Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8020
Revised: June 27, 2013
Accepted: September 16, 2013
Published online: November 28, 2013
Processing time: 298 Days and 8.1 Hours
AIM: To investigate the expression of the hepatitis B virus (HBV) 1.3-fold genome plasmid (pHBV1.3) in an immortalized mouse hepatic cell line induced by SV40 T-antigen (SV40T) expression.
METHODS: Mouse hepatic cells were isolated from mouse liver tissue fragments from 3-5 d old Kunming mice by the direct collagenase digestion method and cultured in vitro. The pRSV-T plasmid was transfected into mouse hepatic cells to establish an SV40LT-immortalized mouse hepatic cell line. The SV40LT-immortalized mouse hepatic cells were identified and transfected with the pHBV1.3 plasmid. The levels of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) in the supernatant were determined by an electrochemiluminescence immunoassay at 24, 48, 72 and 96 h after transfection. The expressions of HBsAg and hepatitis B c antigen (HBcAg) in the cells were investigated by indirect immunofluorescence analysis. The presence of HBV DNA replication intermediates in the transfected cells and viral particles in the supernatant of the transfected cell cultures was monitored using the Southern hybridization assay and transmission electronic microscopy, respectively.
RESULTS: The pRSV-T plasmid was used to immortalize mouse hepatocytes and an SV40LT-immortalized mouse hepatic cell line was successfully established. SV40LT-immortalized mouse hepatic cells have the same morphology and growth characteristics as primary mouse hepatic cells can be subcultured and produce albumin and cytokeratin-18 in vitro. Immortalized mouse hepatic cells did not show the characteristics of tumor cells, as alpha-fetoprotein levels were comparable (0.58 ± 0.37 vs 0.61 ± 0.31, P = 0.37). SV40LT-immortalized mouse hepatic cells were then transfected with the pHBV1.3 plasmid, and it was found that the HBV genome replicated in SV40LT-immortalized mouse hepatic cells. The levels of HBsAg and HBeAg continuously increased in the supernatant after the transfection of pHBV1.3, and began to decrease 72 h after transfection. The expressions of HBsAg and HBcAg were observed in the pHBV1.3-transfected cells. HBV DNA replication intermediates were also observed at 72 h after transfection, including relaxed circular DNA, double-stranded DNA and single-stranded DNA. Furthermore, a few 42 nm Dane particles, as well as many 22 nm subviral particles with a spherical or filamentous shape, were detected in the supernatant.
CONCLUSION: SV40T expression can immortalize mouse hepatic cells, and the pHBV1.3-transfected SV40T-immortalized mouse hepatic cell line can be a new in vitro cell model.
Core tip: This study established a new immortalized mouse hepatic cell line through the transfection of the pRSV-T plasmid. SV40 T-antigen (SV40LT)-immortalized mouse hepatic cells had the same morphology and biological characteristics as primary mouse hepatic cells. SV40LT-immortalized mouse hepatic cells could be transfected with the pHBV1.3 plasmid, which caused the hepatitis B virus (HBV) genes to replicate in SV40LT-immortalized mouse hepatic cells. The expressions of hepatitis B surface antigen and hepatitis B c antigen, as well as the presence of HBV DNA replication intermediates, were observed in the pHBV1.3-transfected cells. This cell model will contribute to the research of HBV and the evaluation of anti-viral drugs in vivo.
- Citation: Song XG, Bian PF, Yu SL, Zhao XH, Xu W, Bu XH, Li X, Ma LX. Expression of hepatitis B virus 1.3-fold genome plasmid in an SV40 T-antigen-immortalized mouse hepatic cell line. World J Gastroenterol 2013; 19(44): 8020-8027
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8020
Chronic hepatitis B (CHB) is a severe public health problem that affects more than 400 million people worldwide and causes more than one million deaths annually[1]. Recent studies have shown that the correlation between serum hepatitis B virus (HBV) DNA levels and the risk of developing cirrhosis and hepatocellular carcinoma (HCC) is stronger than other baseline or virologic parameters[2]. The ultimate long-term goal of therapy is to achieve a “durable response” to prevent hepatic decompensation, reduce or prevent progression to cirrhosis and/or HCC, and prolong survival[3]. Moreover, it has now become clear that continuous suppression of HBV replication can revert liver fibrosis or even cirrhosis in most patients[4].
Treatment of hepatitis B depends on several factors, such as the stage of disease, the presence or absence of the “e” antigen, and the potential for drug resistance and subsequent inability to use a medicine, particularly in the advanced stages of chronic disease of the liver. Therefore, it is very important to evaluate these factors at the time the decision is made regarding the type and duration of treatment[5]. Interferon alpha-2a and interferon alfa-2b (IFN 2a and 2b)-based therapies have been used for many years as the preferential treatment approaches for cases with low levels of HBV DNA and high levels of alanine aminotransferase (ALT)[6-8]. The goal of treatment is to activate an immune response leading to hepatitis B e antigen (HBeAg) seroconversion[9]. This type of treatment, as the first option to modulate the immune system, aims to achieve elimination or remission.
However, this course of treatment results in a high cost, and also produces many adverse side effects, such as anaemia, a significant decrease in hemoglobin, vomiting, cold sweats and nausea[10]. Previous studies have reported that only about one out of three patients receives IFN therapy[11-13]. Furthermore, nucleoside analogues cannot completely eliminate the virus, and may lead to the mutation of the virus[14]. Thus, the development of new antiviral treatments remains a major research task. Additionally, new suitable HBV-infected animal cell models are urgently required to evaluate new treatment strategies. Therefore, this study aimed to establish a new immortalized mouse hepatic cell line induced by SV40 T-antigen (SV40T) expression, and to investigate the expression of the HBV 1.3-fold genome plasmid (pHBV1.3) in the established SV40T-immortalized mouse hepatic cell line.
Kunming mice (3-5 d old) were provided by the clinical drug trial-based Animal Laboratory of Shandong University. Livers were collected from these mice, and mouse hepatic cells were isolated from the liver tissue fragments by the direct collagenase digestion method[15]. The isolated cells were cultured in the 1640 culture medium (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 IU/mL penicillin and 100 mg/mL streptomycin, and placed in the incubator with 5% CO2 and 37 °C. All animals received humane care in compliance with the Principles of Laboratory Animal Care. The protocol was approved by the Animal Care and Use Committee.
The pRSV-T plasmid[16] was provided by Professor Reddel RR of the Australia Children’s Medical Research Institute. The pRSV-T plasmid was transfected into primary mouse hepatic cells according to the instructions of the liposome transfection kit (Invitrogen, Grand Island, United States). Twenty-four hours later, the 1640 culture medium with 10% FBS was added. Forty-eight hours later, it was replaced by the 1640 culture medium supplemented with 10% FBS and 500 μg/mL G418. Cells were passaged every 5 d at a ratio of 1:2.
An SV40 monoclonal antibody (Thermo,Waltham, United States) was used to detect the SV40T antigen and its distribution in SV40T-transfected cells by the indirect immunofluorescence assay. Primary mouse hepatic cells were employed as the negative control.
An inverted phase contrast microscope and an electron microscope were used to observe the morphology and ultrastructure of the SV40T-transfected mouse hepatic cells.
The supernatants of primary and immortalized mouse hepatic cell cultures were collected. The levels of ALT, aspartate aminotransferase (AST) and alpha-fetoprotein (AFP) were determined by an automatic biochemical analyzer (Beckman, Boulevard Brea, United States). Primary cultured mouse hepatic cells were employed as the control.
After total RNA extraction of primary and SV40T-transfected hepatic cells with an RNA extraction kit (Invitrogen), reverse transcription polymerase chain reaction (RT-PCR) was used to determine albumin (ALB) mRNA levels as previously described[17].
Western blotting was used to detect the presence of cytokeratin-18 (CK-18) in primary and SV40T-transfected mouse hepatic cells (22nd generation). Rabbit anti-mouse cell CK-18 was the primary antibody employed and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulins (IgG) was used as the secondary antibody. These two antibodies were purchased from Boster Biological Technology, Ltd (Wuhan, China).
pHBV1.3[18] was provided by Professor Yin-Ping Lu of Huazhong University of Science and Technology. pHBV1.3 contains a 1.3-fold HBV genome (ayw subtype). Following the instructions of the Lipofectamine 2000 transfection kit (Invitrogen), the pHBV1.3 plasmid was transfected into SV40T-immortalized cells (22nd generation).
The supernatants of the pHBV1.3-transfected cell cultures were collected at different times. An AXSYM automatic electrochemiluminescence immunoassay analyzer (Abbott) was used to quantify the levels of HBsAg and HBeAg.
pHBV1.3-transfected cells were seeded in 24-well plates. The cells were washed with PBS three times and fixed with 4% paraformaldehyde at 4 °C for 20 min. The cells were then washed with PBS three times again and incubated with PBS-diluted 10% goat serum for 30 min. Then the indirect immunofluorescence assay was performed using a fluorescence kit. Mouse anti-HBcAg and mouse anti-HBsAg were purchased from Millipore Corporation, while fluorescein Isothiocyanate-conjugated goat anti-mouse IgG was purchased from Southern Biotech (Birmingham, United States).
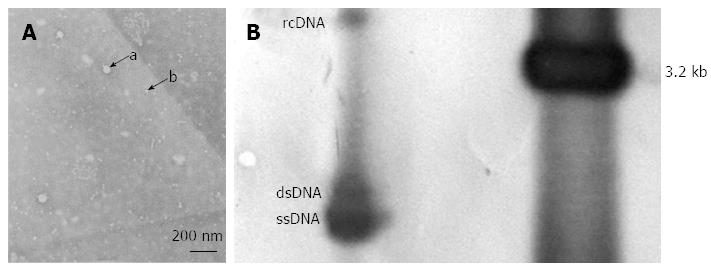
At 72 h post-transfection, a DNA extraction of pHBV1.3-transfected cells was performed for Southern hybridization analysis. The probe was the digoxigenin-labeled 3.2 kb HBV DNA[19]. The Southern kit was purchased from Roche (Indianapolis, United States). Southern hybridization was carried out according to the manufacturer’s instructions.
At 72 h after transfection, the supernatants of pHBV1.3-transfected cell cultures were collected. JEOL transmission electronic microscopy (JEM-1200EX Electron Microscope) was used to visualize the cells and photographs were taken.
Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, Shandong University, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC, United States). The study was approved by the Institutional Animal Care and Use Committee at Shandong University (approval no. SDU003341201).
The data are presented as means ± SD. Comparisons between groups of data were performed using Student’s t test. A difference with P value < 0.05 was considered to be statistically significant. Data were analyzed with the SPSS 11.0 statistical software package (SPSS Inc.; Chicago, IL, United States).
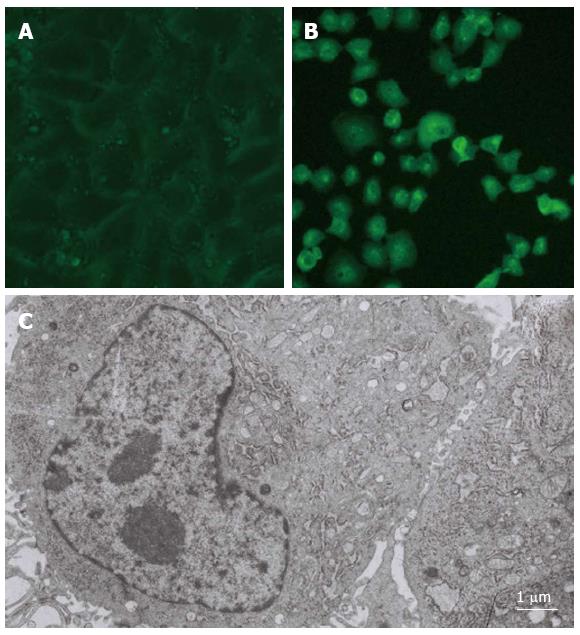
The epithelial cell-like positive clones were found 30 d after the mouse hepatic cells were transfected with a SV40T-expressing plasmid (pRSV-T) by lipofection; these cells were an adherent monolayer and flat-shaped and presented in a polygonal, cluster-like multi-cell arrangement (Figure 1A). The SV40T mouse hepatic cells displayed the typical morphology and structure of hepatic cells, and many glycogen granules, mitochondria and endoplasmic reticulum structures were clearly visible under the electron microscope (Figure 1C). Furthermore, the splitting dual-core cells reflected the in vitro proliferation and differentiation processes of the transfected hepatic cells (Figure 1C). Cells were passaged every five d at a ratio of 1:2 for 38 generations, and no change in cell morphology was observed.
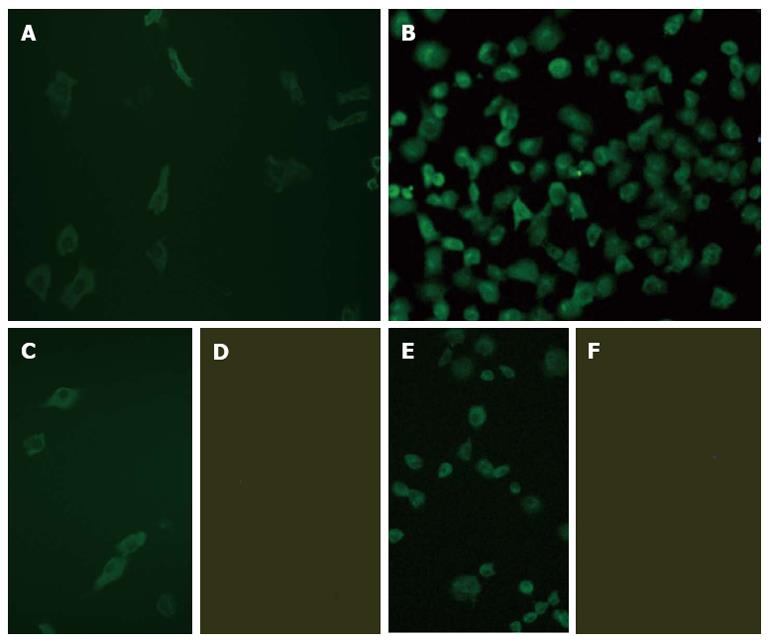
After SV40T transfection, the SV40 T-antigen immunofluorescence of the mouse hepatic cells gradually increased, and was visible 30 d after transfection. Matte-like fluorescence could be clearly detected in the cytoplasm, along with granular-like fluorescence in the nucleus (Figure 1B).
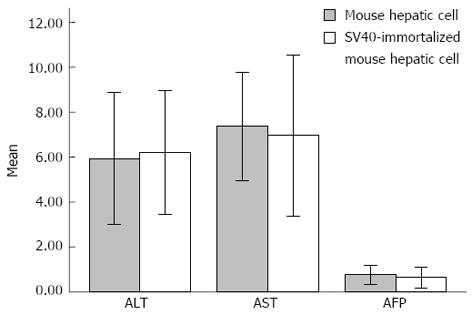
The quantified levels of ALT, AST and AFP in the supernatant of the cultures are shown in Figure 2. The levels of ALT, AST and AFP in the supernatant of mouse hepatic cell and SV40T-transfected hepatic cell cultures were 5.93 ± 1.47 vs 6.21 ± 1.38 (t = 0.481, P = 0.636), 7.36 ± 1.21 vs 6.96 ± 1.79 (t = 0.643, P = 0.527) and 0.76 ± 0.21 vs 0.65 ± 0.24 (t = 1.318, P = 0.201), respectively (n = 12). No significant difference in the levels of ALT, AST and AFP was observed between the mouse hepatic cell and SV40T-transfected hepatic cell cultures (P > 0.05).
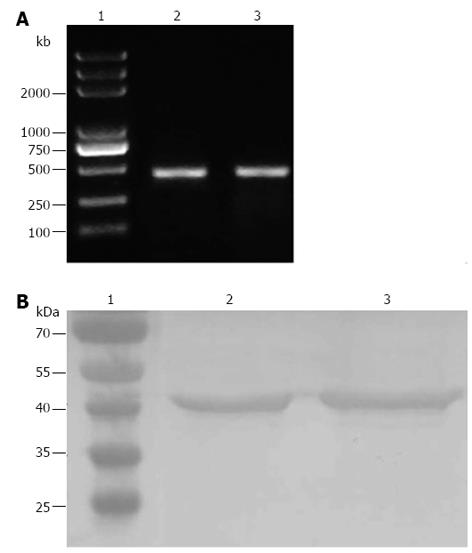
Following the total RNA extraction of SV40T-transfected hepatic cells (22nd generation) and RT-PCR, the ALB mRNA was apparent as a bright band at 475 bp (Figure 3A), indicating that SV40T-immortalized mouse hepatic cells had the ability to express ALB mRNA. Mouse hepatic cells were employed as the positive control.
Following the protein extraction of SV40T-transfected hepatic cells (22 generation), SDS-PAGE and Western blotting were carried out. Immunoblotting of SV40T-transfected hepatic cells demonstrated their expression of CK-18, and mouse hepatic cells, employed as the positive control, also displayed immunoreactivity for CK-18, as expected (Figure 3B).
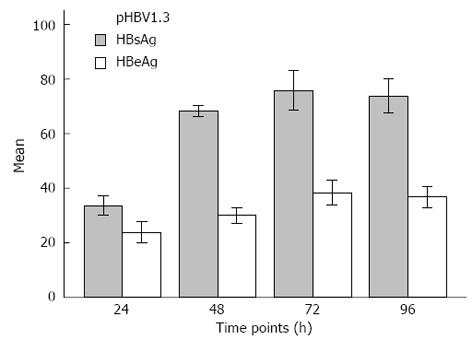
The levels of HBsAg and HBeAg in the supernatant were monitored 24, 48, 72 and 96 h after pHBV1.3 transfection. The results of this analysis are shown in Figure 4. The levels of HBsAg and HBeAg in the supernatant continuously increased after transfection of pHBV1.3, though they both began to gradually decrease after 72 h.
The expression of HBsAg and HBcAg were observed in the pHBV1.3-transfected cells at 24 h after transfection by immunofluorescence microscopy, with expression reaching a peak at 72 h. HBsAg was mainly observed in the cytoplasm, while HBcAg was detected in both the cytoplasm and nucleus, especially in the former (Figure 5). HBV DNA replication intermediates, including rcDNA, dsDNA and ssDNA, were also observed 72 h after transfection (Figure 6). Furthermore, a few 42 nm Dane particles, as well as many 22 nm subviral particles with a spherical or filamentous shape, were observed in the supernatant (Figure 6).
The development of another HBV in vitro cell model would provide a very important tool to study the biological characteristics of HBV, the pathogenesis of hepatitis B, the mechanism of carcinogenesis by HBV infection, and carry out in vitro anti-HBV drug screening 2.2.15 cells, a stable cell line which harbors the HBV genome, and can support HBV replication and the secretion of infectious virus particles[20-23]. However, the low expression of HBV was due to the low viral genome copy number integrated in the host cell chromosome. This drawback limits the application of this cell line as an in vitro cell model of HBV infection, especially as an infection model for antiviral drug-resistant mutant screening and investigation of biological characteristics[24].
Simian vacuolating virus 40 (SV40), which was first obtained from cultured rhesus monkey kidney cells[25], belongs to the Papovaviridae family[26]. The transfection of the SV40 early gene into cells is the most common method for cell immortalization. There are different opinions regarding the mechanism by which SV40LT causes cell immortalization[27,28]. This method has been found to be useful for a variety of human cell types[29]. The use of SV40 for cell immortalization has a long history. It has been used to immortalize epithelial cells of the bronchial intrahepatic bile duct, cervical cells and other cells[30-32]. Many studies of SV40T-immortalized cell lines have shown that the transduction of the SV40T gene can increase the growth rate of cells, while retaining the differentiation phenotype of many original cells and keeping the biological characteristics of the original cells, with the rare expression of a non-original gene[33,34]. These characteristics make the SV40T-immortalized cells suitable for use as an in vitro model. In this study, the pRSV-T plasmid was used to transfect the mouse hepatic cells to establish an SV40T-immortalized mouse hepatic cell line, which was selected through 500 μg/mL G418 screening.
Our experimental results showed that there were no significant differences in morphology between primary and SV40T-immortalized mouse hepatic cells. Furthermore, the same level of expression and secretion of ALB, as well as the CK-18 activity, was observed between the primary and SV40T-immortalized mouse hepatic cells (without any tumorigenic potential). At present, the SV40T-immortalized mouse hepatic cells have been passaged to the 38th generation.
The genome of the HBV 1.3-fold genome plasmid is smaller than that of the HBV 2.0-fold plasmid, and its efficiency of replication and expression is higher than that of the 1.2 and 1.1-fold plasmids[35]. The HBV 1.3-fold genome plasmid contains HBV 5’-end Enh I, Enh II, replication-origin (DR1, DR2), the former genome transcription start site, x and pre-C promoter, and x open reading frame. Hence, the HBV 1.3-fold genome plasmid is used most frequently. After Huh7 and HepG2 cells were transfected with the recombinant plasmid pHBV1.3, the in vitro replication and expression of the HBV gene could be detected, and high levels of HBsAg/HBeAg intermediates and transcripts involved in HBV DNA replication were also found[18,36]. In this study, the pHBV1.3 plasmid was transfected into SV40T-immortalized mouse hepatic cells. After transfection, the expression of HBsAg and HBcAg was observed in the pHBV1.3-transfected cells. Additionally, HBV DNA replication intermediates, including relaxed circular DNA, double-stranded DNA and single-stranded DNA, were also observed 72 h after transfection. Furthermore, a few 42 nm Dane particles, as well as many 22 nm subviral particles with a spherical or filamentous shape, were observed in the supernatant.
In summary, our findings suggest that the expression of the SV40T gene immortalized a mouse hepatic cell line, and subsequent transfection of pHBV1.3 established a new in vitro cell model for anti-HBV drug research.
Chronic hepatitis B (CHB) is a severe public health problem, and treatment of hepatitis B poses many challenges, such as treatment for an advanced stage of disease and the potential for drug resistance. Thus, the development of new antiviral treatments remains a major research task, and new suitable hepatitis B virus (HBV)-infected in vitro cell models are urgently required to evaluate new therapeutic strategies. With this in mind, this study aimed to establish a new immortalized mouse hepatic cell line induced by SV40 T-antigen (SV40T) expression, and to investigate the consequences of HBV 1.3-fold genome plasmid (pHBV1.3) expression in this SV40T-immortalized mouse hepatic cell line.
SV40LT antigen has the ability to immortalize some animal cells. The genome of the HBV 1.3-fold genome plasmid is smaller than that of the HBV 2.0-fold plasmid, and its efficiency of replication and expression is higher than that of the 1.2- and 1.1-fold plasmids. The HBV 1.3-fold genome plasmid contains HBV 5’-end Enh I, Enh II, replication-origin (DR1, DR2), the former genome transcription start site, x and pre-C promoter, and x open reading frame. Hence, the HBV 1.3-fold genome plasmid is used most frequently. The genome of the HBV 1.3-fold genome plasmid has been shown to replicate in HepG2 and Hu7 cell lines. According to the authors, there has been no report concerning the expression of the HBV 1.3-fold genome plasmid in an immortalized mouse hepatic cell line.
The SV40LT antigen immortalized mouse hepatic cells, which was not found in previous reports. In this study, a new immortalized mouse hepatic cell line was established through the transfection of the pRSV-T plasmid into primary mouse hepatic cells. The genome of the HBV 1.3-fold genome plasmid can replicate in human hepatoma cell lines (HepG2 or HUH7 cells). The authors successfully transfected the pHBV1.3 plasmid into the immortalized mouse hepatic cell line and observed the expression of HBV. The new cell model established in this study will contribute to the research of HBV and the evaluation of anti-viral drugs in vivo.
This cell model will contribute to the research of HBV and the evaluation of anti-viral drugs in vivo.
This is a good basic study in which authors transfected the pRSV-T plasmid into primary mouse hepatic cells and established a new immortalized mouse hepatic cell line. The authors transfected the pHBV1.3 plasmid into the immortalized mouse hepatic cell line and observed the expression of HBV. This cell model would contribute to the research of HBV and the evaluation of anti-viral drugs in vivo.
P- Reviewers: Akyuz U, He JY S- Editor: Gou SX L- Editor: Ma JY E- Editor: Zhang DN
| 1. | Lau DT, Bleibel W. Current status of antiviral therapy for hepatitis B. Therap Adv Gastroenterol. 2008;1:61-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] |
| 3. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 4. | Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, Kryczka W, Lurie Y, Gadano A, Kitis G. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Shepherd J, Jones J, Takeda A, Davidson P, Price A. Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation. Health Technol Assess. 2006;10:iii-iv, xi-xiv, 1-183. [PubMed] |
| 6. | Buster EH, Schalm SW, Janssen HL. Peginterferon for the treatment of chronic hepatitis B in the era of nucleos(t)ide analogues. Best Pract Res Clin Gastroenterol. 2008;22:1093-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ozgenc F, Dikici B, Targan S, Doganci T, Akman S, Aydogdu S, Yagci RV. Comparison of antiviral effect of lamivudine with interferon-alpha2a versus -alpha2b in children with chronic hepatitis B infection. Antivir Ther. 2004;9:23-26. [PubMed] |
| 8. | Aoki YH, Ohkoshi S, Yamagiwa S, Yano M, Takahashi H, Waguri N, Igarashi K, Sugitani S, Takahashi T, Ishikawa T. Characterization of elevated alanine aminotransferase levels during pegylated-interferon α-2b plus ribavirin treatment for chronic hepatitis C. Hepatol Res. 2011;41:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, Chutaputti A, Chang WY, Zahm FE, Pluck N. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Gheorghe L, Iacob S, Sporea I, Grigorescu M, Sirli R, Damian D, Gheorghe C, Iacob R. Efficacy, tolerability and predictive factors for early and sustained virologic response in patients treated with weight-based dosing regimen of PegIFN alpha-2b ribavirin in real-life healthcare setting. J Gastrointestin Liver Dis. 2007;16:23-29. [PubMed] |
| 11. | Zuckerman AJ, Lavanchy D. Treatment options for chronic hepatitis. Antivirals look promising. BMJ. 1999;319:799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Lau DT, Everhart J, Kleiner DE, Park Y, Vergalla J, Schmid P, Hoofnagle JH. Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology. 1997;113:1660-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Häussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 574] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Zoulim F. Therapy of chronic hepatitis B virus infection: inhibition of the viral polymerase and other antiviral strategies. Antiviral Res. 1999;44:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zhang LP, Cang GF. Primary hepatocytes Research. Guoji Jianyan Yixue Zazhi. 2004;25:193-196. |
| 16. | De Silva R, Zahra DG, Duncan EL, Reddel RR. Immortalization of human fibroblasts by liposome-mediated transfer of SV4o early region genes. Meth Cell Sci. 1995;17:75-81. |
| 17. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] |
| 18. | Lu YP, Dong JH, Liu Z. Construction of HBV infectious replicon and its significance. Zhongguo Gonggong Weisheng. 2008;24:687-689. |
| 19. | He F, Tang H, Liu L, Liu FJ, Wang S, Zhou TY, Zhao LS, Liu C. Establishment of a highly sensitive chemiluminescent detection system for analysis of hepatitis B virus transcription and replication level in vitro. Shijie Huaren Xiaohua Zazhi. 2006;14:1346-1351. |
| 20. | Gagey D, Ravetti S, Castro EF, Gualdesi MS, Briñon MC, Campos RH, Cavallaro LV. Antiviral activity of 5’-O-carbonate-2’,3’-dideoxy-3’-thiacytidine prodrugs against hepatitis B virus in HepG2 2.2.15 cells. Int J Antimicrob Agents. 2010;36:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Huang KL, Lai YK, Lin CC, Chang JM. Involvement of GRP78 in inhibition of HBV secretion by Boehmeria nivea extract in human HepG2 2.2.15 cells. J Viral Hepat. 2009;16:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Gao LL, Wang XY, Lin JS, Zhang YH, Li Y. Efficacies of beta-L-D4A against Hepatitis B virus in 2.2.15 cells. World J Gastroenterol. 2008;14:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Kayhan H, Karatayli E, Turkyilmaz AR, Sahin F, Yurdaydin C, Bozdayi AM. Inhibition of hepatitis B virus replication by shRNAs in stably HBV expressed HEPG2 2.2.15 cell lines. Arch Virol. 2007;152:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Liu J, Li YH, Xue CF, Ding J, Gong WD, Zhao Y, Huang YX. Targeted ribonuclease can inhibit replication of hepatitis B virus. World J Gastroenterol. 2003;9:295-299. [PubMed] |
| 25. | Sweet BH, Hilleman MR. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960;105:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 357] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Fiers W, Contreras R, Haegemann G, Rogiers R, Van de Voorde A, Van Heuverswyn H, Van Herreweghe J, Volckaert G, Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978;273:113-120. [PubMed] |
| 27. | Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33-39. [PubMed] |
| 28. | Butel JS, Jarvis DL. The plasma-membrane-associated form of SV40 large tumor antigen: biochemical and biological properties. Biochim Biophys Acta. 1986;865:171-195. [PubMed] |
| 29. | Tevethia MJ, Ozer HL. SV40-mediated immortalization. Methods Mol Biol. 2001;165:185-199. [PubMed] |
| 30. | Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 750] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 31. | Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060-G1070. [PubMed] |
| 32. | Gorodeski GI, Merlin D, De Santis BJ, Frieden KA, Hopfer U, Eckert RL, Utian WH, Romero MF. Characterization of paracellular permeability in cultured human cervical epithelium: regulation by extracellular adenosine triphosphate. J Soc Gynecol Investig. 1994;1:225-233. [PubMed] |
| 33. | Harris CC. Human tissues and cells in carcinogenesis research. Cancer Res. 1987;47:1-10. [PubMed] |
| 34. | Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904-1909. [PubMed] |
| 35. | Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158-6169. [PubMed] |
| 36. | Tang N, Huang AL, Zhang BQ, Yan G, Xiang MQ, Pu D, Guo H. Construction of recombinant eukaryotlc expression plasmid containing 1.3.fold.overlength genome of HBV and its expression in HepG2 cells. Zhonghua Ganzangbing Zazhi. 2003;8:464-466. |