Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7820
Revised: September 8, 2013
Accepted: October 13, 2013
Published online: November 21, 2013
Processing time: 126 Days and 10.9 Hours
Sarcomatoid carcinoma of the pancreas (SCP) is a very rare pathological type of carcinoma that usually has a poor prognosis. Its pathogenesis has not been elucidated. We herein report a case of an early-stage SCP involving successful treatment and a good prognosis. The patient was a 48-year-old Chinese man with a 5-mo history of vague abdominal pain. Ultrasonography revealed a 93 mm × 94 mm × 75 mm mass of mixed echogenicity in the tail of the pancreas. Laboratory test results were within the normal range, with the exception of an obviously increased pretreatment neuron-specific enolase level. The plasma transforming growth factor (TGF)β1 and interleukin-11 levels were obviously increased according to enzyme-linked immunosorbent assay. Microscopically, the excised tumor tissue comprised cancer cells and mesenchymal cells. Immunohistochemical analysis was positive for α-1-antichymotrypsin, pan-cytokeratin, cytokeratin 19, cytokeratin 8/18, and vimentin and negative for CD68 and lysozyme. The pathogenetic mechanism of this case shows that TGFβ1 may regulate the epithelial-to-mesenchymal transition in SCP. With early eradication of the tumor and systemic therapy, this patient has been alive for more than 3 years without tumor recurrence or distant metastasis. This case is also the first to show that TGFβ1 may regulate the epithelial-to-mesenchymal transition in early-stage SCP.
Core tip: We herein report a case of an early-stage sarcomatoid carcinoma of the pancreas (SCP) involving successful treatment and a good prognosis. The plasma transforming growth factor (TGF)β1 and interleukin-11 levels were obviously increased according to enzyme-linked immunosorbent assay. Immunohistochemical analysis was positive for pan-cytokeratin, cytokeratin 8/18, and vimentin and negative for CD68 and lysozyme. The pathogenetic mechanism of this case shows that TGFβ1 may regulate the epithelial-to-mesenchymal transition in SCP. This case is also the first to show that TGFβ1 may regulate the epithelial-to-mesenchymal transition in early-stage SCP.
- Citation: Ren CL, Jin P, Han CX, Xiao Q, Wang DR, Shi L, Wang DX, Chen H. Unusual early-stage pancreatic sarcomatoid carcinoma. World J Gastroenterol 2013; 19(43): 7820-7824
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7820.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7820
Microscopically, sarcomatoid carcinoma of the pancreas (SCP) comprises mostly anaplastic cells and is strikingly sarcoma-like in appearance[1]. SCP may originate from many different organs, such as the pancreas, lung, liver, and esophagus[2-8]. Confirmation of this disease is often based on the pathological diagnosis. Advanced radiographic studies are also good tools with which to support the diagnosis of sarcomatoid carcinoma[2]. Early diagnosis and eradication of the tumor is important for a better prognosis of malignant sarcomatoid carcinomas.
It has been proposed that during malignant progression, carcinoma cells undergo an epithelial-to-mesenchymal transition (EMT), which is a vital step in the formation of pancreatic ductal adenocarcinoma (PDAC)[9]. The etiology of SCP is unknown. The EML4-ALK fusion gene was reportedly involved in the development of a sarcomatoid carcinoma of the lung[10]. ALK gene amplification is a nonrandom and clonally related event in a subset of pulmonary sarcomatoid carcinomas, but its biologic rationale deserves further investigation[11]. The mechanism of the formation of SCP and its metastasis remains unknown.
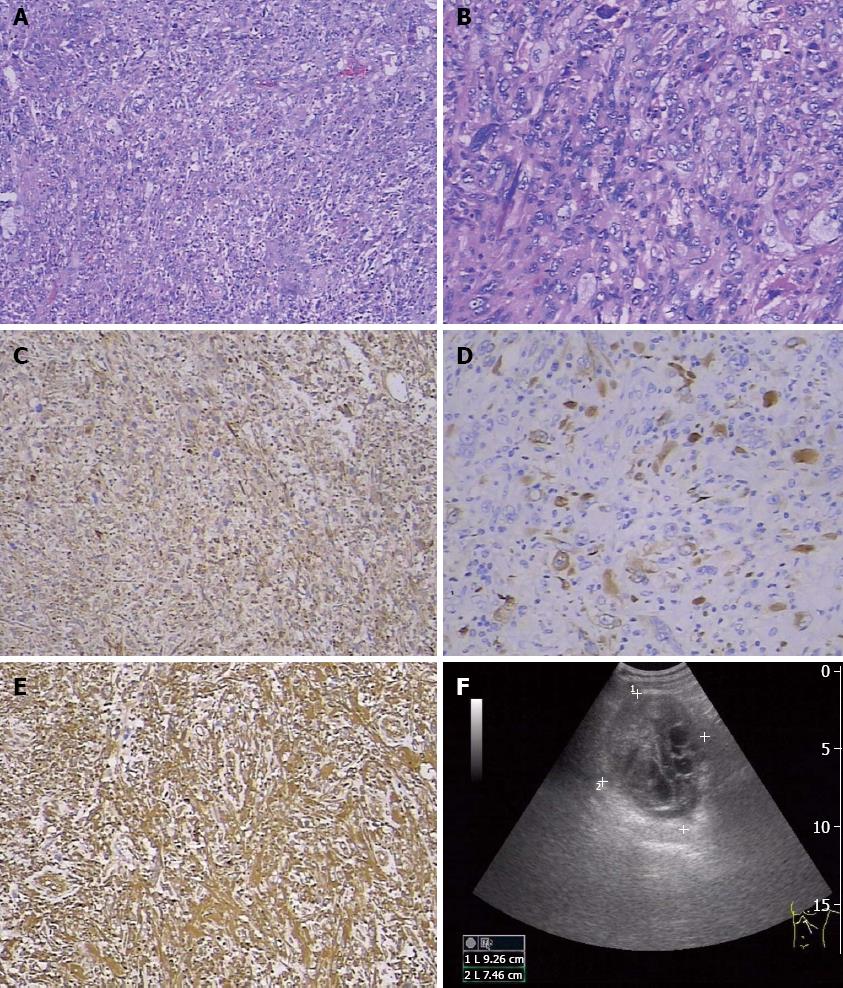
A 48-year-old Chinese man suffered from vague abdominal pain for 5 mo. He had no evidence of jaundice, hematuria, vomiting, or fever, but abdominal swelling and chest distress were present. He had no smoking or drinking habits, and no history of malignant or other diseases. Ultrasonography revealed a 93 mm × 94 mm × 75 mm mass of mixed echogenicity in the tail of the pancreas (Figure 1). Computed tomography (CT) showed displacement of the retroperitoneal organs by the mass (Figure not shown).
Laboratory test results, including a blood count, serum biochemistry, and urinalysis, were within the normal ranges. The levels of 11 common serum tumor markers, including CA19-9, CEA, and CA242, were normal, except that NSE was obviously increased before any treatment (Table 1). The plasma transforming growth factor (TGF)β1 and Interleukin (IL)-11 levels were higher than those of the healthy controls, patients with PDAC, and patients with pancreatic intraepithelial neoplasias (PanINs) (Table 2).
| Tumor markers | Index | Normal range |
| CA19-9 (KU/L) | 1.21 | < 35.00 |
| CA242 (KU/L) | 1.14 | < 20.00 |
| CA125 (KU/L) | 11.71 | < 35.00 |
| CA15-3 (KU/L) | 2.32 | < 35.00 |
| NSE (ng/mL) | 23.42 | < 13.00 |
| CEA (ng/mL) | 0.24 | 5 |
| Ferritin (ng/mL) | 161.47 | < 322.00 |
| β-HCG (MIU/mL) | 0.12 | < 3.00 |
| AFP (ng/mL) | 1.07 | < 20.00 |
| Free-PSA (ng/mL) | 0.32 | < 1.00 |
| PSA (ng/mL) | 1.53 | < 5.00 |
| HGH (ng/mL) | 0.36 | < 7.50 |
| Tumor (pg/mL) | Index (median) | n | |
| TGFβ1 | SCP | 35688 | 1 |
| PDAC | 10475 (5142-30865) | 20 | |
| PanINs | 7949 (6655-11404) | 10 | |
| HC | 6865 (3272-22463) | 11 | |
| IL-11 | SCP | 58 | 1 |
| HC | 22 (10-42) | 11 |
Surgery was performed, and the tumor was completely resected. The mass measured 10 cm × 8 cm × 3.5 cm and had cystic features after the excision. The section containing the solid tumorous tissue was pale in color. Microscopically, the excised tumor tissue comprised cancer cells and mesenchymal cells, with dispersion of atypical cells and obvious karyokinesis. Some were fusiform in shape and some were multinucleated giant cells (Figure 1A and B). Therefore, SCP was pathologically diagnosed. The neighboring lymph nodes and incisal margin were free of tumor cells. Immunohistochemical study results showed that the tumor cells were positive for vimentin, α-1-antichymotrypsin (AACT), cytokeratin 19, cytokeratin 18 (Figure 1C), and pan-cytokeratin (Figure 1D) and negative for CD68 and lysozyme (data not shown). Thus, an early-stage SCP was diagnosed.
The preoperative diagnosis was cystadenoma in the tail of the pancreas. Seven months after surgical excision, there was no evidence of tumor recurrence or metastasis. Digital subtraction angiography interventional chemotherapy was then implemented. Gemcitabine (1.4 g), oxaliplatin (150 mg), and floxuridine (1.0 g) were intravenously injected via the superior mesenteric artery and celiac trunk artery. After 28 mo of follow-up, a routine check-up and CT scan revealed that the patient was in good condition and free of tumor recurrence and metastasis. Because the patient had the opportunity to be treated in the early stage of the disease, he is in good condition and has been alive for more than 3 years without tumor recurrence or metastasis.
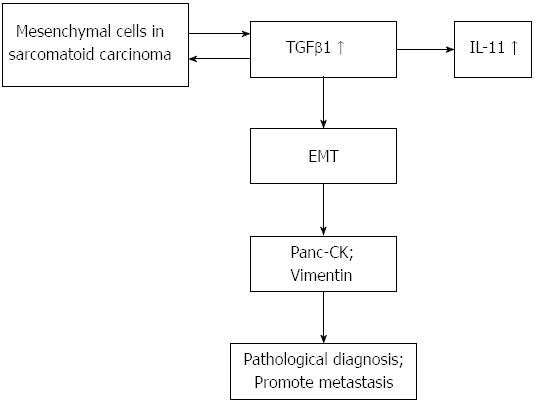
Sarcomatoid carcinoma is a rare and very aggressive malignant tumor comprising a mixture of carcinomatous and sarcomatous elements[12]. Areas of spindle cells arranged in a storiform pattern were present[13]. The tumor demonstrated cellular patterns similar to those present in tumors of mesenchymal origin in the case. In the present case, many cells undergoing heterotypic division were seen in the tissue specimen, and karyokinesis was frequent. Some cells were fusiform in shape, and some were pleomorphic giant cells. This change into pleomorphic giant cells was the most frequent sarcomatoid transformation encountered[1]. Compared with ordinary pancreatic carcinomas, malignant giant cell tumors of the pancreas appear to have a distinctive behavior characterized by local invasiveness, a reluctance to metastasize, and a more favorable prognosis when resected[1]. Immunohistochemical study results showed that the tumor cells were positive for vimentin, AACT, pan-cytokeratin, cytokeratin 19, and cytokeratin 8/18 and negative for CD68 and lysozyme. Some authors reported that the tumor cells in sarcomatoid carcinoma were positive for CK, S-100 protein, α1-antitrypsin, α1-chymotrypsin, anti-CA19-9, and SMA. No cells were positive for vimentin or desmin[14]. Vimentin-positive tumor cells are of mesenchymal origin in sarcomatoid carcinoma and are also seen in inflammatory myofibroblastic tumor of the prostate, another rare malignant disease[15]. In the present case, both epithelial and mesenchymal markers were positive. The process of EMT may play an important role in the formation of SCP. TGFβ signaling plays a dual role in oncogenesis. TGFβ can sometimes function as a tumor suppressor gene that inhibits the proliferation of normal epithelial cells, while in other tumor types it functions as an oncogenic gene. This dual function implies that the activity of TGFβ is highly dependent on the cellular context, pathological type, and specific environment[16-21]. In this case, the TGFβ1 level was markedly higher than those in patients with PDAC and PanINs and in HCs. TGFβ1 may regulate the EMT pathway in pancreas cells and promote the formation of SCP. The plasma IL-11 level in the present patient was obviously higher than that of the healthy controls. IL-11 is a TGFβ target gene. IL-11 stimulates the production of the osteoclastogenic factors RANKL and granulocyte macrophage-colony stimulating factor in osteoblasts. Induction of IL-11 and CTGF expression by TGFβ is mediated by the SMAD pathway[22]. TGFβ1 could be an important driving force during the sarcomatoid transdifferentiation of clear cell renal cell carcinoma[23]. The combination of early diagnosis of sarcomatoid carcinoma, eradication of the tumor, and systemic therapy may provide a chance of a good prognosis. Whether postoperative patients in the early tumor stage require chemotherapy may be controversial. High levels of NSE, TGFβ1, and IL-11 in the serum or plasma may help in the early diagnosis of SCP. TGFβ1 may play an important role in tumor metastasis (Figure 2) and some papers support our hypothesis[24,25]. In view of the complex biologic behavior of SCP, continued real-time monitoring of the clinical course of the disease is strongly recommended.
P- Reviewers: Azuma YT, Maeda S, Osawa S S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Alguacil-Garcia A, Weiland LH. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer. 1977;39:1181-1189. [PubMed] |
| 2. | Mi B, Wan W, Yu C, You X, Xu Q. Sarcomatoid hepatocellular carcinoma diagnosed by FDG PET/CT. Clin Nucl Med. 2011;36:925-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Raza MA, Mazzara PF. Sarcomatoid carcinoma of esophagus. Arch Pathol Lab Med. 2011;135:945-948. [PubMed] |
| 4. | Jung LY, Jeon SY, Kim SR, Chung MJ, Lee YC. Pulmonary sarcomatoid carcinoma accompanying duodenal involvement. Am J Respir Crit Care Med. 2012;185:899-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Lu HS, Gan MF, Zhou T, Wang SZ. Sarcomatoid thymic carcinoma arising in metaplastic thymoma: a case report. Int J Surg Pathol. 2011;19:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Molina AM, Tickoo SK, Ishill N, Trinos MJ, Schwartz LH, Patil S, Feldman DR, Reuter VE, Russo P, Motzer RJ. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. Am J Clin Oncol. 2011;34:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Shuch B, Said J, LaRochelle JC, Zhou Y, Li G, Klatte T, Pouliot F, Kabbinavar FF, Belldegrun AS, Pantuck AJ. Histologic evaluation of metastases in renal cell carcinoma with sarcomatoid transformation and its implications for systemic therapy. Cancer. 2010;116:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1664] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 10. | Alì G, Proietti A, Niccoli C, Pelliccioni S, Borrelli N, Giannini R, Lupi C, Valetto A, Bertini V, Lucchi M. EML4-ALK translocation in both metachronous second primary lung sarcomatoid carcinoma and lung adenocarcinoma: a case report. Lung Cancer. 2013;81:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Pelosi G, Gasparini P, Cavazza A, Rossi G, Graziano P, Barbareschi M, Perrone F, Barberis M, Takagi M, Kunimura T. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer. 2012;77:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | De la Riva S, Muñoz-Navas MA, Betés M, Súbtil JC, Carretero C, Sola JJ. Sarcomatoid carcinoma of the pancreas and congenital choledochal cyst. Gastrointest Endosc. 2006;64:1005-1006; discussion 1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Cresson DH, Reddick RL. Sarcomatoid carcinoma of the pancreas presenting as gastric carcinoma: clinicopathologic and ultrastructural findings. J Surg Oncol. 1987;36:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Higashi M, Takao S, Sato E. Sarcomatoid carcinoma of the pancreas: a case report with immunohistochemical study. Pathol Int. 1999;49:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Liu C, Zhao X, Zhao Z, Lu P, Jin F, Li G. Malignant inflammatory myofibroblastic tumor of the prostate. J Clin Oncol. 2013;31:e144-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2613] [Cited by in RCA: 2505] [Article Influence: 192.7] [Reference Citation Analysis (0)] |
| 17. | Akhurst RJ. Large- and small-molecule inhibitors of transforming growth factor-beta signaling. Curr Opin Investig Drugs. 2006;7:513-521. [PubMed] |
| 18. | Cha Y, Kim DK, Hyun J, Kim SJ, Park KS. TCEA3 binds to TGF-beta receptor I and induces Smad-independent, JNK-dependent apoptosis in ovarian cancer cells. Cell Signal. 2013;25:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Arteaga CL. Inhibition of TGFbeta signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Massagué J. TGFbeta in Cancer. Cell. 2008;134:215-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2725] [Cited by in RCA: 3126] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 21. | Seoane J. The TGFBeta pathway as a therapeutic target in cancer. Clin Transl Oncol. 2008;10:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massagué J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA. 2005;102:13909-13914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Boström AK, Möller C, Nilsson E, Elfving P, Axelson H, Johansson ME. Sarcomatoid conversion of clear cell renal cell carcinoma in relation to epithelial-to-mesenchymal transition. Hum Pathol. 2012;43:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Bonnomet A, Syne L, Brysse A, Feyereisen E, Thompson EW, Noël A, Foidart JM, Birembaut P, Polette M, Gilles C. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene. 2012;31:3741-3753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 896] [Article Influence: 68.9] [Reference Citation Analysis (0)] |