin-8 (IL-8) is overexpressed in gastric mucosa exposed to H. pylori. The expression of IL-8 directly correlates with a poor prognosis in gastric cancer. IL-8 is multifunctional. In addition to its potent chemotactic activity, it can induce proliferation and migration of cancer cells. In this review, we focus on recent insights into the mechanisms of IL-8 signaling associated with gastric cancer. The relationship between IL-8 and H. pylori is discussed. We also summarize the current therapeutics against IL-8 in gastric cancer.
Core tip: There is a close association between gastric cancer and Helicobacter pylori (H. pylori) infection. H. pylori upregulates interleukin-8 (IL-8) gene expression in gastric epithelial cells and the levels of IL-8 may be indicative of poor prognosis. We propose that IL-8 overexpression induced by H. pylori plays a major role in gastric cancer development and progression, and that targeting IL-8 may be a promising strategy for gastric cancer treatment.
-
Citation: Lee KE, Khoi PN, Xia Y, Park JS, Joo YE, Kim KK, Choi SY, Jung YD.
Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol 2013; 19(45): 8192-8202 - URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8192
Gastric cancer has affected humans for millennia. The risk of gastric cancer appears to evolve over a lifetime as a possible result of changes in diet and lifestyle. In 1984, Marshall and Warren were first to describe the association between peptic ulcer disease and Helicobacter pylori (H. pylori)[1]. H. pylori was subsequently causally linked with the development of gastric cancer.
Despite the improved prognosis of gastric cancer resulting from the early diagnosis and development of adjuvant therapy, overall 5-year survival rates for patients with gastric cancer remain disappointing, with a mortality rate of 20% in Western countries and up to 60% in Asian countries[2]. Although current combinatory chemotherapeutic regimes result in a median overall survival of up to 11 mo, toxicity is increased[3,4]. To overcome the adverse effects, novel chemotherapeutic concepts have focused on the development of targeted therapies for gastric cancer. An understanding of the detailed mechanisms of invasion and metastasis in gastric cancer would be helpful in improving the treatment outcome.
H. pylori infection is usually asymptomatic in most hosts, as virtually all carriers develop superficial chronic active gastritis, whereas only about 10% suffer gastric or duodenal ulceration and 0.5%-2.0% develop gastric adenocarcinoma or B cell lymphoma of mucosa-associated lymphoid tissue[5]. H. pylori colonize the gastric mucosa of 35%-70% of people worldwide and infection with H. pylori is the main etiologic factor for development of chronic active gastritis and peptic ulcers[6,7]. Epidemiologic data indicate that gastric cancer occurs more frequently in populations with higher rates of H. pylori infection, and the World Health Organization has classified this bacterium as a class 1 carcinogen for gastric cancer[8]. Animal models have also demonstrated the importance of H. pylori in gastric carcinogenesis[9]. H. pylori infection is important in the process of tissue remodeling, angiogenesis, tumor invasion and metastasis[10], and induces a number of genes in host cells that are potential determinants of inflammation, angiogenesis, and metastasis including interleukin-8 (IL-8), cyclooxygenase-2[11], monocyte chemoattractant protein-1[12], vascular endothelial growth factor[13], and matrix metalloproteinase (MMP)-9[14]. However, it remains unclear how H. pylori infection activates specific transcription factors and induces gene expression.
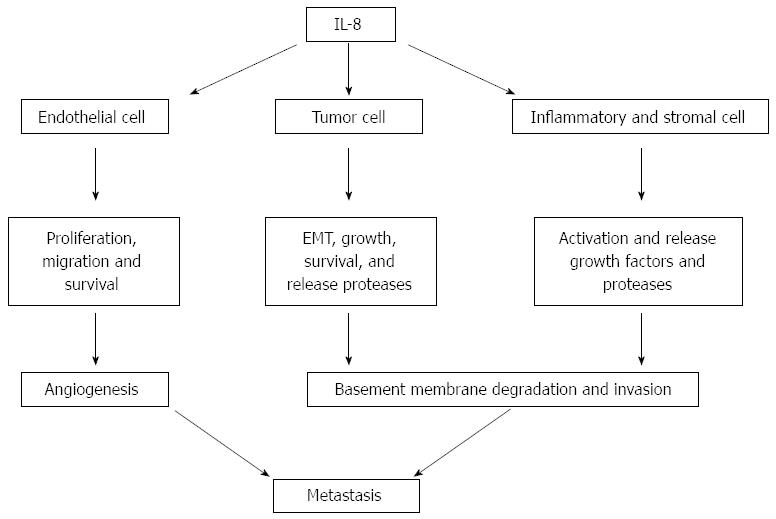
IL-8 seems to have significant potential as a prognostic and predictive cancer biomarker. IL-8 was originally identified as a chemoattractant for neutrophils that release angiogenic growth factors, stimulating angiogenesis as a part of cancer progression. As shown in Figure 1, IL-8 increases the proliferation, migration and survival of endothelial cells, potentiates the epithelial-mesenchymal transition and survival of cancer cells, and activates macrophage and immune responses at the tumor site[15]. IL-8 enhances the production and secretion of MMP-2 and MMP-9[16,17], suggesting that it can modulate invasiveness and/or extracellular matrix remodeling in normal physiological conditions and in cancer progression.
An understanding of the basic principles and underlying signals by which H. pylori regulates IL-8 may lead to the development of new therapeutic strategies in gastric cancer. With this in mind, we present a brief review.
A significant correlation between high expression levels of IL-8 in gastric mucosa and risk of gastric cancer has been reported[18]. Macrì et al[19] reported that the serum levels of IL-8 act as markers of gastric cancer. Increased expression of IL-8 mRNA in tissue extracts from gastric cancer patients has been associated with some clinicopathological aspects of the disease, including poor prognosis[20]. In IL-8 transgenic mice, where expression of human IL-8 is controlled by its own regulatory elements, expression of IL-8 increased tumorigenesis, suggesting that IL-8 might have a crucial role in gastrointestinal cancers[21]. These observations indicate that high levels of IL-8 may be associated with poor prognosis as judged by stage and histology, and that IL-8 may be indicative of more aggressive gastric cancers.
The roles for IL-8 in the angiogenesis of gastric cancer have drawn much interest. Since invasion and angiogenesis are all involved in the metastatic process, IL-8 expression in gastric cancer can influence their metastatic capabilities. Upregulation of IL-8 in human gastric carcinomas correlates closely with their angiogenesis[22]. Kitadai et al[23] reported that the expression of IL-8 directly correlated with the vascularity of human gastric carcinomas and that IL-8-transfected cells produced rapidly growing, highly vascular neoplasms, compared to control cells. In contrast, inhibition of IL-8 decreases angiogenesis in gastric cancer. Wang et al[24] reported that CHIP, a protein that interacts with the carboxy terminus of Hsc70, also interacted with nuclear factor-kappa B (NF-κB), terminating NF-κB activity and inhibiting IL-8-induced angiogenesis. IL-8 stimulates vascular endothelial growth factor (VEGF) expression in endothelial cells via CXCR-2 and thereby promotes the activation of VEGF receptors in an autocrine fashion[25]. IL-8 has a direct role in angiogenesis by enhancing endothelial cell proliferation and survival in CXCR1- and CXCR2-expressing endothelial cells[26]. IL-8 stimulates both endothelial proliferation and capillary tube formation in vitro, and both of these effects can be blocked by monoclonal anti-bodies to IL-8. H. pylori-derived heat shock protein 60 (HpHSP60) enhances angiogenesis by a CXCR2-mediated signaling pathway[27]. Use of an angiogenic array showed that HpHSP60 markedly induced IL-8 and that inhibition of CXCR2, the receptor for IL-8, significantly abolished HpHSP60-induced tube formation. IL-8 has also been linked with cell adhesion and migration in gastric cancer[23]. IL-8 activates NF-κB and Akt signals, and induces adhesion molecules including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 and CD44 expression in gastric cancer cells[28]. Inhibition of IL-8 with small interfering RNA reportedly decreased the adhesion, migration and invasion functions in cancer cells[23].
IL-8 polymorphisms may increase the risk of gastric cancer. Taguchi et al[29] reported the association of the IL-8-251 A/T polymorphism with higher expression of IL-8 protein, severe neutrophil infiltration and increased risk of atrophic gastritis and gastric cancer. IL-8-251 T/A and IL-8-251 A/A polymorphisms may be associated with angiogenesis in gastric carcinogenesis in H. pylori-infected Koreans[30]. In the study, there were significant correlations between MMP-9, angiopoietin-1 concentrations and disease progression in IL-8-251 A/A and IL-8-251 A/T genotypes. Felipe et al[31] reported that patients with the heterozygous IL-8-251 A/T genotype, high fat intake and smokers or ex-smokers presented an increased risk of gastric cancer in a Brazilian population. However, the association of IL-8 polymorphisms and gastric cancer is controversial. The IL-8 polymorphism was not consistently associated with gastric cancer risk in a Polish population[32]. Furthermore, a meta-analysis of epidemiological studies revealed an overall lack of association between IL-8-251 gene polymorphisms and risk of gastric cancer; any association is likely to be variable depending on histological type, tumor location, H. pylori infection, and ethnicity/country[33].
The downstream signals of IL-8 produced by H. pylori have been intensively studied. All biological effects of IL-8 are mediated by two receptors designated CXCR1 and CXCR2. IL-8 binds with high specificity to CXCR1[34] and with less specificity to CXCR2[35] expressed on stromal, endothelial and tumor cells. CXCR1, a cell-surface G-protein-coupled receptor, has been associated with tumorigenesis, development and progression of some tumors. Hu et al[36] documented that CXCR1 overexpression is associated with late-stage gastric cancer. They reported that knockdown of CXCR1 could inhibit cell proliferation in vitro and in vivo. Lin et al[37] reported that enforced expression of the cysteine-rich 61 (Cyr61) gene or treatment with recombinant Cyr61 protein enhanced expression of CXCR1 and CXCR2 in gastric cancer cells. The upregulated functionality of CXCR1 and CXCR2 could facilitate their chemotactic migration toward IL-8 and contribute to transendothelial migration, as well as intravasation. The interaction between IL-8 and epidermal growth factor receptor (EGFR) promotes cell proliferation through transactivation of the receptor by activation of a disintegrin and metalloproteinase[38]. IL-8 could induce EGFR phosphorylation, while anti-IL-8 and anti-IL-8 receptor antibodies suppressed EGFR phosphorylation, indicating that H. pylori-stimulated IL-8 accelerates the processing of EGFR ligands, and that cleaved EGFR ligands bind and stimulate EGFR in paracrine and autocrine manners to induce cell proliferation.
A whole genome analysis of the epithelial response to H. pylori exposure revealed IL-8 as the most markedly upregulated gene[39]. IL-8 appears to play a paramount role in the epithelial cell response to H. pylori infection and in the pathological processes leading to gastric disease. IL-8, a CXC chemokine specific for neutrophil granulocyte chemotaxis, has been correlated with the histological severity of gastritis[40]. The majority of gastric cancers are end products of an inflammatory process. A chronic H. pylori infection is characterized by an inflammation of the gastric mucosa and is accepted as the major cause of chronic gastritis.
IL-8 induction in gastric epithelial cells has been clearly correlated with a functional cagA gene[41]. In H. pylori strains that express cagA, cytokine expression has been linked with an elevated inflammatory response in vivo[42]. H. pylori strains are classified as cagA-positive or cagA-negative according to the presence or absence of cagA, respectively[43]. CagA protein is a major virulence factor of H. pylori that has attracted clinical interest as a marker of H. pylori-associated disease, having been shown to confer increased gastric cancer risk[6,44]. The cagA gene is located at one end of the cag pathogenicity island (cagPAI). The island contains two segments: an upstream cag II region and a downstream cag I region[45]. PAI comprises a gene cluster of 40 kbps that encodes a type IV secretion system (T4SS) that functions to translocate cagA from epithelium-adherent bacteria into gastric epithelial cells[45]. Once inside the cells, cagA is phosphorylated by host cellular kinases, Src[46,47] and Abl[48], on a repeating glutamic acid proline-isoleucine-tyrosine-alanine tyrosine phosphorylation motif located at the carboxyl terminus of the protein. In vitro examinations of H. pylori infection of gastric epithelial cells revealed the requirement of proteins encoded by the cagPAI, with the exception of cagA, for IL-8 secretion, and the regulation of IL-8 induction by the NF-κB pathway[44,49]. However, H. pylori-induced pro-inflammatory responses remain controversial[50-52]. Ando et al[51] observed upregulated IL-8 expression in gastric epithelial cells infected with H. pylori containing an inactivated cagA gene, while Peng et al[52] reported upregulation of IL-8 expression in gastric epithelial cells in response to treatment with extracts of cagA-positive and cagA-negative strains. Bacterial cagA expression may not be essential for the upregulation of IL-8 expression in H. pylori-infected gastric epithelial cells.
Although it is well known that H. pylori upregulates IL-8 expression in gastric cancer cells, the underlying molecular mechanism is not fully understood. Analyses of the genomic structure of IL-8 have revealed many potential targets for regulation at both the transcriptional and post-transcriptional levels. Within its 3’-flanking region, the IL-8 gene contains a repetitive ATTTA motif, which is responsible for destabilization of various cytokine mRNAs[53]. Within the 5’-flanking region, the gene contains multiple cis elements including a CCAAT box, steroid-responsive element, hepatocyte nuclear factor-1 element, two interferon regulatory factor-1 elements and binding sites for activator protein-1 (AP-1), CCAAT/enhancer binding protein and NF-κB, all of which have been implicated in the induction of IL-8 gene transcription by the aforementioned stimuli[54]. As demonstrated by mutation and deletion analyses, these promoter elements are regulated in cell type-specific manners[55]. A myriad of intracellular signals have been suggested to mediate the effects of H. pylori including production of reactive oxygen species (ROS), and activation of transcription factor NF-κB, AP-1 and mitogen-activated protein kinase (MAPK).
ROS are involved in the pathogenesis of H. pylori-associated gastric diseases that include gastric cancer[56,57]. Park et al[57] reported that ROS are produced by NADPH oxidase (NOX1) and induce apoptotic cell death of H. pylori-infected gastric epithelial cells. NOX1 induced by H. pylori in gastric disease functions in the constitutive production of superoxide anion and hydrogen peroxide[58]. Increased expression of NOX1 mRNA moderately increases the generation of superoxide anion, which leads to a reduction in aconitase activity, making NOX1 a good marker of oxidative stress. ROS induced by H. pylori stimulate MAPKs, such as extracellular signal-related kinases (ERKs), c-Jun NH2-terminal kinases (JNKs) and p38 MAPK, and upregulate transcription of NF-κB[59]. Interestingly, IL-8 contributes to the generation of copious quantities of ROS, and can elicit the induction of IL-1β, IL-6, IL-8, IL-12, tumor necrosis factor-alpha[60,61], and interferon-gamma[60]. IL-8 activates the CD11b/CD18 dimer, which forms a complex with neutrophils. The complex activates ICAM-1 on the vascular endothelial cell membrane. The resulting tetramer (CD11b/CD18/neutrophil/ICAM-1) infiltrates gastric epithelial cells and facilitates the copious release of ROS through neutrophil NADPH oxidase, resulting in an oxidative burst[62,63]. The ROS released from gastric epithelial cells may mediate the chemotactic action of neutrophils and monocytes in H. pylori-infected gastric tissues[56].
Co-culture of H. pylori with cells can induce IL-8 through the activation of the oxidant-sensitive transcriptional factor NF-κB. ROS are important in this process in H. pylori -infected cancer cells[64]. NF-κB exists in a latent form in the cytoplasm, bound to the inhibitory protein, IκB. IκB kinase (IKK) directly phosphorylates IκB molecules, leading to the ubiquitin-mediated proteolysis of IκB. The NF-κB dimer that is released from IκB translocates to the nucleus where it activates target genes by binding to the promoter/enhancer region. In addition to ROS, several mechanisms for NF-κB activation by H. pylori have been proposed. H. pylori induces the phosphorylation of heat shock protein 90 (Hsp90) in gastric epithelial cells[65,66]. Hsp90 associates stoichiometrically with the IKK complex, which contributes to the stabilization, activation and shuttling of IKKs to the plasma membrane, because Hsp90 regulates the stability and function of a unique complement of signaling molecules[67]. Given that Hsp90 is associated with IKK-α and IKK-γ in H. pylori-infected gastric epithelial cells[65], the Hsp90-IKK complex may be a target for the pharmacological inhibition of the H. pylori-mediated activation of NF-κB signaling. Takeshima et al[68] reported that NF-κB activation by H. pylori requires Akt-mediated phosphorylation of p65. Phosphorylated Akt is detected in epithelial cells of H. pylori positive gastric tissues. The application of phosphoinositol-3-kinase inhibitor, dominant-negative Akt and small interfering RNA for Akt suppresses H. pylori-induced p65 phosphorylation as well as IL-8 expression, suggesting that Akt signals are involved in H. pylori-induced NF-κB activation.
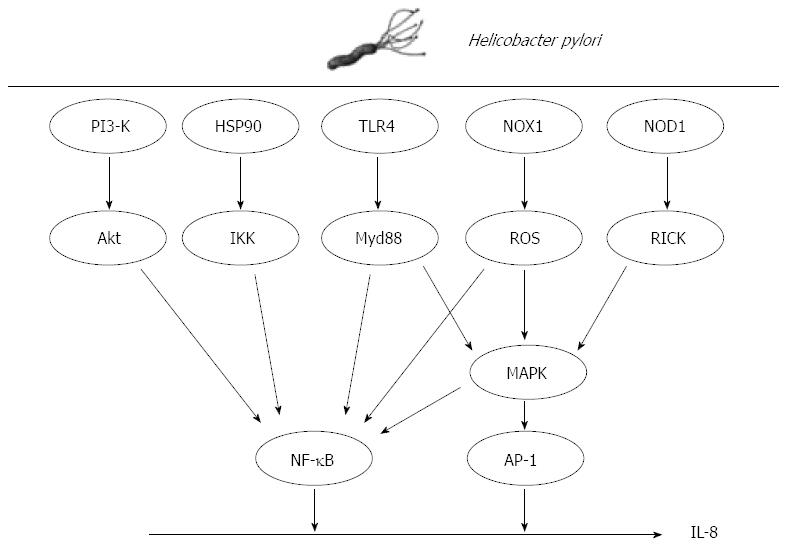
H. pylori also activates the transcription factor AP-1 in a cagPAI-dependent manner[69,70]. The AP-1 complex activated during H. pylori infection is composed primarily of c-jun and c-fos heterodimers[71]. AP-1 is activated by MAPK and is capable of inducing a strong pro-inflammatory response, often in concert with NF-κB[71]. H. pylori rapidly activate MAPKs upon contact with gastric epithelial cells[72]. MAPK cascades are well characterized pathways that transduce signals from the cell surface to the nucleus. The family includes distinct subgroups: ERKs, JNKs and p38 MAPK. A number of bacterial factors have been implicated in MAPK activation including vacA[73] and cagA[72]. The signaling events leading to rapid MAPK phosphorylation during H. pylori infection are not well understood, although T4SS is required for ERK phosphorylation of p38 MAPK and JNK[67]. CagA is capable of activating ERK[73], though ERK can also be activated by cagA-independent mechanisms[74], suggesting that cagA has an additive role in transcription factor activation. JNK activation during H. pylori infection also requires a functional T4SS[72]. H. pylori peptidoglycan is delivered to the host cell via the T4SS, where it is recognized by cytosolic nucleotide binding and oligomerization domain 1 (NOD1)[75]. Upon stimulation with purified agonist, NOD1 associates with the receptor-interacting protein serine-threonine kinase 2, triggering a pro-inflammatory response that is characterized by NF-κB activation and IL-8 production[76]. In addition to activation of the classical NF-κB pathway, NOD1 is reported to be required for MAPK activation in response to bacterial pathogens. This NOD1-dependent p38 MAPK activation induces IL-8 production[77]. Allison et al[77] observed that NOD1 was necessary for MAPK activation in the early stages of infection and that NOD1 was essential for the activation of both NF-κB and AP-1, as well as the release of IL-8 in response to H. pylori. These observations support previous findings that cagA induces IL-8 induction via the Ras→Raf→Mek→ERK→NF-κB signaling pathway[78] and that cagA can activate the Ras→ERK pathway[79]. Understanding the signals involved in IL-8 expression by H. pylori may be beneficial to develop new therapeutics in gastric cancer (Figure 2).
Gastric cancer features increased IL-8 expression, suggesting that IL-8 might be a promising therapeutic targeting to prevent cancer progression. Many inhibitors that prevent H. pylori-induced IL-8 expression and regulate the IL-8 downstream signals have been proposed (Table 1).
| Inhibitors | Mechanisms | Ref. |
| Resveratrol | Reduces ROS, inhibits MAPK, AP-1 and NF-κB | [82,83] |
| Anthocyanin | Reduces ROS, inhibits MAPK, AP-1 and NF-κB | [84-86] |
| Apigenin | Increases the IκBα and thus inhibits NF-κB | [88,89] |
| RK-I-123 | Reduces ROS and inhibits AP-1 and NF-κB | [91] |
| DA-6034 | Dissociates IKK/HSP90 complex and inhibits NF-κB | [92,93] |
| Rebamipide | Prevents PLD expression via NF-κB | [94,95] |
| Gefitinib [Iressa™] | Inhibits EGFR | [98,99] |
| L. bulgaricus | Inhibits TLR4 | [101] |
| L. acidophilus | Dissociates IKK/Hsp90 complex and inhibits NF-κB | [65,103] |
| NRF peptide | Disrupts interaction of NRF and NF-κB | [105] |
| miR-146 | Negatively regulates IL-8 | [110,111] |
| miR-155 | Inhibits MyD88 via NF-κB | [112,115] |
| G31P | Synthetic derivative of IL-8 | [117] |
| SCH-527123 | CXCR2 inhibitor | [118] |
Polyphenols derived from natural products that include resveratrol, apigenin and anthocyanins inhibit IL-8 induced by H. pylori. Resveratrol suppresses the secretion of IL-8 from H. pylori-infected gastric epithelial cells. IL-8 secretion is usually regulated by the transcription factor NF-κB and H. pylori can induce IL-8 expression by activating a NF-κB pathway in gastric epithelial cells[80,81]. Since resveratrol inhibits NF-κB[82], its suppressive effect on IL-8 secretion may correlate with its NF-κB inhibitory activity. Inhibition of IL-8 expression by resveratrol may also be due to modulation of regulatory enzymes like MAPK[83]. Anti-oxidant anthocyanins from black soybean may inhibit IL-8 production[84]. Cyanidin-3-glucoside, which is abundant in anthocyanins, is reportedly an effective anti-oxidant that inactivates NF-κB by inhibiting phosphorylation of IκB[85,86]. Anthocyanins have anti-oxidant effects and the ability to downregulate ROS generation, and decrease the activation of MAPKs induced by H. pylori. Apigenin, one of the most common flavonoids, increases IκBα expression, and thus inhibits NF-κB activation and decreases IL-8 expression[87]. Apigenin’s anti--inflammatory activity has been characterized in vitro and in vivo[88,89].
Phenyl-thiophenyl propenone RK-I-123 is a small molecule that reportedly reduces the level of ROS and suppresses the activation of NF-κB and AP-1, and the expression of IL-8 in H. pylori-infected gastric epithelial cells[90]. RK-I-123 was synthesized as a novel propenone compound in an attempt to develop a dual inhibitor of COX-2 and 5-LOX[91]. 7-Carboxymethyloxy-3’,4’,5-trimethoxy flavone, abbreviated as DA-6034, is a synthetic derivative of eupatilin that also inhibits IL-8 induced by H. pylori[92]. DA-6034 promotes the dissociation of the IKK-HspP90 complex and suppresses NF-κB signaling, leading to the inhibition of IL-8 expression in H. pylori-infected cells. DA-6034 also inhibits ERK in such cells[93].
Rebamipide [2-[4-chlorobenzoylamino]-3-[2[1H] quinolinon-4-yl] propionic acid; OPC-12759], a mucosal-protective anti-ulcer agent, was reported to inhibit IL-8 in gastric cancer by the regulation of phospholipase D (PLD) expression. Gastric cancer cells infected with H. pylori display significant induction of PLD1 expression via activation of NF-κB[94]. The level of PLD1 protein and IκBα phosphorylation is aberrantly upregulated in H. pylori-infected human gastric tissues. Rebamipide is a gastroprotective agent used in the treatment of gastritis and gastric ulcers[95]. It protects against gastric mucosa inflammation induced by H. pylori by inhibiting neutrophil function[96]. Moreover, rebamipide inhibits the growth of gastric cancer cells[97]. PLD and IL-8 might be novel targets of rebamipide in H. pylori-associated gastric cancer.
Gefitinib (Iressa™, ZD1839) reportedly inhibits epidermal growth factor (EGF) signals and IL-8 production in gastric cancer cells[98]. Gefitinib is an orally active, quinazoline-derived agent that inhibits EGF receptor (EGFR)-tyrosine kinase[99]. Previous studies have shown that EGFR-mediated signals contribute to the expression of IL-8 and that IL-8 may be involved, at least in part, in EGF/EGFR-induced cancer progression[100]. Kishida et al[98] employed SN38 (an active metabolite of CPT-11) for activation of EGFR-tyrosine kinase. SN38 activates the EGF/EGFR autocrine loop and induces IL-8 in gastric cancer cells. SN38 induces binding activities in both NF-κB and AP-1, critical transcription factors for the expression of IL-8, and this reaction is inhibited by gefitinib.
Interestingly, Zhou et al[101] suggested that the probiotic application of lactobacilli may inhibit IL-8 production induced by H. pylori-activated Toll-like receptor 4 (TLR4). Lactobacillus bulgaricus (LBG), a bacterium used in the production of yogurt, is one of the best-studied probiotic microbes. Probiotics are living microorganisms with no or low pathogenicity, which exert beneficial effects on the host. H. pylori induces mucosal inflammation including IL-8 production via TLR4 signaling[102]. Conjugated linoleic acids (CLA) produced by Lactobacillus acidophilus (LBA) also decreases the activation of NF-κB and IL-8 expression in H. pylori-infected gastric epithelial cells[103]. Kim et al[65] demonstrated that CLA-containing conditioned medium produced by LBA has anti-inflammatory effects on H. pylori infection. In their study, conditioned medium produced by LBA significantly inhibited the activation of the core inflammatory gene signal NF-κB in gastric epithelial cells by dissociation of the complex between Hsp90 and the IκB kinase-subunit. CLA-containing conditioned medium also inhibited the expression of IL-8[65]. There is increasing evidence[104] that Lactobacillus has therapeutic effects on H. pylori-related diseases, including enhanced eradication of H. pylori, amelioration of resistance to antibiotics, downregulated side effects of antibiotic-based therapy, decreased recurrence of H. pylori infection, and inhibition of H. pylori-induced apoptosis.
Bartel et al[105] suggested that a peptide capable of disrupting the interaction between NF-κB and NF-κB repressing factor (NRF) inhibits H. pylori-induced IL-8 expression. In IL-8 gene expression, exclusively, NRF had two functions. It repressed the basal transcription of IL-8 gene in unstimulated cells[106], but, following cell stimulation, it was required for the transcriptional activation of the IL-8 gene. A synthetic peptide corresponding to amino acid 223-238 of NRF interfered with the binding of endogenous NF-κB to NRF interaction, which significantly decreased endogenous IL-8 gene transcription in response to H. pylori infection
Several microRNAs (miR) are reported to regulate IL-8 gene expression[107]. miRNAs are central regulators of various physiologic processes and their disruption is associated with human diseases[108]. Recently, Liu et al[109] reported that miR-146a negatively regulated H. pylori-induced IL-8 via reduced NF-κB activity. miR-146a reportedly suppresses NF-κB activity through the reduction of metastatic potential in cancer cells[110]. The authors also reported that miR-146a is the negative regulator of NF-κB activity through the downregulation of IRAK1 and TRAF6 in cancer cells. Perry et al[111] found that miR-146a was able to negatively regulate the release of IL-1β-induced IL-8, independent of IRAK1 and TRAF6 signals. miR-155 was also suggested to regulate IL-8 in H. pylori-infected gastric epithelial cells. Overexpression of miR-155 reportedly reduced the H. pylori-induced IL-8 expression[112]. miR-155 has been indicated to play a key role in the regulation of normal immunity or inflammation response[113,114]. Among a number of targets of miR-155, MyD88 is suggested for IL-8 regulation[115]. miR-155 may downregulate the protein MyD88 through inhibition of translation. Most TLRs activate MyD88 leading to the nuclear translocation of transcription factors, such as NF-κB and AP-1, and thus transcriptionally regulate IL-8[116]. The function of miRNAs during H. pylori infection is complex and miR-155 may cooperate with other H. pylori-induced miRNAs including miR-146a in response to H. pylori. There may be crosstalk between miR-146a and miR-155 in the signal pathways leading to the downregulation of H. pylori-induced IL-8 in gastric cancer.
Small molecule inhibitors targeting IL-8 receptors (CXCR1 and CXCR2) have been developed to suppress prostate and colon cancers[117,118]. Inhibition of these receptors reduces cell migration and invasion, while increasing apoptosis in cancer cells[119]. Liu et al[117] synthesized a derivative of the human cytokine IL-8, G31P, with high-affinity for human CXCR1 and CXCR2. G31P treatment significantly reduced prostate cancer cell viability, adhesion and migration capacity. Additionally, G31P inhibited tumor tissue vascularization, which was associated with the decreased expression of vascular endothelial growth factor and NF-κB in orthotopic xenograft tissues. Another small molecule inhibitor targeting CXCR2 is SCH-527123[118]. SCH-527123 is able to suppress CXCR2-mediated signal transduction as shown through decreased phosphorylation of the NF-κB, MAPK and Akt pathways in colon cancer cells. The anti-tumor activity of SCH-527123 resulted from inhibition of cancer cell growth, motility, and angiogenesis. In addition to having a direct anti-angiogenic and anti-tumor effect, targeting IL-8 or CXCR2 may also increase chemosensitivity to chemotherapeutics. Wilson et al[120] also showed that IL-8/CXCR2 signaling confers resistance to chemotherapeutics (oxaliplatin) through NF-κB activity, which is an important determinant of cancer cell sensitivity to chemotherapeutics.
There is a close association between H. pylori infection and gastric cancer. IL-8 is overexpressed in gastric epithelial cells exposed to H. pylori. IL-8 is significantly upregulated in both the tumor and its microenvironment, and acts as a key regulator of proliferation, angiogenesis and metastasis. IL-8 expression also contributes to the resistance of gastric cancer to chemotherapeutics. Although anti-IL-8 therapeutic agents are yet to enter preclinical and clinical trials, a large body of published evidence suggests that targeting IL-8 in gastric cancer could have broad-spectrum anti-tumor effects. Many advances have been made since the discovery that IL-8 regulates cell signaling in cancer development and progression independently of chemotaxis during the inflammatory process. Thus, we propose that IL-8 induced by H. pylori plays a major role in gastric cancer and that targeting IL-8 may be a promising strategy for the treatment of cancer.
P- Reviewers: Arsenijevic T, Guo P, Kanda T, Shimoyama S, Takahashi Y, Tiberio GAM S- Editor: Zhai HH L- Editor: Cant MR E- Editor: Ma S
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] |
| 2. | Lee YY, Derakhshan MH. Environmental and lifestyle risk factors of gastric cancer. Arch Iran Med. 2013;16:358-365. [PubMed] |
| 3. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1691] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 4. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1457] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 5. | Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63-96. [PubMed] |
| 6. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [PubMed] |
| 7. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 9. | Kodama M, Murakami K, Sato R, Okimoto T, Nishizono A, Fujioka T. Helicobacter pylori-infected animal models are extremely suitable for the investigation of gastric carcinogenesis. World J Gastroenterol. 2005;11:7063-7071. [PubMed] |
| 10. | Iwamoto J, Mizokami Y, Takahashi K, Nakajima K, Ohtsubo T, Miura S, Narasaka T, Takeyama H, Omata T, Shimokobe K. Expressions of urokinase-type plasminogen activator, its receptor and plasminogen activator inhibitor-1 in gastric cancer cells and effects of Helicobacter pylori. Scand J Gastroenterol. 2005;40:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Futagami S, Hiratsuka T, Tatsuguchi A, Suzuki K, Kusunoki M, Shinji Y, Shinoki K, Iizumi T, Akamatsu T, Nishigaki H. Monocyte chemoattractant protein 1 (MCP-1) released from Helicobacter pylori stimulated gastric epithelial cells induces cyclooxygenase 2 expression and activation in T cells. Gut. 2003;52:1257-1264. [PubMed] |
| 12. | Watanabe N, Shimada T, Ohtsuka Y, Hiraishi H, Terano A. Proinflammatory cytokines and Helicobacter pylori stimulate CC-chemokine expression in gastric epithelial cells. J Physiol Pharmacol. 1997;48:405-413. [PubMed] |
| 13. | Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue N, Yasui W, Aihara M, Imagawa K, Haruma K, Chayama K. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun. 2003;311:809-814. [PubMed] |
| 14. | Göõz M, Göõz P, Smolka AJ. Epithelial and bacterial metalloproteinases and their inhibitors in H. pylori infection of human gastric cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G823-G832. [PubMed] |
| 15. | Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853-865. [PubMed] |
| 16. | Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104-2119. [PubMed] |
| 17. | Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Yamada S, Kato S, Matsuhisa T, Makonkawkeyoon L, Yoshida M, Chakrabandhu T, Lertprasertsuk N, Suttharat P, Chakrabandhu B, Nishiumi S. Predominant mucosal IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer. World J Gastroenterol. 2013;19:2941-2949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Macrì A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, Teti D, Famulari C. Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006;11:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH, Song SK, Kim HS. Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology. 2004;66:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Beales IL, Calam J. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1beta and TNF-alpha requires tyrosine kinase activity, but not protein kinase C. Cytokine. 1997;9:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93-100. [PubMed] |
| 24. | Wang S, Wu X, Zhang J, Chen Y, Xu J, Xia X, He S, Qiang F, Li A, Shu Y. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human gastric cancer. Gut. 2013;62:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038-6042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369-3376. [PubMed] |
| 27. | Lin CS, He PJ, Hsu WT, Wu MS, Wu CJ, Shen HW, Hwang CH, Lai YK, Tsai NM, Liao KW. Helicobacter pylori-derived Heat shock protein 60 enhances angiogenesis via a CXCR2-mediated signaling pathway. Biochem Biophys Res Commun. 2010;397:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 28. | Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R, Tang XJ. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol. 2012;18:979-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487-2493. [PubMed] |
| 30. | Song JH, Kim SG, Jung SA, Lee MK, Jung HC, Song IS. The interleukin-8-251 AA genotype is associated with angiogenesis in gastric carcinogenesis in Helicobacter pylori-infected Koreans. Cytokine. 2010;51:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Felipe AV, Silva TD, Pimenta CA, Kassab P, Forones NM. lnterleukin-8 gene polymorphism and susceptibility to gastric cancer in a Brazilian population. Biol Res. 2012;45:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Savage SA, Hou L, Lissowska J, Chow WH, Zatonski W, Chanock SJ, Yeager M. Interleukin-8 polymorphisms are not associated with gastric cancer risk in a Polish population. Cancer Epidemiol Biomarkers Prev. 2006;15:589-591. [PubMed] |
| 33. | Liu L, Zhuang W, Wang C, Chen Z, Wu XT, Zhou Y. Interleukin-8 -251 A/T gene polymorphism and gastric cancer susceptibility: a meta-analysis of epidemiological studies. Cytokine. 2010;50:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278-1280. [PubMed] |
| 35. | Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 567] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 36. | Hu W, Wang J, Luo G, Luo B, Wu C, Wang W, Xiao Y, Li J. Proteomics-based analysis of differentially expressed proteins in the CXCR1-knockdown gastric carcinoma MKN45 cell line and its parental cell. Acta Biochim Biophys Sin (Shanghai). 2013;45:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Lin BR, Chang CC, Chen LR, Wu MH, Wang MY, Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ. Cysteine-rich 61 (CCN1) enhances chemotactic migration, transendothelial cell migration, and intravasation by concomitantly up-regulating chemokine receptor 1 and 2. Mol Cancer Res. 2007;5:1111-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Joh T, Kataoka H, Tanida S, Watanabe K, Ohshima T, Sasaki M, Nakao H, Ohhara H, Higashiyama S, Itoh M. Helicobacter pylori-stimulated interleukin-8 (IL-8) promotes cell proliferation through transactivation of epidermal growth factor receptor (EGFR) by disintegrin and metalloproteinase (ADAM) activation. Dig Dis Sci. 2005;50:2081-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Eftang LL, Esbensen Y, Tannæs TM, Bukholm IR, Bukholm G. Interleukin-8 is the single most up-regulated gene in whole genome profiling of H. pylori exposed gastric epithelial cells. BMC Microbiol. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJ. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61-66. [PubMed] |
| 41. | Peek RM, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760-770. [PubMed] |
| 42. | Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, Fukatsu A, Ichiyama S, Ohta M. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150-1156. [PubMed] |
| 43. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [PubMed] |
| 44. | Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111-2115. [PubMed] |
| 45. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [PubMed] |
| 46. | Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 248] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777-1780. [PubMed] |
| 48. | Hsu PI, Hwang IR, Cittelly D, Lai KH, El-Zimaity HM, Gutierrez O, Kim JG, Osato MS, Graham DY, Yamaoka Y. Clinical presentation in relation to diversity within the Helicobacter pylori cag pathogenicity island. Am J Gastroenterol. 2002;97:2231-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Nomura AM, Pérez-Pérez GI, Lee J, Stemmermann G, Blaser MJ. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am J Epidemiol. 2002;155:1054-1059. [PubMed] |
| 50. | Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 51. | Ando T, Peek RM, Lee YC, Krishna U, Kusugami K, Blaser MJ. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin Diagn Lab Immunol. 2002;9:167-175. [PubMed] |
| 52. | Peng YC, Ho SP, Shyu CL, Chang CS, Huang LR. Clarithromycin modulates Helicobacter pylori-induced activation of nuclear factor-κB through classical and alternative pathways in gastric epithelial cells. Clin Exp Med. 2012;Nov 6; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465-470. [PubMed] |
| 54. | Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153-164. [PubMed] |
| 55. | Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506-22511. [PubMed] |
| 56. | Handa O, Naito Y, Yoshikawa T. Redox biology and gastric carcinogenesis: the role of Helicobacter pylori. Redox Rep. 2011;16:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Park KJ, Lee CH, Kim A, Jeong KJ, Kim CH, Kim YS. Death receptors 4 and 5 activate Nox1 NADPH oxidase through riboflavin kinase to induce reactive oxygen species-mediated apoptotic cell death. J Biol Chem. 2012;287:3313-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007;43:1627-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Kim MH, Cho HS, Jung M, Hong MH, Lee SK, Shin BA, Ahn BW, Jung YD. Extracellular signal-regulated kinase and AP-1 pathways are involved in reactive oxygen species-induced urokinase plasminogen activator receptor expression in human gastric cancer cells. Int J Oncol. 2005;26:1669-1674. [PubMed] |
| 60. | Lamb A, Chen LF. The many roads traveled by Helicobacter pylori to NFκB activation. Gut Microbes. 2010;1:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 62. | Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1451] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 63. | Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1343] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 64. | Kim JM, Kim JS, Jung HC, Oh YK, Chung HY, Lee CH, Song IS. Helicobacter pylori infection activates NF-kappaB signaling pathway to induce iNOS and protect human gastric epithelial cells from apoptosis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1171-G1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Kim JM, Kim JS, Kim YJ, Oh YK, Kim IY, Chee YJ, Han JS, Jung HC. Conjugated linoleic acids produced by Lactobacillus dissociates IKK-gamma and Hsp90 complex in Helicobacter pylori-infected gastric epithelial cells. Lab Invest. 2008;88:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Yeo M, Park HK, Lee KM, Lee KJ, Kim JH, Cho SW, Hahm KB. Blockage of HSP 90 modulates Helicobacter pylori-induced IL-8 productions through the inactivation of transcriptional factors of AP-1 and NF-kappaB. Biochem Biophys Res Commun. 2004;320:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23:5378-5386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 68. | Takeshima E, Tomimori K, Kawakami H, Ishikawa C, Sawada S, Tomita M, Senba M, Kinjo F, Mimuro H, Sasakawa C. NF-kappaB activation by Helicobacter pylori requires Akt-mediated phosphorylation of p65. BMC Microbiol. 2009;9:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Meyer-ter-Vehn T, Covacci A, Kist M, Pahl HL. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem. 2000;275:16064-16072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 70. | Naumann M, Wessler S, Bartsch C, Wieland B, Covacci A, Haas R, Meyer TF. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J Biol Chem. 1999;274:31655-31662. [PubMed] |
| 71. | Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003;48:257-265. [PubMed] |
| 72. | Naumann M, Crabtree JE. Helicobacter pylori-induced epithelial cell signalling in gastric carcinogenesis. Trends Microbiol. 2004;12:29-36. [PubMed] |
| 73. | Hisatsune J, Nakayama M, Isomoto H, Kurazono H, Mukaida N, Mukhopadhyay AK, Azuma T, Yamaoka Y, Sap J, Yamasaki E. Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-kappaB activation. J Immunol. 2008;180:5017-5027. [PubMed] |
| 74. | Pillinger MH, Marjanovic N, Kim SY, Lee YC, Scher JU, Roper J, Abeles AM, Izmirly PI, Axelrod M, Pillinger MY. Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J Biol Chem. 2007;282:18722-18731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Kim EJ, Lee JR, Chung WC, Jung SH, Sung HJ, Lee YW, Oh YS, Kim SB, Paik CN, Lee KM. Association between genetic polymorphisms of NOD 1 and Helicobacter pylori-induced gastric mucosal inflammation in healthy Korean population. Helicobacter. 2013;18:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Alber T. Signaling mechanisms of the Mycobacterium tuberculosis receptor Ser/Thr protein kinases. Curr Opin Struct Biol. 2009;19:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol. 2009;183:8099-8109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 78. | Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300-9305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 420] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 79. | Zhu Y, Zhong X, Zheng S, Du Q, Xu W. Transformed immortalized gastric epithelial cells by virulence factor CagA of Helicobacter pylori through Erk mitogen-activated protein kinase pathway. Oncogene. 2005;24:3886-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Martin T, Cardarelli PM, Parry GC, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur J Immunol. 1997;27:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Nakamura H, Yoshimura K, Jaffe HA, Crystal RG. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611-19617. [PubMed] |
| 82. | Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509-6519. [PubMed] |
| 83. | Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Kim JM, Kim KM, Park EH, Seo JH, Song JY, Shin SC, Kang HL, Lee WK, Cho MJ, Rhee KH. Anthocyanins from black soybean inhibit Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Microbiol Immunol. 2013;57:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Speciale A, Canali R, Chirafisi J, Saija A, Virgili F, Cimino F. Cyanidin-3-O-glucoside protection against TNF-α-induced endothelial dysfunction: involvement of nuclear factor-κB signaling. J Agric Food Chem. 2010;58:12048-12054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Cimino F, Ambra R, Canali R, Saija A, Virgili F. Effect of cyanidin-3-O-glucoside on UVB-induced response in human keratinocytes. J Agric Food Chem. 2006;54:4041-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Wang YC, Huang KM. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem Toxicol. 2013;53:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 89. | Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res. 2007;30:1318-1327. [PubMed] |
| 90. | Jang SH, Cho S, Lee ES, Kim JM, Kim H. The phenyl-thiophenyl propenone RK-I-123 reduces the levels of reactive oxygen species and suppresses the activation of NF-κB and AP-1 and IL-8 expression in Helicobacter pylori-infected gastric epithelial AGS cells. Inflamm Res. 2013;62:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Jahng Y, Zhao LX, Moon YS, Basnet A, Kim EK, Chang HW, Ju HK, Jeong TC, Lee ES. Simple aromatic compounds containing propenone moiety show considerable dual COX/5-LOX inhibitory activities. Bioorg Med Chem Lett. 2004;14:2559-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Ko SH, Yoo DY, Kim YJ, Choi SM, Kang KK, Kim H, Kim N, Kim JS, Kim JM. A mechanism for the action of the compound DA-6034 on NF-κB pathway activation in Helicobacter pylori-infected gastric epithelial cells. Scand J Immunol. 2011;74:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Lee JS, Kim HS, Hahm KB, Sohn MW, Yoo M, Johnson JA, Surh YJ. Inhibitory effects of 7-carboxymethyloxy-3’,4’,5-trimethoxyflavone (DA-6034) on Helicobacter pylori-induced NF-kappa B activation and iNOS expression in AGS cells. Ann N Y Acad Sci. 2007;1095:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Kang DW, Hwang WC, Park MH, Ko GH, Ha WS, Kim KS, Lee YC, Choi KY, Min DS. Rebamipide abolishes Helicobacter pylori CagA-induced phospholipase D1 expression via inhibition of NFκB and suppresses invasion of gastric cancer cells. Oncogene. 2013;32:3531-3542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S-13S. [PubMed] |
| 96. | Arakawa T, Higuchi K, Fujiwara Y, Watanabe T, Tominaga K, Sasaki E, Oshitani N, Yoshikawa T, Tarnawski AS. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50 Suppl 1:S3-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Tarnawski A, Pai R, Chiou SK, Chai J, Chu EC. Rebamipide inhibits gastric cancer growth by targeting survivin and Aurora-B. Biochem Biophys Res Commun. 2005;334:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Kishida O, Miyazaki Y, Murayama Y, Ogasa M, Miyazaki T, Yamamoto T, Watabe K, Tsutsui S, Kiyohara T, Shimomura I. Gefitinib (Iressa, ZD1839) inhibits SN38-triggered EGF signals and IL-8 production in gastric cancer cells. Cancer Chemother Pharmacol. 2005;55:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, De Placido S, Bianco AR, Tortora G. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459-1465. [PubMed] |
| 100. | Hirata A, Ogawa S, Kometani T, Kuwano T, Naito S, Kuwano M, Ono M. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res. 2002;62:2554-2560. [PubMed] |
| 101. | Zhou C, Ma FZ, Deng XJ, Yuan H, Ma HS. Lactobacilli inhibit interleukin-8 production induced by Helicobacter pylori lipopolysaccharide-activated Toll-like receptor 4. World J Gastroenterol. 2008;14:5090-5095. [PubMed] |
| 102. | Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496-3502. [PubMed] |
| 103. | Hwang SW, Kim N, Kim JM, Huh CS, Ahn YT, Park SH, Shin CM, Park JH, Lee MK, Nam RH. Probiotic suppression of the H. pylori-induced responses by conjugated linoleic acids in a gastric epithelial cell line. Prostaglandins Leukot Essent Fatty Acids. 2012;86:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095-1102. [PubMed] |
| 105. | Bartel M, Hänsch GM, Giese T, Penzel R, Ceyhan G, Ketterer K, von Knebel-Döberitz M, Friess HM, Giese NA. Abnormal crosstalk between pancreatic acini and macrophages during the clearance of apoptotic cells in chronic pancreatitis. J Pathol. 2008;215:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Nourbakhsh M, Kalble S, Dorrie A, Hauser H, Resch K, Kracht M. The NF-kappa b repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-kappa b-flanking sequence element. J Biol Chem. 2001;276:4501-4508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Li N, Xu X, Xiao B, Zhu ED, Li BS, Liu Z, Tang B, Zou QM, Liang HP, Mao XH. H. pylori related proinflammatory cytokines contribute to the induction of miR-146a in human gastric epithelial cells. Mol Biol Rep. 2012;39:4655-4661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 108. | Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 843] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 109. | Liu Z, Xiao B, Tang B, Li B, Li N, Zhu E, Guo G, Gu J, Zhuang Y, Liu X. Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells. Microbes Infect. 2010;12:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 110. | Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643-5647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 518] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 111. | Perry MM, Williams AE, Tsitsiou E, Larner-Svensson HM, Lindsay MA. Divergent intracellular pathways regulate interleukin-1beta-induced miR-146a and miR-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett. 2009;583:3349-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 112. | Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li BS, Xie QH, Zhuang Y, Zou QM, Mao XH. Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett. 2010;584:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 113. | O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1483] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 114. | Worm J, Stenvang J, Petri A, Frederiksen KS, Obad S, Elmén J, Hedtjärn M, Straarup EM, Hansen JB, Kauppinen S. Silencing of microRNA-155 in mice during acute inflammatory response leads to derepression of c/ebp Beta and down-regulation of G-CSF. Nucleic Acids Res. 2009;37:5784-5792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 115. | Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27:474-482. [PubMed] |
| 116. | Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282:28929-28938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 117. | Liu X, Peng J, Sun W, Yang S, Deng G, Li F, Cheng JW, Gordon JR. G31P, an antagonist against CXC chemokine receptors 1 and 2, inhibits growth of human prostate cancer cells in nude mice. Tohoku J Exp Med. 2012;228:147-156. [PubMed] |
| 118. | Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli Venkata KC. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11:1353-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 119. | Singh S, Sadanandam A, Varney ML, Nannuru KC, Singh RK. Small interfering RNA-mediated CXCR1 or CXCR2 knock-down inhibits melanoma tumor growth and invasion. Int J Cancer. 2010;126:328-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7:2649-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |