Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8078
Revised: September 16, 2013
Accepted: October 19, 2013
Published online: November 28, 2013
Processing time: 187 Days and 21.3 Hours
AIM: To explore the clinical characteristics and prognosis of young patients with colorectal cancer patients in Eastern China.
METHODS: A total of 1335 patients with colorectal cancer treated from December 1985 to December 2005 at the Second Affiliated Hospital of Zhejiang University School of Medicine were studied retrospectively. The patients were divided into two groups, a younger group (aged ≤ 30 years) and an older group (aged > 30 years), and comparison was made in the clinical characteristics and prognosis between the two groups. Chi-square test was used for data analysis of all categorical variables, and overall survival (OS) was calculated by the Kaplan-Meier method. A multivariate analysis was performed using the Cox model.
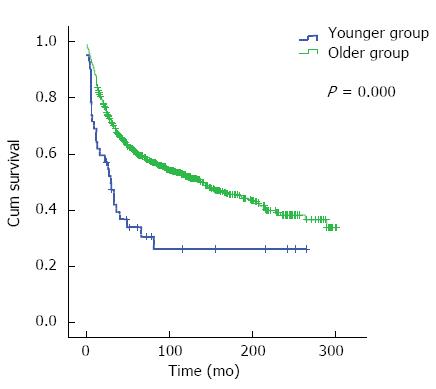
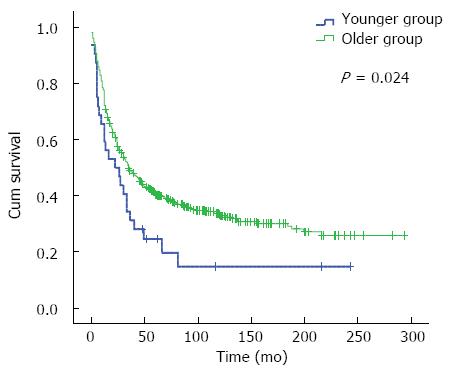
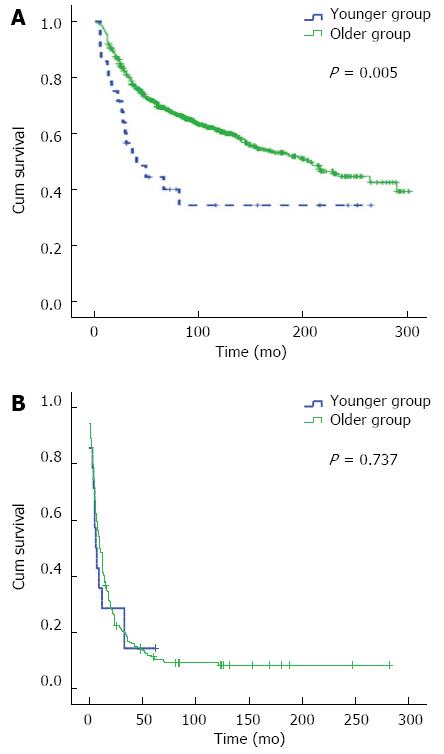
RESULTS: There were 42 (3.1%) and 1293 (96.9%) cases in the younger group and older group, respectively. Univariate analysis showed that the 5- and 10-year OS in the younger group were 33.9% and 26.1%, respectively, and those in the older group were 60.1% and 52.2%, respectively. Younger group had poor survival (χ2 = 14.146, P = 0.000). Multivariate analysis revealed that age was not a dependent factor for prognosis (OR = 0.866, 95%CI: 0.592-1.269, P = 0.461). Stratified analysis indicated that in stage III and IV disease, the 5- and 10-year OS were 24.6% and 14.8% in the younger group, and 40.4% and 33.3% in the older group, respectively, with a significant difference between the two groups (χ2 = 5.101, P = 0.024). In the subgroup of radical surgery, the 5- and 10-year OS were 44.3% and 34.2% in the younger group, and 69.6% and 60.5% in the older group, with a difference being significant between the two groups (χ2 = 7.830, P = 0.005).
CONCLUSION: Compared with older patients, the younger patients have lower survival, especially in the subgroups of stage III and IV disease and radical surgery.
Core tip: We firstly described the clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. The incidence rate of colorectal cancer in young patients was higher than that in other reports. Younger patients with colorectal cancer had more poorly differentiated and advanced tumors, and worse prognosis, especially patients with stage III and IV disease.
- Citation: Fu JF, Huang YQ, Yang J, Yi CH, Chen HL, Zheng S. Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol 2013; 19(44): 8078-8084
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8078.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8078
As a kind of common cancer, colorectal cancer severely threatens the health of people. Colorectal cancer is the fourth common cancer and the second leading cause of cancer death in the world[1]. The majority of patients are affected in their 50s to 70s, but the age at diagnosis is getting younger[2]. The annual percentage of colorectal cancer in young people is increasing[2]. There has been an increasing number of reports about young colorectal cancer patients in recent years. The outcomes of young colorectal cancer patients varied widely among different regions[2-4]. The incidence rate of colorectal cancer in young patients has also been increasing in recent years in China[5]. Nearly all reports showed that young colorectal cancer patients had specific clinicopathologic characteristics, including poor histological feature, and more mucinous tumors, signet ring cell tumors, and advanced tumors[6-8]. However, the relationship between the age and survival was not confirmed. Some reports documented that young colorectal cancer patients had worse survival compared with the older counterparts[2,7-9]. But the others indicated opposite results[10-15]. There are still controversies about the definition of the age of young population. This study was to retrospectively analyze the data of patients with colorectal cancer who received surgery at our center over the past 30 years. Based on the distribution of the age, the population with colorectal cancer aged < 30 years was considered as a special subgroup in our center. Therefore, the young population was defined as those aged ≤ 30 years in our study. This study was designed to explore the clinicopathologic characteristics and prognosis of young colorectal cancer patients in Eastern China.
A total of 1335 consecutive patients with colorectal cancer (aged 19-92 years, mean 58 ± 13.3 years) treated from December 1985 to December 2005 at the Second Affiliated Hospital of Zhejiang University School of Medicine, located in Eastern China, were studied retrospectively. The patients were divided into two groups, a younger group (42 cases, aged ≤ 30 years, average age, 26.0 ± 3.5 years) and an older group (1293 cases, aged > 30 years, average age 58.0 ± 12.3 years). The criteria for inclusion were as follows: (1) patients with pathologically confirmed colorectal cancer; and (2) patients who underwent operations, including palliative surgeries. Patients with anal cancer or non-adenomas were excluded. Following the approval by the ethics committee of the hospital, the data including age, gender, tumor location, histological grade, approach of surgery, tumor infiltration, number of metastatic lymph nodes, distant metastasis and survival were obtained. Follow-up was made every 3 mo for 2 years, 6 mo for 5 years, then every one year. The follow-up proceeded through telephone calls or mail correspondence. The events of relapse and death in all patients were recorded.
The deadline of follow-up was November 2011. The follow-up lasted 0-302 mo (median, 57.0 ± 68.1 mo). Finally, 1335 patients who had complete data were analyzed; 267 patients (20.0%) were lost to follow-up, with 5 patients (11.9%) in the younger group and 262 patients (20.3%) in the older group. There was no significant difference in the percentage of lost patients between two groups (P = 0.183). The lost patients were taken as censors when the survival was analyzed. Twenty-nine patients died of colorectal cancer in the younger group and 604 patients died in the older group, including 51 patients who died due to other causes. They were considered as censors when cancer-related survival was calculated. All 1335 cases were included when we analyzed the clinicopathologic difference between the two groups.
The tumor was staged according to the 7th pathologic TNM staging system of AJCC[16]. Tumor location was described in detail as the cecum, ascending colon, liver flexure colon, transverse colon, descending colon, sigmoid, sigmorectal junction and rectum. Overall survival was calculated from the time of operation to death. Cancer-related survival was from the time of operation to the date of death because of the colorectal cancer. Causes of non-special cancer-related death included benign disease, accident, and secondary cancer. Radical surgery was classified as a procedure for no residual tumor left behind microscopically at resection margins. Palliative surgery was defined as a procedure for the residual tumor left macroscopically, which also included bypass or ileostomy. All palliative surgeries were considered as non-radical surgery.
Data of all categorical variables are summarized using frequencies and percentages. The data were analyzed with χ2 test. Overall survival was calculated according to the Kaplan-Meier method. Survival rates were compared by the log-rank test. A multivariate analysis was performed using the Cox model. When a P-value was less than 0.05, the difference was considered significant. SPSS 16.0 statistical software was used for data analysis.
The patient age ranged from 19 to 92 years, with a median of 58 ± 13.3 years. There were 42 (3.1%) and 1293 (96.9%) cases in the younger group and older group, respectively. The ratio of male to female was 1.3:1 in both groups.
The rectum was the frequent location in colorectal cancer, with a slightly higher rate in the younger group than in the older group (59.5% vs 49.3%, P > 0.05). Compared with the older group, significantly more patients in the younger group had mucinous tumor (33.3% vs 13.8%, P = 0.000), signet ring cell cancer (7.1% vs1.7%, P = 0.010) and poorly differentiated tumor (59.5% vs 15.7%, P = 0.000).
As for tumor infiltration, no tumor in situ (Tis) was found in the younger group, but 17 (13.1%) patients in older group were diagnosed with tumor in situ (Tis). Interestingly, there was no significant difference between the two groups in the tumor infiltration (P = 0.264). The percentages of patients with lymph node metastasis (≥ 4 lymph nodes), distance metastasis, stage IV and stage I disease and radical surgery were 35.7%, 28.6%, 31.0%, 2.4% and 66.7%, respectively, in the younger group, and 14.2%, 15.4%, 15.5%, 30.2% and 83.7%, respectively, in the older group, with significant differences between the two groups (P = 0.021, 0.021, 0.007, 0.008 and 0.008, respectively) (Table 1).
| Variable | Younger group (n = 42) ( ≤30 yr) | Older group (n = 1293) (> 30 yr) | P value |
| Gender | NS | ||
| Male | 24 (57.1) | 738 (57.1) | |
| Female | 18 (42.9) | 555 (42.9) | |
| Location of tumor | |||
| Cecum | 1 (2.4) | 69 (5.3) | |
| Ascending colon | 1 (2.4) | 154 (11.9) | |
| Hepatic flexure | 1 (2.4) | 86 (6.7) | |
| Transverse colon | 2 (4.8) | 52 (4.0) | |
| Splenic flexure | 1 (2.4) | 29 (2.2) | |
| Descending colon | 6 (14.3) | 49 (3.8) | |
| Sigmoid | 5 (11.9) | 201 (15.5) | |
| Rectosigmoid junction | 0 (0) | 16 (1.2) | |
| Rectum | 25 (59.5) | 637 (49.3) | 0.1911 |
| Histology | |||
| Mucinous cancer | 14 (33.3) | 179 (13.8) | 0.0002 |
| Signet ring cell cancer | 3 (7.1) | 22 (1.7) | 0.0103 |
| Papillary adenocarcinoma | 5 (11.9) | 237 (18.3) | |
| Tubular adenocarcinoma | 17 (40.5) | 732 (56.6) | |
| Undifferentiated adenocarcinoma | 1 (2.4) | 6 (0.5) | |
| Adenosquamous cancer | 0 (0) | 2 (0.2) | |
| Adenocarcinoma (unclassified) | 2 (4.8) | 115 (8.9) | |
| Differentiation | 0.000 | ||
| Well | 2 (9.5) | 276 (21.3) | |
| Moderate | 14 (33.3) | 630 (48.7) | |
| Poor | 25 (28.6) | 203 (15.7) | |
| Undifferentiated | 1 (2.4) | 184 (14.2) | |
| Stage T | 0.264 | ||
| Tis | 0 (0) | 17 (1.3) | |
| T1 | 1 (2.4) | 41 (3.2) | |
| T2 | 4 (9.5) | 232 (17.9) | |
| T3 | 16 (38.1) | 526 (40.7) | |
| T4 | 21 (50.0) | 477 (36.9) | |
| Number of metastatic lymph nodes | 0.001 | ||
| 0 | 11 (26.2) | 669 (51.7) | |
| 1-3 | 10 (23.8) | 337 (26.1) | |
| > 4 | 15 (35.7) | 184 (14.2) | |
| Nx | 6 (14.3) | 103 (8.0) | |
| Distant metastasis | 0.021 | ||
| M0 | 30 (71.4) | 1094 (84.6) | |
| M1 | 12 (28.6) | 99 (15.4) | |
| AJCC stage | 0.001 | ||
| 0 | 0 (0) | 17 (1.3) | |
| I | 1 (2.4) | 221 (17.1) | 0.0084 |
| II | 9 (21.4) | 427 (33.0) | |
| III | 19 (45.2) | 428 (33.1) | |
| IV | 13 (31.0) | 200 (15.5) | 0.0075 |
| Approach of surgery | 0.028 | ||
| Radical surgery | 28 (66.7) | 1084 (83.8) | |
| Palliate surgery | 10 (23.8) | 151 (11.7) | |
| Unresectable | 4 (9.5) | 58 (4.5) | |
Univariate analysis showed that there was a significant difference in total overall survival between the two groups (χ2 = 14.146, P = 0.000) (Figure 1, Table 2). Multivariate analysis revealed that age was not an independent factor for the prognosis of colorectal cancer (OR = 0.866, 95%CI: 0.592-1.269, P = 0.461). TNM stage III/IV, the approach of palliative surgery, rectal cancer, mucinous cancer and poorly differentiated tumor were independent factors for worse prognosis (Table 2).
| Variable | OR | 95%CI | P value |
| Stage (III + IV/I + II) | 2.196 | 1.827-2.639 | 0.000 |
| Approach of surgery (non-radical/radical) | 4.496 | 3.718-5.437 | 0.000 |
| Age (> 30 yr/ ≤ 30 yr) | 0.866 | 0.592-1.269 | 0.461 |
| Gender (male/female) | 0.997 | 0.852-1.167 | 0.970 |
| Tumor location (rectum/colon) | 1.270 | 1.084-1.488 | 0.003 |
| Differentiation (moderate + well/low ) | 0.802 | 0.650-0.990 | 0.041 |
| Histology (others/mucinous) | 0.791 | 0.632-0.990 | 0.041 |
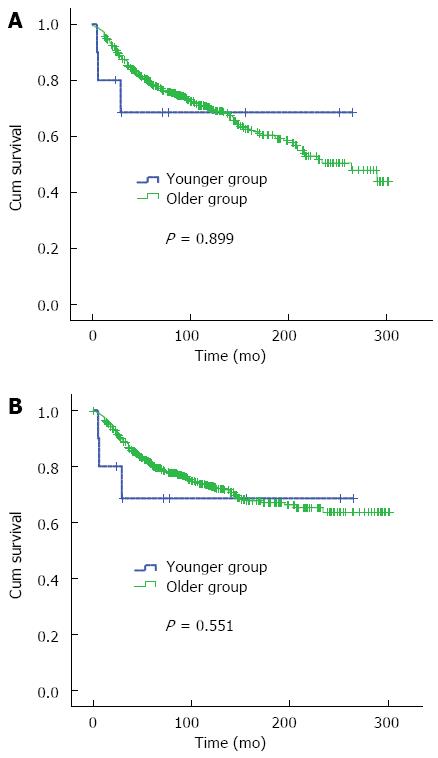
As for stage I and II disease, the 10-year overall survival and median survival time had not reached until the deadline in the younger group, which might be due to the small sample size of the study. There was no significant difference in the 10-year overall survival and median survival time between the two groups (χ2 = 0.016, P = 0.899) (Figure 2A, Table 3). Fifty-one patients died of other diseases in the older group. In order to diminish the influence of the non-cancer death, the cases in the subgroup of stage I and II disease were analyzed; as a result, there was also no difference in cancer-related survival between the two groups (χ2 = 0.356, P = 0.551) (Figure 2B). For stage III and IV disease, the outcome was worse in the younger group than in the older group (χ2 = 5.101, P = 0.024) (Figure 3, Table 3). For the subgroup of radical surgery, in the older group, the median survival time had not reached until the deadline. There was a significant difference in median survival time between the two groups (χ2 = 7.830, P = 0.005) (Figure 4A, Table 3). In the non-radical surgery subgroup, there was no significant difference in median survival time between the two groups (χ2 = 0.112, P = 0.737) (Figure 4B, Table 3).
| Age (yr) | n | 5-yr OS | 10-yr OS | Median survival time (mo, 95%CI) | |
| Total1 | ≤ 30 | 42 | 33.90% | 26.10% | 29.0 (18.0-40.0) |
| > 30 | 1293 | 60.10% | 52.20% | 140.0 (111.6-168.4) | |
| Stage I and II | ≤ 30 | 10 | 68.60% | /4 | /4 |
| > 30 | 665 | 78.60% | 69.80% | 264.0 (203.5-324.5) | |
| Stage III and IV2 | ≤ 30 | 32 | 24.60% | 14.80% | 22 (2.6-41.4) |
| < 30 | 628 | 40.40% | 33.30% | 35 (27.9-42.1) | |
| Radical surgery3 | ≤ 30 | 28 | 44.30% | 34.20% | 40.0 (10.1-69.9) |
| > 30 | 1082 | 69.60% | 60.50% | /5 | |
| Non-radical surgery | ≤ 30 | 14 | 14.30% | 0% | 6 (2.3-9.7) |
| > 30 | 211 | 11.80% | 0% | 11 (9.2-12.8) |
A total of 1335 patients with colorectal cancer were analyzed retrospectively in this study, including 42 (3.1%) patients in the younger group (aged ≤ 30 years). In other studies, the incidence rate was less than 1% and 3% if young patients with colorectal cancer were defined as those aged ≤ 30 years[17-19] and ≤ 40 years[4,15], respectively. The incidence rate in this study was higher than in other regions, suggesting an obvious regional difference. In this study, Eastern China refers to Yangtze River delta region where people enjoy a similar lifestyle and economic status. Consequently, the epidemiological characteristics of colorectal cancer in this region are similar. Therefore, data from our center could represent the features of this tumor in Eastern China. There might be statistical biases about the incidence rate of colorectal cancer in young patients because the data were collected retrospectively by a single medical center.
There was no significant difference in gender ratio between the two groups. The percentage of female patients is becoming higher with the trend of younger age in gastric cancer. This phenomenon was not seen in colorectal cancer. Estrogen was considered to be related with gastric cancer in younger patients[20]. It is not clear whether estrogen was related to the occurrence of colorectal cancer in young people[21,22]. On the other hand, this study indicated that female patients with colorectal cancer had better outcome than male patients, but with no significant difference (OR = 0.969, P = 0.708) in survival as shown by the multivariate analysis.
In this study, the rectum and sigmoid were common sites of the tumor in both groups. The proportion of rectal cancer was higher in the younger group (59.5%) than in older group (49.3%), but without significant difference (P = 0.191). Some reports indicated that the rate of rectal cancer in younger population was higher than in older one[2]. That might be related to the epidemics of colorectal cancer that rectal cancer is more common than colonic cancer in China. Eating habit and lifestyle might contribute more to the occurrence of colorectal cancer than age.
In this study, mucinous tumor and signet ring cell cancer were more common in the younger group than in the older group. A majority of patients in the younger group had poor histologic grade compared with the older group. Studies on gastric cancer also indicated that there were more poorly differentiated cancers in younger population than in older population, especially signet ring cell cancer[23]. It was not clear about the age impact on the occurrence of gastrointestinal cancer.
Compared with the older population, the percentage of patients with stage IV disease increased and that of patients with stage I disease decreased in the younger group. As for the infiltration of tumor and nodal metastasis, the patients in the younger group presented with more aggressive findings. Some studies found that 66.0% of younger patients with colorectal cancer were diagnosed with stage III or IV disease, which was obviously lower (32.0%) in the older patients[24]. This may result from the poor differentiation and high aggressiveness of tumors which were often diagnosed in younger patients with colorectal cancer. Besides, younger patients with colorectal cancer often had delayed diagnosis, but the older ones would be diagnosed earlier through screening program.
Univariate analysis revealed that the patients in the younger group had poorer survival than those in the older group. The impact of young age on the prognosis of colorectal cancer is not confirmed. Some studies showed that young patients with colorectal cancer had more mucinous cancer and signet ring cancer, poorer histologic grade, later stage and worse prognosis[2,7-9]. But results were contradictory from other studies which indicated that young age had no impact on the prognosis[10-15]. In our study, young colorectal cancer patients had worse prognosis, while multivariate analysis indicated that age was not an independent factor for prognosis. Furthermore, multivariate analysis also showed that disease stage and approach of surgery were strongly related to the prognosis. Worse prognosis might result from stage III and IV disease and non-radical surgery. Therefore, stratified analyses with these two factors were carried out.
The result of stratified analysis with stage indicated that younger patients had poor prognosis, and univariate analysis showed that younger patients presented with mainly stage III and IV disease. The reasons might be that young patients had more poorly differentiated tumor, and mucinous carcinoma and signet ring cell cancer, which were more aggressive in the same stage. As for patients with stage I and II disease, age exerted no effect on the survival. In this study, there were more patients in the older group who did not die of colorectal caner. In order to exclude the influence of the non-cancer special death, the cancer-related survival in patients with stage I and II disease was analyzed. The result showed no significant difference in cancer-related survival in stage I and II tumor between the two groups. The study of Quah et al[25] considered that patients with an earlier stage disease had better survival in younger group than older group; young patients were more tolerable to surgery and aggressive adjuvant chemotherapy and radiotherapy[26]. And the study of McMillan et al[27] indicated that in the older group, non-special cancer factors were major causes of death.
The stratified analysis with approach of surgery revealed that patients had poorer prognosis in the younger group than in the older group with radical surgery, but there was no significant difference between the two groups without radical surgery. In stratified analysis with stage, patients with stage I and II disease had similar prognosis between the two groups. Stage I and II tumors were often considered to be resectable. For patients with resectable stage III and stage IV tumors, younger age strongly contributed to poor survival. For patients who received operation without adjuvant chemotherapy in the 1980s and 1990s, the value of postoperative adjuvant therapy should be highlighted for patients with resectable stage III or more advanced colorectal cancer[28].
The current study had some limitations. The clinical data did not include the signs and symptoms of colorectal cancer patients. It was impossible to identify the alarming symptoms for younger patients. Family histories were not described, which were routinely detected in young population as the other studies[29]. The percentage of lost patients was 20%, which might influence the result of survival. In China, there are several medical centers owning elaborate clinical data, but few centers carried out the systemic follow-up. The data of 10-year follow-up are rare.
In summary, compared with older patients, the younger ones have specific clinicopathologic characteristics that are worthy to be explored and managed differentially. Younger patients with colorectal cancer tend to be diagnosed at later stage. For younger patients who have poor survival, especially those with stage III and IV disease and treated by radical surgery, more aggressive adjuvant therapies are recommended.
The incidence rate of colorectal cancer has been increasing in recent years. The onset age of colorectal cancer is getting younger. Should the young colorectal cancer patients be treated as a heterogeneous group? It is important to explore the phenotype of young patients with colorectal cancer.
Age is an independent prognostic factor for many cancers such as breast cancer, thyroid cancer and gastric cancer. Young patients have more triple negative breast cancers and worse prognosis. Lymph node-positive thyroid cancers are commonly diagnosed in adolescent patients, who have satisfactory prognoses. Young patients with gastric cancer in early stage have better prognosis than old ones, while their prognoses are worse in advanced gastric cancer. It is unknown about the age impact on the prognosis of colorectal cancer. Some studies showed that young patients with colorectal cancer had more mucinous cancer and signet ring cancer, poorer histologic grade, later stage and worse prognosis. But results were contradictory in other studies which indicated that the young age had no impact on prognosis.
The authors described systematically for the first time the clinical characteristics and prognosis of young colorectal cancer patients in Eastern China. The incidence rate of young colorectal cancer was higher than in other reports. Colorectal cancer in younger patients was characterized by poorer differentiation and advanced stage. Young colorectal cancer patients had worse prognosis, especially those with stage III and IV disease, rather than stage I and II disease.
Relapse risk of postoperative stage II colonic cancer is a crucial factor for decision-making in postoperative treatment. This study showed that age was not an independent risk factor for stage II colorectal cancer. On the other hand, young colorectal cancer patients with stage III and IV disease had worse prognosis, and more aggressive adjuvant therapy is recommended for these patients.
Young patients with colorectal cancer: Onset age of colorectal cancer was less than or equal to 30 years. Eastern China refers to Yangtze River delta region where people have similar lifestyle and economic conditions. Epidemiological characteristics of colorectal cancer in this region are also similar.
The study described the detailed clinicopathologic characteristics of 1335 cases of colorectal cancer in Eastern China and analyzed the significance of prognosis by many statistical methods. The major goal of authors was to analyze the difference between younger patients (≤ 30 year-old) and older patients (> 30 year-old). The information enclosed in this manuscript is very plentiful and clear, and the authors applied many different statistical methods to perform the analysis. Although the results are not novel and methodology was orthodox, it is worth reporting the present results.
P- Reviewers: Catena F, Hung LY, Tsuda H S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1505] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 2. | You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 3. | Neufeld D, Shpitz B, Bugaev N, Grankin M, Bernheim J, Klein E, Ziv Y. Young-age onset of colorectal cancer in Israel. Tech Coloproctol. 2009;13:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | McMillan DC, McArdle CS. The impact of young age on cancer-specific and non-cancer-related survival after surgery for colorectal cancer: 10-year follow-up. Br J Cancer. 2009;101:557-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wu QJ, Vogtmann E, Zhang W, Xie L, Yang WS, Tan YT, Gao J, Xiang YB. Cancer incidence among adolescents and young adults in urban Shanghai, 1973-2005. PLoS One. 2012;7:e42607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Endreseth BH, Romundstad P, Myrvold HE, Hestvik UE, Bjerkeset T, Wibe A. Rectal cancer in the young patient. Dis Colon Rectum. 2006;49:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Chan KK, Dassanayake B, Deen R, Wickramarachchi RE, Kumarage SK, Samita S, Deen KI. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: analysis of survival and prognostic markers. World J Surg Oncol. 2010;8:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Kaplan MA, Isikdogan A, Gumus M, Arslan UY, Geredeli C, Ozdemir N, Koca D, Dane F, Suner A, Elkiran ET. Childhood, adolescents, and young adults (≤25 y) colorectal cancer: study of Anatolian Society of Medical Oncology. J Pediatr Hematol Oncol. 2013;35:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Do young colon cancer patients have worse outcomes? World J Surg. 2004;28:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Yeo SA, Chew MH, Koh PK, Tang CL. Young colorectal carcinoma patients do not have a poorer prognosis: a comparative review of 2,426 cases. Tech Coloproctol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Taggarshe D, Rehil N, Sharma S, Flynn JC, Damadi A. Colorectal cancer: are the “young” being overlooked? Am J Surg. 2013;205:312-36; discussion 316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Ko CY. Are survival rates different for young and older patients with rectal cancer? Dis Colon Rectum. 2004;47:2064-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Chung YF, Eu KW, Machin D, Ho JM, Nyam DC, Leong AF, Ho YH, Seow-Choen F. Young age is not a poor prognostic marker in colorectal cancer. Br J Surg. 1998;85:1255-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Li M, Li JY, Zhao AL, Gu J. Do young patients with colorectal cancer have a poorer prognosis than old patients? J Surg Res. 2011;167:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Enblad G, Enblad P, Adami HO, Glimelius B, Krusemo U, Påhlman L. Relationship between age and survival in cancer of the colon and rectum with special reference to patients less than 40 years of age. Br J Surg. 1990;77:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Ueno H, Mochizuki H, Akagi Y, Kusumi T, Yamada K, Ikegami M, Kawachi H, Kameoka S, Ohkura Y, Masaki T. Optimal colorectal cancer staging criteria in TNM classification. J Clin Oncol. 2012;30:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Al-Barrak J, Gill S. Presentation and outcomes of patients aged 30 years and younger with colorectal cancer: a 20-year retrospective review. Med Oncol. 2011;28:1058-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Rodriguez-Bigas MA, Mahoney MC, Weber TK, Petrelli NJ. Colorectal cancer in patients aged 30 years or younger. Surg Oncol. 1996;5:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Kam MH, Eu KW, Barben CP, Seow-Choen F. Colorectal cancer in the young: a 12-year review of patients 30 years or less. Colorectal Dis. 2004;6:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Kim JH, Boo YJ, Park JM, Park SS, Kim SJ, Kim CS, Mok YJ. Incidence and long-term outcome of young patients with gastric carcinoma according to sex: does hormonal status affect prognosis? Arch Surg. 2008;143:1062-107; discussion 1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Koo JH, Jalaludin B, Wong SK, Kneebone A, Connor SJ, Leong RW. Improved survival in young women with colorectal cancer. Am J Gastroenterol. 2008;103:1488-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Olofinlade O, Adeonigbagbe O, Gualtieri N, Freiman H, Ogedegbe O, Robilotti J. Colorectal carcinoma in young females. South Med J. 2004;97:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Park JC, Lee YC, Kim JH, Kim YJ, Lee SK, Hyung WJ, Noh SH, Kim CB. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol. 2009;99:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Quah HM, Joseph R, Schrag D, Shia J, Guillem JG, Paty PB, Temple LK, Wong WD, Weiser MR. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol. 2007;14:2759-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Pedrazzani C, Cerullo G, De Marco G, Marrelli D, Neri A, De Stefano A, Pinto E, Roviello F. Impact of age-related comorbidity on results of colorectal cancer surgery. World J Gastroenterol. 2009;15:5706-5711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | McMillan DC, Hole DJ, McArdle CS. The impact of old age on cancer-specific and non-cancer-related survival following elective potentially curative surgery for Dukes A/B colorectal cancer. Br J Cancer. 2008;99:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Lin JT, Wang WS, Yen CC, Liu JH, Yang MH, Chao TC, Chen PM, Chiou TJ. Outcome of colorectal carcinoma in patients under 40 years of age. J Gastroenterol Hepatol. 2005;20:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Boardman LA, Morlan BW, Rabe KG, Petersen GM, Lindor NM, Nigon SK, Goldberg J, Gallinger S. Colorectal cancer risks in relatives of young-onset cases: is risk the same across all first-degree relatives? Clin Gastroenterol Hepatol. 2007;5:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |