Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7701
Revised: August 2, 2013
Accepted: September 13, 2013
Published online: November 21, 2013
Processing time: 170 Days and 2.9 Hours
AIM: To analyze the difference in disease course and need for surgery in patients with Crohn’s disease (CD).
METHODS: Data of 506 patients with incident CD were analyzed (age at diagnosis: 31.5 ± 13.8 years). Both hospital and outpatient records were collected prospectively with a complete clinical follow-up and comprehensively reviewed in the population-based Veszprem province database, which includes incident CD patients diagnosed between January 1, 1977 and December 31, 2008. Follow-up data were collected until December 31, 2009. All patients included had at least 1 year of follow-up available. Patients with indeterminate colitis at diagnosis were excluded from the analysis.
RESULTS: Overall, 73 patients (14.4%) required resective surgery within 1 year of diagnosis. Steroid exposure and need for biological therapy were lower in patients with early limited surgery (P < 0.001 and P = 0.09). In addition, surgery rates during follow-up in patients with and without early surgery differed significantly after matching on propensity scores (P < 0.001, HR = 0.23). The need for reoperation was also lower in patients with early limited resective surgery (P = 0.038, HR = 0.42) in a Kaplan-Meier and multivariate Cox regression (P = 0.04) analysis. However, this advantage was not observed after matching on propensity scores (PLogrank = 0.656, PBreslow = 0.498).
CONCLUSION: Long-term surgery rates and overall exposure to steroids and biological agents were lower in patients with early limited resective surgery, but reoperation rates did not differ.
Core tip: An alternative approach may be early limited resective surgery in a well-selected group of patients with Crohn’s disease. In this population-based study, we found that overall exposure to steroids and biological agents was lower in patients with early limited resective surgery; observed surgery rates were also lower, yet reoperation rates did not differ in the two groups after matching on propensity scores.
- Citation: Golovics PA, Lakatos L, Nagy A, Pandur T, Szita I, Balogh M, Molnar C, Komaromi E, Lovasz BD, Mandel M, Veres G, Kiss LS, Vegh Z, Lakatos PL. Is early limited surgery associated with a more benign disease course in Crohn’s disease? World J Gastroenterol 2013; 19(43): 7701-7710
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7701
Crohn’s disease (CD) has a variable course, but the majority of patients eventually develop penetrating or stricturing complications. In addition, several environmental risk factors (diet, smoking, measles, or appendectomy) may contribute to its etiology and course. A significant adverse outcome is the need for surgery. Nevertheless, surgery is not curative in CD. Surgical resection is typically performed for emergency indications (e.g., obstructive symptoms and hemorrhage) or for failure to respond to medical therapy.
Some years ago, a review article reported that the probability of first resective surgery ranged from 38% to 96% in the first 15 years after diagnosis[1]. The overall clinical relapse and reoperation rates after initial resective surgery are 50%-60% and 28%-45%, respectively, during the following 15 years. Surgical resection rates over time vary widely among published studies, ranging between 25 and 61% in the first 5 years. Until recently, there was little evidence that disease outcomes for CD had changed over recent decades. Recently, Peyrin-Biroulet et al[2] published a systematic review of the natural history of CD in population-based cohorts. According to the authors’ conclusions, the impact of changing treatment paradigms with the increased use of immunosuppressants and biological agents on the natural history of the disease was poorly understood. Available data did not suggest a significant change in outcome of CD, with approximately half of patients requiring surgery within 10 years of diagnosis. The risk of postoperative clinical recurrence within 10 years was 44%-55%.
A recent meta-analysis from International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) Epidemiology Task Force reported that the risk of surgery in CD in prebiologic population-based cohorts has been decreasing during the past decade[3]. One of the most striking changes was reported by Jess et al[4] in a Danish study. The rate of early surgery (within 1 year of diagnosis) has fallen from 35% (1962-1987) to 12% (2003-2004). During this time, there was a significant change in patient management; namely, increased and earlier use of immunosuppressants and the introduction of biological therapies. The effect of azathioprine (AZA) on disease prognosis was until recently controversial. Two recent population-based reports confirmed that early AZA use is associated with reduced need for surgery according to a Cox regression analysis and propensity score matching in two population-based cohorts from Wales and Hungary[5,6]. Furthermore, in a study from France, an association was reported between the duration of anti-tumor necrosis factor (TNF) and AZA therapy and risk for surgery[7]. In contrast, in a previous referral center study from France, the need for intestinal surgery did not decrease despite the increased use of immunosuppressants[8]. Of note, in this study, AZA therapy was started only after surgery in the majority of patients. Earlier AZA use is only one of the complex changes in patient management. Other changes have also occurred, including a trend toward tight patient monitoring. Moreover, whether the risk of surgery is affected by the more widespread use of biological agents has yet to be demonstrated by population-based studies.
An alternative approach to the predominant strategy of initially using conservative therapy: using limited resective surgery in a selected group of patients as a primary therapeutic option, may prove advantageous. In a study by Aratari et al[9], early surgery at diagnosis in 207 CD patients with ileocecal disease was associated with a more benign postoperative disease course, in comparison to patients receiving delayed surgery. Nevertheless, reoperation rates were not reduced. Thus, in a subgroup of CD patients, early surgery may represent a valid alternative to medical therapy; particularly in patients with limited, isolated, stenotic ileocecal disease.
Therefore, our aim was to analyze the disease course, drug exposure and need for surgery and reoperation in patients with and without early (within 1 year of diagnosis) limited resective surgery in a population-based cohort from Eastern Europe with a complete clinical follow-up.
A well-characterized Hungarian cohort of 506 patients with incident CD (male/female: 251/255; age at diagnosis: 31.5 ± 13.8 years) diagnosed between January 1, 1977 and December 31, 2008 were included. Follow-up data were collected until December 31, 2009. All patients included had at least 1 year follow-up available. Patients with indeterminate colitis at diagnosis were excluded from the analysis. The clinical data of CD patients are summarized in Table 1.
| Characteristics | n = 506 |
| Male/female | 251/255 |
| Age at presentation (yr) | 31.5 ± 13.8 |
| Median follow-up (yr) | 11.4 ± 7.8 |
| Familial IBD | 12.9% |
| Location (n) | |
| L1 | 166 |
| L2 | 182 |
| L3 | 155 |
| L4 only | 3 |
| Behavior (n) | |
| B1 | 288 |
| At diagnosis B2 | 100 |
| B3 | 118 |
| Perianal disease | 25.5% |
| Total steroid exposure/dependent-refractory | 68.6%/11.2% |
| Total azathioprine exposure | 45.8% |
| Total biological exposure | 9.1% |
| Smoking habits (n) | |
| No | 224 |
| At diagnosis Ex | 38 |
| Yes | 244 |
Clinical data were collected every year from the seven general hospitals (departments of internal medicine, surgery, and pediatrics) and gastroenterology outpatient units. The majority of patients [76% of ulcerative colitis (UC) and 94% of CD patients] were monitored at the Csolnoky F. Province Hospital in Veszprem, where data were also registered. Disease behavior was updated yearly. A more detailed description of the data collection method, case assessment, the geographical and socioeconomic background of the province, and the results of surgical and medical management, as well as a detailed description of the Veszprem Province inflammatory bowel disease (IBD) Group, was published in previous epidemiological studies[10-12].
The disease phenotype (age at onset, duration, location, and behavior) was determined according to the Montreal Classification[13]. The presence of perianal disease and behavior change during follow-up were also registered. Medical therapy was recorded in detail (as defined by the European Crohn’s and Colitis Organisation Consensus Report[14]). The need for surgery or reoperation and smoking habits were investigated by review of medical files and by questionnaire.
The study was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics and by the Csolnoky F. Province Hospital Institutional Committee of Science and Research Ethics.
The majority of the patients received maintenance therapy with sulfasalazine or a 5-aminosalicylic acid derivative (mesalazine or olsalazine), if tolerated, especially until the mid-1990s. AZA or 6-mercaptopurine (6-MP) was used in selected cases as maintenance therapy for steroid-dependent and -refractory patients or for patients with fistulizing disease. AZA and 6-MP were typically used following resective surgery until the late 1980s, and later on a more widespread basis beginning in the mid-1990s. Short-term oral corticosteroid treatment was used for clinical exacerbations, usually prednisone 40-60 mg/d, tapered and discontinued over the course of 2-3 mo. Beginning in the mid-1990s, methotrexate was used as second-line immunosuppressive therapy in limited cases. Since the late 1990s, infliximab has been used for both induction and maintenance therapy in selected cases.
Surgical resection was performed for emergency indications (e.g., obstructive symptoms and hemorrhage) and for failure to respond to medical therapy. Surgical techniques have also changed during the follow-up period of this study; laparoscopic surgery became available and more widely used from the late 1990s. Limited resections were more widely used from the mid-1990s than in the past, and there is very limited use of defunctioning ileostomy (n = 4) formation in routine management of CD. For the majority of patients in the present study, one of the most experienced IBD surgeons in Hungary performed the operations, while laparoscopic surgery and stricturoplasty were performed only in a minority of cases. The definition of early limited surgery was resection of the terminal ileum or ileocecum within 1 year of diagnosis.
Due to Hungarian health authority regulations, a follow-up visit is obligatory for IBD patients at a specialized gastroenterology center every 6 mo. Otherwise, under the regulations of the Hungarian National Health Insurance (OEP) system, the right to subsidized therapy is forfeited. Consequently, the relationship between IBD patients and specialists is a close one.
The authors used two propensity score models to control further for possible confounders, to quantify the probability of surgery and reoperation in patients who had early limited resective surgery versus those who did not.
In the first propensity model, covariates included in the propensity score were selected according to a two-step process. We first constructed an outcome model identifying independent predictors of early limited resective surgery. Subsequently, we included in the model additional predictors known to be associated with surgical outcome (e.g., age at onset and smoking) irrespective of P value. We used multivariate logistic regression to estimate propensity scores of early limited resection for each individual. Goodness-of-fit was evaluated by the Hosmer-Lemeshow test, the P values of which were not significant. Using the predicted propensity scores from our model, we attempted to match all early limited surgery to identical CD patients without early limited surgery by 5-to-1 greedy matching[15]. Additional analysis in patients with ileum-only disease was also performed.
In the second propensity model, we aimed to analyze the probability of reoperation in patients with and without early surgery in a group of CD patients with a history of at least one resective operation. Using the above two-step process and predicted propensity scores from our model, we attempted to match all early surgery CD patients to identical CD patients with a non-early surgical resection through 5-to-1 greedy matching[15].
The study protocol was approved by Semmelweis University Regional and Institutional Committee of Science and Research Ethics and the Csolnoky F. Province Hospital Institutional Committee of Science and Research Ethics
Variables were tested for normality by the Shapiro-Wilk W test. Wilcoxon rank sum test, χ2 test, and χ2 test with Yates correction and logistic regression were used to test differences in disease phenotype between subgroups of UC and CD patients for dichotomous variables. Kaplan-Meier survival curves were plotted for analysis with log rank and Breslow tests to determine probability of surgical resection. Additionally, Cox regression analysis using the enter method was used to assess the association between categorical clinical variables and time to AZA use and surgical requirements. Variables with P < 0.2 in univariate analysis were included in the multivariate testing. To control further for possible confounders, we developed a propensity score models (see below) for quantifying the probability of reoperation in patients with and without early limited resective surgery[15]. Matching on propensity scores is one technique commonly used to control for measured confounders in observational studies. P < 0.05 was considered significant. Results for continuous variables were expressed as median (lower and upper quartile) unless otherwise stated. For the statistical analysis, SPSS version 20.0 was used (SPSS, Chicago, IL, United States).
Five hundred and six residents in Veszprem Province were diagnosed with CD in the 32-year period. Follow-up information was collected up to December 31, 2009, equaling 5758 patient-years of follow-up. The clinical characteristics and disease course according to the year of diagnosis is shown in Table 1. There were significant differences in disease phenotype, drug exposure, and smoking habits in the patient groups diagnosed in the early period and thereafter. Overall, exposure to AZA, systemic steroids and biological agents (after 1998 only) was 45.8%, 68.6% and 9.5%, respectively. Total AZA exposure increased in the subsequent cohorts despite shorter follow-up.
A total of 204 (40.7%) patients had at least one resective operation (5 patients with resective surgery due to malignant disease were excluded from analysis). The most common surgical procedures were ileocecal resection (n = 93), right hemicolectomy (n = 59), segmental colonic resection (n = 19), and subtotal colectomy/left hemicolectomy (n = 11 and 8, respectively).
A further 36 (7.1%) patients had other surgical procedures (abscess drainage or fistulectomy). Forty-two (8.4%) patients had two resective operations and 17 (3.4%) had three or more operations for CD during follow-up. Ileocecal resection was the most common procedure overall.
The probability of first intestinal surgery due to non-malignant disease after 1, 5 and 10 years was 14.6%, 30.1%, and 51.6%, respectively, in a Kaplan-Meier analysis. The cumulative probability of resective surgery rate decreased in patients diagnosed in the last decade [Group 1 (1977-1998), Group 2 (1999-2008); PLogrank = 0.022, and PBreslow = 0.07].
Overall, 73 patients (14.4%) required resective surgery within 1 year of diagnosis. Ten patients were excluded from further analysis from the early surgery group in whom extensive index surgery was performed. The prevalence of early limited resective surgery did not differ significantly in the three cohorts [Cohort A (diagnosed 1977-1989): 11.3%; Cohort B (1990-1998): 12.8%; and Cohort C (1999-2008): 13.4%]. Predictors of early limited resective surgery were ileal location (P < 0.001), colonic location (P < 0.001), complicated behavior at diagnosis (P < 0.001), and age of onset (P = 0.06, Table 2).
| Early surgery | No early surgery | P value | OR (95%CI) | |
| Age at onset (A1) | 28.4% | 19.0% | 0.060 | 1.69 (0.97-2.96) |
| Ileal location | 59.5% | 28.1% | < 0.001 | |
| Colonic location | 13.5% | 39.9% | < 0.001 | |
| Complicated behavior at diagnosis | 85.1% | 35.9% | < 0.001 | 10.2 (5.2-20.1) |
| Overall steroid exposure | 52.7% | 71.3% | 0.001 | 0.45 (0.27-0.74) |
| Steroid-dependent course | 2.6% | 12.3% | 0.070 | 0.19 (0.03-1.40 |
| Overall biological exposure | 4.1% | 10.0% | 0.090 | 0.38 (0.12-1.26) |
| Overall azathioprine exposure | 45.9% | 45.8% | > 0.050 |
Overall steroid exposure was significantly lower (P = 0.001) in patients with early limited resection despite similar follow-up (median: 12 years). The same trend was observed for steroid dependency (P = 0.07) and overall biological agent exposure (P = 0.09). In contrast, overall AZA exposure was similar between the two groups. Of note, AZA exposure before surgery was 0% vs 28.8% (P < 0.001), while TNF-antagonist exposure before surgery was 0% vs 4.9% (P = 0.05) in patients with and without early limited surgery. In logistic regression analysis, disease location (P < 0.001) and disease behavior (P < 0.001) were associated with the need for early resective surgery (Table 3).
| P value | OR | 95%CI | |
| Age at diagnosis | |||
| A1 | 0.172 | 1.62 | 0.81-3.25 |
| A2 | Reference | ||
| Disease location | < 0.001 | ||
| L1 | < 0.001 | 7.88 | 2.92-21.3 |
| L3 | 0.035 | 3.21 | 1.09-9.48 |
| L2 | Reference | ||
| Disease behavior at diagnosis | < 0.001 | ||
| B2 | < 0.001 | 4.91 | 2.15-11.2 |
| B3 | < 0.001 | 7.66 | 3.52-16.7 |
| B1 | Reference | ||
| Smoking | |||
| Yes | 0.487 | 1.24 | 0.68-2.27 |
| No | Reference | ||
In a propensity score model, we compared surgery rates between patients with and without early resective surgery. In the early limited surgery model, propensity scores ranged from 0.02 to 0.56 (median: 0.29) in patients with early limited resection (n = 63), and from 0.02-0.55 (median: 0.14) in patients without early limited surgery (n = 428). Goodness-of-fit was evaluated by the Hosmer-Lemeshow test, and P values were non-significant (P = 0.653).
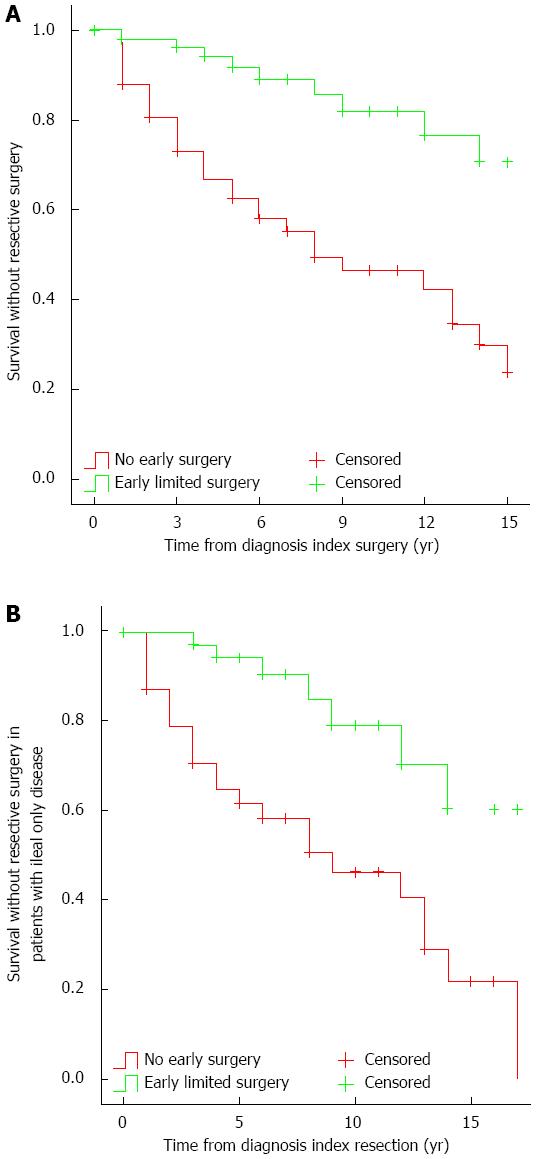
Using a 5-to-1 greedy matching algorithm, we were able to match 58 patients from the early resective surgery group to patients with comparable phenotype without early limited resections (Table 4). The observed first resective surgery rate was 12.1%, 33.2%, and 53.6% after 2, 5 and 10 years of disease duration, respectively, in the latter group. In contrast, the reoperation rate in the former group was 1.8%, 5.8%, and 17.9% after 2, 5 and 10 years (PLogrank < 0.001, HR = 0.23, 95%CI: 0.11-0.48; Figure 1A).
If the analysis was restricted to patients with ileum-only location (n = 38), the observed first resective surgery rate was 21%, 35.3%, and 59.4% in patients without early surgery after 2, 5 and 10 years of disease duration, respectively. In contrast, the observed reoperation rate in the other group was 0%, 5.8%, and 20.8% after 2, 5 and 10 years (PLogrank < 0.001, HR = 0.25, 95%CI: 0.11-0.58) (Figure 1B).
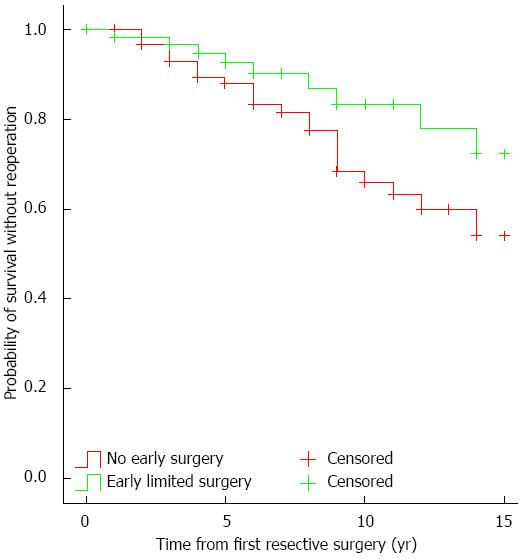
The need for reoperation in patients with early limited resection at 5 years was 7.5%, at 10 years it was 16.5%, while for those without early resective surgery at 5 years it was 12.9%, and 36.3% at 10 years in a Kaplan-Meier analysis (PLogrank = 0.038, Figure 2). In a Cox regression analysis, early limited surgery (P = 0.04) was the only factor independently associated with the need for reoperation (Table 5).
| P value | HR | 95%CI | |
| Early surgery1 | |||
| Yes | 0.04 | 0.42 | 0.19-0.95 |
| No | Reference | ||
| Age at diagnosis | |||
| A1 | 0.75 | - | - |
| A2 | Reference | ||
| Disease location | 0.95 | ||
| L2 | 0.77 | - | - |
| L3 | 0.95 | - | - |
| L1 | Reference | ||
| Disease behavior at diagnosis | 0.56 | ||
| B2 | 0.30 | - | - |
| B3 | 0.65 | - | - |
| B1 | Reference | ||
| Smoking | |||
| Yes | 0.29 | - | - |
| No | Reference | ||
In addition, we developed a propensity score model to assess further the need for reoperation in patients with and without early resective surgery. After identifying predictors, multivariate logistic regression was used to estimate propensity scores of early limited resection for each individual. Goodness-of-fit was evaluated by the Hosmer-Lemeshow test and P values were non-significant (P = 0.812). In the early limited surgery model, propensity scores ranged from 0.10 to 0.83 (median: 0.45) in patients with early limited resection (n = 63), and from 0.02 to 0.69 (median: 0.30) in patients with non-early surgery (n = 126). Using a 5-to-1 greedy matching algorithm, we were able to match 54 out of 63 (85.7%) patients with early limited surgery to patients with non-early surgery. As expected, the prevalence of factors included in the propensity score model was well balanced across surgical groups (data not shown).
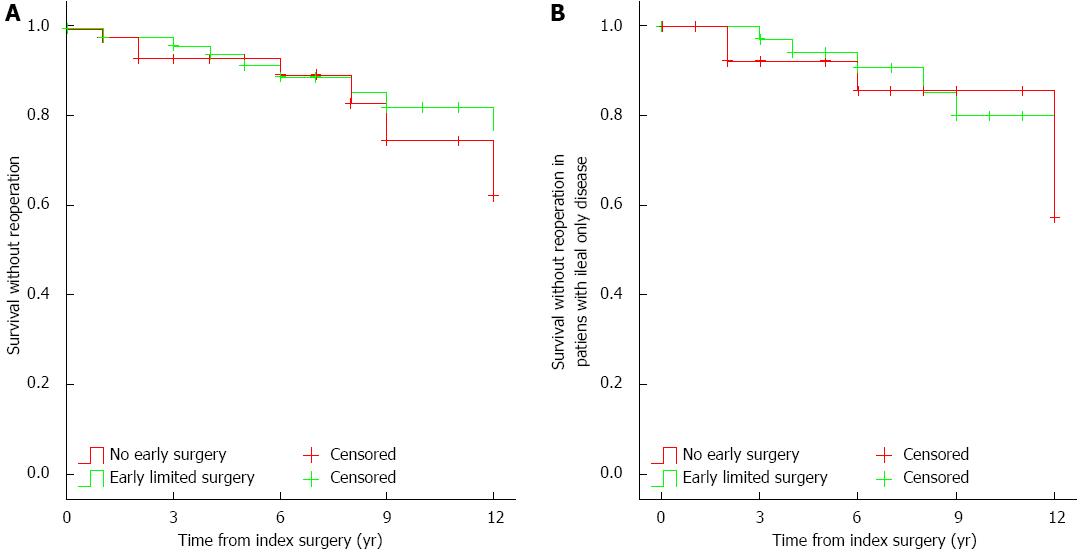
The observed reoperation rates did not differ between the two groups (early surgery: 1.9%, 5.9%, and 17.7%; vs non-early surgery: 2%, 6.7%, and 25.1%, after 1, 5 and 10 years, respectively, PLogrank > 0.05, Figure 3A). Similar results were found if the analysis was restricted to patients with disease limited to the ileum only (n = 33, early surgery: 0%, 5.8%, and 20%, vs non-early surgery: 0%, 7.7%, and 14.3%, after 1, 5 and 10 years, respectively, PLogrank > 0.05, Figure 3B).
In the present study, we studied the benefits of early limited resective surgery in patients in a population-based Veszprem Province database. Results from this population-based inception cohort have shown that surgery rates and overall exposure to steroids and biological agents were lower in patients with early limited resective surgery. In contrast, although patients with early limited resective surgery needed less reoperation, by Kaplan-Meier analysis and multivariate Cox regression analysis, in the final propensity-score-matched model, this advantage was lost, and the probability of reoperation was similar in patients with early limited resection and non-early surgery. To the best of our knowledge, this is the first time that the association between early limited resective surgery and reoperation were studied using a propensity score model.
The rates of resective surgery vary significantly according to previous studies with a range from 25% to 61% in the first 5 years. An earlier review article by Wolters et al[1] reported that the probability of first resective surgery was as high as 38%-96% within the first 15 years of diagnosis. The overall recurrence and reoperation rates after first resective surgery is 50%-60% and 28%-45%, respectively, during the subsequent 15 years. More recently, in the IBSEN Study, the cumulative probability of surgery was 13.6%, 27.0% and 37.9%, at 1, 5 and 10 years after diagnosis, respectively, while the risk of reoperation was also lower (9%)[16].
Similarly, in a recent meta-analysis from the IOIBD Epidemiology Task Force, the authors reported that the probability of surgery in CD decreased gradually between 1955 and 2003, even before the advent of the biological era[3]. An association with increased and earlier use of immunosuppressants was also suggested in studies from Wales[6] and Hungary[5]. Ramadas et al[6] found that the rates of intestinal surgery decreased during the study period from 59% to 25% within 5 years of diagnosis. There was also a significant reduction in patients having any surgical procedure, from 60% to 35%. Likewise, in a previous referral center study from Hungary, early monotherapy with AZA or combination AZA/biological therapy was associated with a reduced risk for surgery. In earlier published studies from this cohort, the probability of surgical resection was 9.8%, 18.5% and 21.3% after 1, 3 and 5 years, respectively, in patients diagnosed between 2002 and 2006, and a recent decrease in surgical rates (in patients diagnosed after 1998) was observed[12]. These changes were associated with increased and earlier use of immunosuppressants[5]. Notwithstanding, the change in the use of immunosuppressants could be regarded as a marker of the complex changes in patient management rather than an exclusive factor itself.
Additional predictors of surgery include ileal or colonic disease location, complicated disease behavior, and age at onset - as reported previously in Sweden[17] and more recently from the IBSEN cohort[16]. Similarly, ileal (HR = 2.35) or ileocolonic (HR = 1.79) location compared to isolated colonic disease, as well as stricturing (HR = 4.33) or penetrating (HR = 3.44) disease at diagnosis, but not perianal disease, were independently associated with time to first surgery in a population-based cohort study from our research group[18]. Interestingly, we observed a similar phenotype pattern associated with early limited resection (ileal location, P < 0.001; complicated behavior, P < 0.001; and age at onset, P = 0.06).
However, the risk of surgery, as well as the disease course in patients with primary ileocecal CD, is somewhat different. In an earlier Swedish study, the risk of resective surgery in patients with primary ileocecal CD was 61%, 77% and 83%, after 1, 5 and 10 years of diagnosis, respectively, in 907 patients[19]. Relapse rates were 28% and 36% within 5 and 10 years of the first resection, respectively. In an Italian study, clinical and surgical recurrence rates after 5 years were 30.6% and 49.4%, and after 10 years they were and 3.6% and 28%, respectively[20]. In addition, early surgery (within 3 years of diagnosis) was associated with a longer postoperative course free from clinical recurrence compared with late surgery, but not with reoperation. In the present study, despite higher rates of ileal and complicated disease in patients with early limited surgery, the overall need of steroids (OR = 0.45, P < 0.001) during follow-up was lower, and there was a similar tendency for steroid-dependent disease course (P = 0.07) and need for biological agents (P = 0.09). In contrast, the overall use of AZA was similar in both groups, which may represent an active therapeutic decision by the treating physician rather than a marker of negative disease outcome. Of note, median follow-up was similar for both groups (12 years). We defined early surgery as that performed within 1 year of diagnosis, to avoid a potential bias due to early medical therapy. Of note, a positive effect of early aggressive medical therapy was already observed in patients in whom AZA was started within 18 mo of diagnosis, in a previous study in this cohort[5]. In addition, since reoperation rates may be lower in patients with extensive initial surgery due to the anatomical situation, we excluded these patients from the final analysis.
Comparable data were reported by Aratari et al[9]. In that study, early limited surgery at diagnosis was associated with less clinical recurrence - defined as need for steroids and lesions documented by endoscopy or radiology (P = 0.01), and less need for immunosuppressants (P = 0.05), but reoperation rates were not significantly different. Immunosuppressants were started in only 16.2% of patients with early or late surgery, confirming that the medical approach reported by the authors was much more conservative compared to the present study. In addition, the need for immunosuppressants was interpreted as a negative outcome. In another Italian study, the disease course of CD patients diagnosed during emergency surgery was compared to patients without emergency surgery[21]. The authors reported that the disease course was more benign in patients requiring surgery at diagnosis, by both univariate and multivariate analysis. Similar to the present study, surgery rates were significantly lower in patients operated on at diagnosis (in the present study with early limited surgery) compared to patients without surgery at diagnosis (in the present study without early limited surgery after matching on propensity scores). Observed surgery rates in the Italian study were 14% and 30% in the surgery-at-diagnosis group and 30% and 44% in the non-early surgery group, respectively. Of note, this design mimics clinical trials comparing two treatment algorithms: (1) early limited resective surgery; and (2) medical therapy but no early surgery. Observed surgery and drug exposure rates were clearly different. However, a different interpretation of our results may be that 40%-45% of patients could avoid surgery despite a similar patient phenotype within 10 years of diagnosis, while in the other group, all patients started with a limited operation. In addition, in the non-early surgery group, only 25.6% of patients received early AZA therapy. Thus, their therapeutic strategy was far from optimized. Moreover, reoperation rates were also analyzed in the present study, which would be very difficult in a clinical trial due to the need for long term follow-up. These were lower in patients with early limited resection versus those without early limited resection by Kaplan-Meier (PLogrank = 0.038) and Cox regression (P = 0.04) analysis but the difference was lost after matching on propensity scores. Cost-benefit was not analyzed but with the increasing exposure to biological agents in certain CD populations[22], these studies are urgently awaited.
The authors are aware of the possible limitations of the present study. One such potential limitation is the partially retrospective nature of the study, which may have led to bias in data interpretation. However, data were collected prospectively since 1985 and intestinal resection can be considered an unbiased and solid criterion, even retrospectively, because the indications for surgical intervention are well established. Another possible criticism may be the definition of early surgery. In this subgroup, patients presented partly with a short history of subacute or even acute symptoms. In these patients, the timing of surgery was determined by the clinical presentation and not by the physician’s strategic decision. However, this was also the case for a proportion of non-early surgical patients. Patient management has also changed significantly with regard to surgery techniques (laparoscopy) and imaging (availability of computed tomography and magnetic resonance imaging) that could have potentially affected therapeutic decision making, including indication for surgery. However, in the present study, one leading surgeon performed the majority of the operations and laparoscopic surgery and stricturoplasty was performed only in a minority of the cases. Similarly, there was only limited use of defunctioning ileostomy formation in routine management of CD during the follow-up period. Finally, postoperative management has significantly changed during the follow-up period, including routine endoscopy evaluation and prophylactic therapy; although in our analysis, we matched the groups for decade of diagnosis. In addition, there is accumulating evidence that anti-TNF therapies may reduce the number of operations[7,23], although this was not a universal finding[24,25]. Exposure to biological agents was limited in the present study, and most of these patients received induction-only or intermittent infliximab therapy. In contrast, the strengths of this study include the long-term, comprehensive, validated data capture, and the use of both propensity score matching and multiple Cox regression analysis to overcome the limitations present in any partly retrospective study, thereby enabling unbiased analysis. Moreover, a follow-up of several years is needed to assess the reoperation rates, especially in patients without early limited resection, which is almost impossible in a clinical trial.
In conclusion, early limited resective surgery was associated with a lower risk for surgery and lower overall exposure to steroids and biological agents in this population-based cohort but it was not preventive for reoperations after matching on propensity scores. In addition, the similar exposure to immunosuppressants in the two groups may be interpreted as an active medical decision rather than a negative disease outcome. Conversely, resective surgery could be avoided in 40%-45% of CD patients with a similar disease phenotype without an early limited surgery within 10 years of diagnosis.
The optimal initial therapy in patients with limited, isolated, stenotic ileocecal Crohn’s disease (CD) is debated. In some cases, early surgery may represent a valid alternative to medical therapy.
There are only limited data available on the disease course, including drug exposures, operation and reoperation rates in patients with and without an early limited resective surgery. In addition, data are lacking from population-based cohorts.
The present long-term, population-based study in a well-characterized cohort of patients with CD has found that the overall exposure to steroids and biologicals was lower in patients with early limited resective surgery, observed surgery rates were also lower, yet reoperation rates were not different in the two groups after matching on propensity scores
Understanding the disease course in this subgroup of patients with CD may lead to more optimized patient managment and follow-up.
Disease phenotype is categorized according to the Montreal classification and includes age at onset location and. Early limited surgery was defined as a resective surgery within the year of diagnosis and affecting only the terminal ileum and coecum.
This is good research. To add impact, adding in other centres in Europe should be considered in future.
P- Reviewer: Nash GF S- Editor: Zhai HH L- Editor: Kerr C E- Editor: Ma S
| 1. | Wolters FL, Russel MG, Stockbrügger RW. Systematic review: has disease outcome in Crohn’s disease changed during the last four decades? Aliment Pharmacol Ther. 2004;20:483-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 756] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 3. | Bernstein CN, Loftus EV, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Jess T, Riis L, Vind I, Winther KV, Borg S, Binder V, Langholz E, Thomsen OØ, Munkholm P. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Lakatos PL, Golovics PA, David G, Pandur T, Erdelyi Z, Horvath A, Mester G, Balogh M, Szipocs I, Molnar C. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol. 2012;107:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 7. | Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 490] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Aratari A, Papi C, Leandro G, Viscido A, Capurso L, Caprilli R. Early versus late surgery for ileo-caecal Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Lakatos L, Mester G, Erdelyi Z, Balogh M, Szipocs I, Kamaras G, Lakatos PL. Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977-2001. World J Gastroenterol. 2004;10:404-409. [PubMed] |
| 11. | Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300-2307. [PubMed] |
| 12. | Lakatos L, Kiss LS, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011;17:2558-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 14. | Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas L, Geboes K, Barakauskiene A, Villanacci V, Von Herbay A, Warren BF. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55 Suppl 1:i1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7528] [Article Influence: 537.7] [Reference Citation Analysis (0)] |
| 16. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 17. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [PubMed] |
| 18. | Szamosi T, Banai J, Lakatos L, Czegledi Z, David G, Zsigmond F, Pandur T, Erdelyi Z, Gemela O, Papp M. Early azathioprine/biological therapy is associated with decreased risk for first surgery and delays time to surgery but not reoperation in both smokers and nonsmokers with Crohn’s disease, while smoking decreases the risk of colectomy in ulcerative colitis. Eur J Gastroenterol Hepatol. 2010;22:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Margagnoni G, Aratari A, Mangone M, Moretti A, Spagnolo A, Fascì Spurio F, Luchetti R, Papi C. Natural history of ileo-caecal Crohn’s disease after surgical resection. A long term study. Minerva Gastroenterol Dietol. 2011;57:335-344. [PubMed] |
| 21. | Latella G, Cocco A, Angelucci E, Viscido A, Bacci S, Necozione S, Caprilli R. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis. 2009;41:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, Mahmmod N, van de Meeberg PC, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk CJ, Vermeijden JR, Siersema PD, van Oijen MG, Oldenburg B; on behalf of the COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2012;Nov 7; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 23. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 24. | Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Domènech E, Zabana Y, Garcia-Planella E, López San Román A, Nos P, Ginard D, Gordillo J, Martínez-Silva F, Beltrán B, Mañosa M. Clinical outcome of newly diagnosed Crohn’s disease: a comparative, retrospective study before and after infliximab availability. Aliment Pharmacol Ther. 2010;31:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |