Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7680
Revised: September 13, 2013
Accepted: September 16, 2013
Published online: November 21, 2013
Processing time: 209 Days and 12.2 Hours
AIM: To evaluate whether 8-bromo-7-methoxychrysin (BrMC), a synthetic analogue of chrysin, inhibits the properties of cancer stem cells derived from the human liver cancer MHCC97 cell line and to determine the potential mechanisms.
METHODS: CD133+ cells were sorted from the MHCC97 cell line by magnetic activated cell sorting, and amplified in stem cell-conditioned medium to obtain the enriched CD133+ sphere forming cells (SFCs). The stem cell properties of CD133+ SFCs were validated by the tumorsphere formation assay in vitro and the xenograft nude mouse model in vivo, and termed liver cancer stem cells (LCSCs). The effects of BrMC on LCSCs in vitro were evaluated by MTT assay, tumorsphere formation assay and transwell chamber assay. The effects of BrMC on LCSCs in vivo were determined using a primary and secondary xenograft model in Balb/c-nu mice. Expressions of the stem cell markers, epithelial-mesenchymal transition (EMT) markers and β-catenin protein were analyzed by western blotting or immunohistochemical analysis.
RESULTS: CD133+ SFCs exhibited stem-like cell properties of tumorsphere formation and tumorigenesis capacity in contrast to the parental MHCC97 cells. We found that BrMC preferentially inhibited proliferation and self-renewal of LCSCs (P < 0.05). Furthermore, BrMC significantly suppressed EMT and invasion of LCSCs. Moreover, BrMC could efficaciously eliminate LCSCs in vivo. Interestingly, we showed that BrMC decreased the expression of β-catenin in LCSCs. Silencing of β-catenin by small interfering RNA could synergize the inhibition of self-renewal of LCSCs induced by BrMC, while Wnt3a treatment antagonized the inhibitory effects of BrMC.
CONCLUSION: BrMC can inhibit the functions and characteristics of LCSCs derived from the liver cancer MHCC97 cell line through downregulation of β-catenin expression.
Core tip: We successfully obtained liver cancer stem cells (LCSCs) from the liver cancer MHCC97 cell line by employing the combination of magnetic activated cell sorting and tumorsphere culture. We showed for the first time that 8-bromo-7-methoxychrysin (BrMC), a synthetic analogue of chrysin, could preferentially inhibit proliferation and self-renewal, suppress epithelial-mesenchymal transition and invasion of LCSCs, and further eradicate LCSCs in vivo. The results of this study support the use of BrMC for liver cancer chemoprevention or chemotherapy.
-
Citation: Quan MF, Xiao LH, Liu ZH, Guo H, Ren KQ, Liu F, Cao JG, Deng XY. 8-bromo-7-methoxychrysin inhibits properties of liver cancer stem cells
via downregulation of β-catenin. World J Gastroenterol 2013; 19(43): 7680-7695 - URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7680
Human liver cancer is the fifth most common cancer in the world and the third leading cause of cancer-related death[1,2]. Although surgery, liver transplantation or chemotherapy offers the possibility of prolonged survival for liver cancer patients, mortality still remains high, largely due to recurrence and drug-resistance[3,4]. According to the cancer stem cell hypothesis, this is thought to be due to the survival of a population of chemoresistant cells within the tumor, the cancer stem cells (CSCs) in liver cancer, that are able to regenerate the tumor following chemotherapy[5]. However, most currently available therapeutic approaches, including chemotherapy and radiotherapy, lack the ability to effectively kill these CSCs, which may eventually lead to the disease relapse and metastasis[6,7]. A number of previous studies have suggested that CD133, originally identified as a hematopoietic stem cell marker, could be used to isolate liver cancer stem cells (LCSCs) from human liver cancer cell lines, xenograft tumors and primary liver cancer specimens[8-13]. These CD133+ liver cancer cells possess many stem cell properties, including extensive proliferation, self-renewal, and differentiation into the bulk of cancer cells. Thus, this minor subpopulation of CD133+ LCSCs may contribute to the high recurrence rate of liver cancer. Therefore, the identification of a compound that can target LCSCs is one of the main steps in improving overall survival of liver cancer patients.
More recently, a number of studies have found that several dietary compounds can directly or indirectly inhibit cancer stem cell self-renewal pathways[14]. For example, natural flavonoid, genistein and a synthetic derivative of daidzein, N-t-boc-daidzein, have been reported to possess inhibitory activity against prostate and epithelial ovarian CSCs, respectively[15,16]. Chrysin (5,7-dihydroxyflavone), a naturally widely distributed flavonoid, has been shown to possess promising effects on the inhibition of proliferation and induction of apoptosis in a variety of cancer cells[17]. 8-bromo-7-methoxychrysin (BrMC) is a synthetic derivative of chrysin, and our previous study demonstrated the effect of BrMC in inhibiting proliferation and induction of apoptosis in colon, gastric and liver cancer cells was stronger than that of chrysin[18-21].
In this study, we investigated the inhibitory effects of BrMC on the characteristics of LCSCs. We showed for the first time that BrMC was able to inhibit cancer stem cell-like properties of LCSCs and eliminate LCSCs in vivo. We also found that BrMC significantly decreased β-catenin expression in LCSCs and knockdown of β-catenin expression could synergize the inhibition of self-renewal of LCSCs induced by BrMC. Together, our results indicated that the downregulation of β-catenin expression appeared to contribute to the inhibitory effects of BrMC on the properties of LCSCs.
The human liver cancer MHCC97 cell line was purchased from Fuxiang Biotechnology Co., Ltd. (Shanghai, China). MHCC97 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen Life Technologies, Carlsbad, CA, United States) in an incubator containing 5% CO2 at 37 °C. Wnt3a-conditioned medium was prepared as described by Willert et al[22]. BrMC was synthesized as described previously[18]. MTT was purchased from Sigma (St. Louis, MO, United States). Fetal bovine serum was from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, China). Trypsin and DMSO were from Amersco Company (Solon, OH, United States). Antibodies used in this study were as follows: rabbit polyclonal antibodies against ZO-1 (Abcam, Cambridge, MA, United States), mouse monoclonal antibodies against N-cadherin (Upstate Co., Lake Placid, NY, United States), Vimentin (Neo Markers, Fremont, CA, United States), E-cadherin (BD Transduction Labs, Lexington, KY, United States), β-catenin and CD44 (Cell Signaling Technology Inc., Danvers, MA, United States), β-actin (Sigma Chemical Co., St Louis, MO, United States), and horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States).
Cell sorting was performed on MHCC97 cells using the cell surface marker CD133+ with magnetic activated cell sorting (MACS) separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. Cells were trypsinized and washed with PBS, and suspended in Phosphate buffered saline (PBS) containing 0.5% Bovine Serum Albumin (BSA). 100 μL Fc receptor (FCR) Blocking Reagent (anti-CD133 antibody) and 100 μL CD133-conjugated MicroBeads (AC133, Cell Isolation Kit, Miltenyi Biotec) per 108 cells were subsequently added to the sample and incubated in parallel for 30 min on ice. After washing the cells, CD133 positive and negative fractions were each isolated through MACS separation columns. The quality of sorting was controlled by flow cytometry analysis for CD133 expression using PE-conjugated anti-human CD133 antibody and isotype control mouse IgG2b-PE (Biolegend, San Diego, CA, United States). The single cell suspension was cultured in stem cell-conditioned medium (DMEM/F12 medium supplemented with 1 × B27, 20 ng/mL EGF, 20 ng/mL bFGF, 0.4% BSA, 4 μg/mL Insulin, 100 U/mL penicillin and 100 μg/mL streptomycin; Invitrogen) for the following assays.
Single-cell suspensions were suspended at a density of 2000 cells/mL in stem cell-conditioned medium and seeded into ultralow attachment 24-well plates (Corning, NY, United States). When the diameter of the spheroid reached 50 μm, suspension cultures were passaged every 6 d. Colonies were counted at 10 different views under a microscope (Nikon, Japan). The volume of the spheroid was estimated using V = (4/3) π R3. Experiments were repeated 3 times with duplication in each experiment.
The procedures for preparation of whole cell lysates and western blotting analysis have been previously described[23]. Mouse anti-human β-catenin, N-cadherin, vimentin, E-cadherin, ZO-1, CD133, CD44 and β-actin antibodies were used as primary antibodies. Signals were visualized using chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL, United States). β-actin was used as an internal control. Images were scanned, followed by densitometry analysis with UN-SCAN-IT software (Silk Scientific Inc., Orem, UT, United States).
CD133+ sphere-forming cells (SFCs) or parental MHCC97 cells were seeded in a 96-well plate pre-coated with 0.6% agarose at a density of 5000 cells/well as described previously[24]. One day after plating, various concentrations of BrMC (0.1, 0.3 1.0, 3.0 or 10.0 μmol/L) were added to each well and the culture continued for 48 h. After removal of the medium, cells were incubated with 5 mg/mL of MTT for 4 h. Cells were then extracted with acidic isopropanol and the absorbance at 570 nm (A570) was measured by means of an enzyme-labeling instrument (EXL-800 type). The relative cell proliferation inhibition rate = (average A570 of the experimental group/average A570 of the control group) × 100%.
The invasion ability of tumor cells was examined in vitro using a transwell chamber system with 8.0 μm pore polycarbonate filter inserts (Corning Coster, Cambridge, MA, United States). The lower side of the filter was coated with 10 μL gelatin (1 mg/mL), and the upper side was coated with 10 μL of Matrigel. Parental MHCC97 cells or LCSCs (2 × 103) were placed in the upper part of the filter. 10% fetal bovine serum was added in the lower part of the chamber as a chemical attractant. The chamber was then incubated at 37 °C for 48 h. Cells that could not invade through the filter were removed with a cotton swab. The cells in the lower part of the chamber were fixed with methanol and stained with crystal violet. The invasiveness of tumor cells was determined by counting the total number of cells on the lower side of the filter at 100 × magnification. In the drug-intervention experiment, cells were pretreated with different concentrations of BrMC for 24 h prior to the transwell chamber assay.
Pathogen-free Balb/c-nu mice aged 5-6 wk were purchased from Shanghai Laboratory Animal Center (Shanghai, China). All animal studies were performed in accordance with the standard protocols approved by the Ethical Committee of Hunan Normal University and the Committee of Experimental Animal Feeding and Management. Mice were randomly divided into 3 groups (4 mice/group) and maintained under standard conditions, according to the standard protocols. Cells were suspended in serum free-DMEM/Matrigel (BD Biosciences, San Jose, CA, United States) mixture (1:1 volume). Each recipient Balb/c-nu mouse was inoculated subcutaneously with various numbers of CD133+ SFCs (2 × 103, 1 × 104 and 1 × 105 cells) in one flank and parental MHCC97 cells (1 × 104, 1 × 105 and 1 × 106) in the other. Tumorigenicity experiments were terminated 2 mo after cell inoculation. Tumor size were measured with a caliper, and the volume was calculated using V (mm3) = L × W2× 0.5. Harvested tumors were imaged and weighed immediately. Specimens from tumor tissue samples were fixed in 10% neutral buffered formalin, processed in paraffin blocks, and sectioned. The sections were stained with hematoxylin and eosin (HE) and examined for the histopathology.
For BrMC treatment studies, 5 × 104 LCSCs per mouse were injected subcutaneously. Two weeks after inoculation, animals were randomly divided into 4 groups. One group underwent daily gastric lavage with refined olive oil as control, and the other 3 groups were treated with 12.5, 25 or 50 mg/kg BrMC. After 20 d of treatment, living cells from the primary tumors were dissociated and injected into 3 groups of mice (4 mice per group). Each mouse was implanted with 5 × 104 cells from the control group and from the 50 mg/kg BrMC treated group in each flank. The growth of tumors was monitored, and tumor volumes were measured every 3 d. Animals were humanely sacrificed when the larger of the two tumors reached 500 mm3.
For immunohistochemical analysis of CD44 and CD133, tissues of the LCSCs derived-tumors in the nude mouse xenograft model were performed with formalin-fixed, paraffin-embedded sectioning as previously described by Moinfar et al[25]. After incubation with 1% non-fat dry milk in PBS (pH 7.4), the sections were then reacted with mouse anti-CD44 monoclonal antibody (1:250, Cell Signaling Technology Inc.) or mouse anti-CD133 monoclonal antibody (1:200, Abzoom, Dallas, TX, United States) for 1 h at room temperature followed by incubation with the secondary biotinylated antibody for 30 min. After washing, sections were subsequently incubated with streptavidin-peroxidase for 30 min. Finally, the results were visualized after a 15-min incubation with diaminobenzidine.
A control non-specific small interfering RNA (siRNA) (5’-GACTTCATAAGGCGCATGC-3’) and β-catenin siRNA (5’-AGCUGAUAUUGAUGGACAGTT-3’) were synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). Transfection of siRNA was carried out with Lipofectamine 2000 (Invitrogen Life Technologies) according to the procedure recommended by the manufacturer. Twenty-four hours after transfection, the cells were treated with DMSO (control) or BrMC at the indicated concentrations for 24 h. The cells were then collected and processed for western blotting and the tumorsphere formation assay.
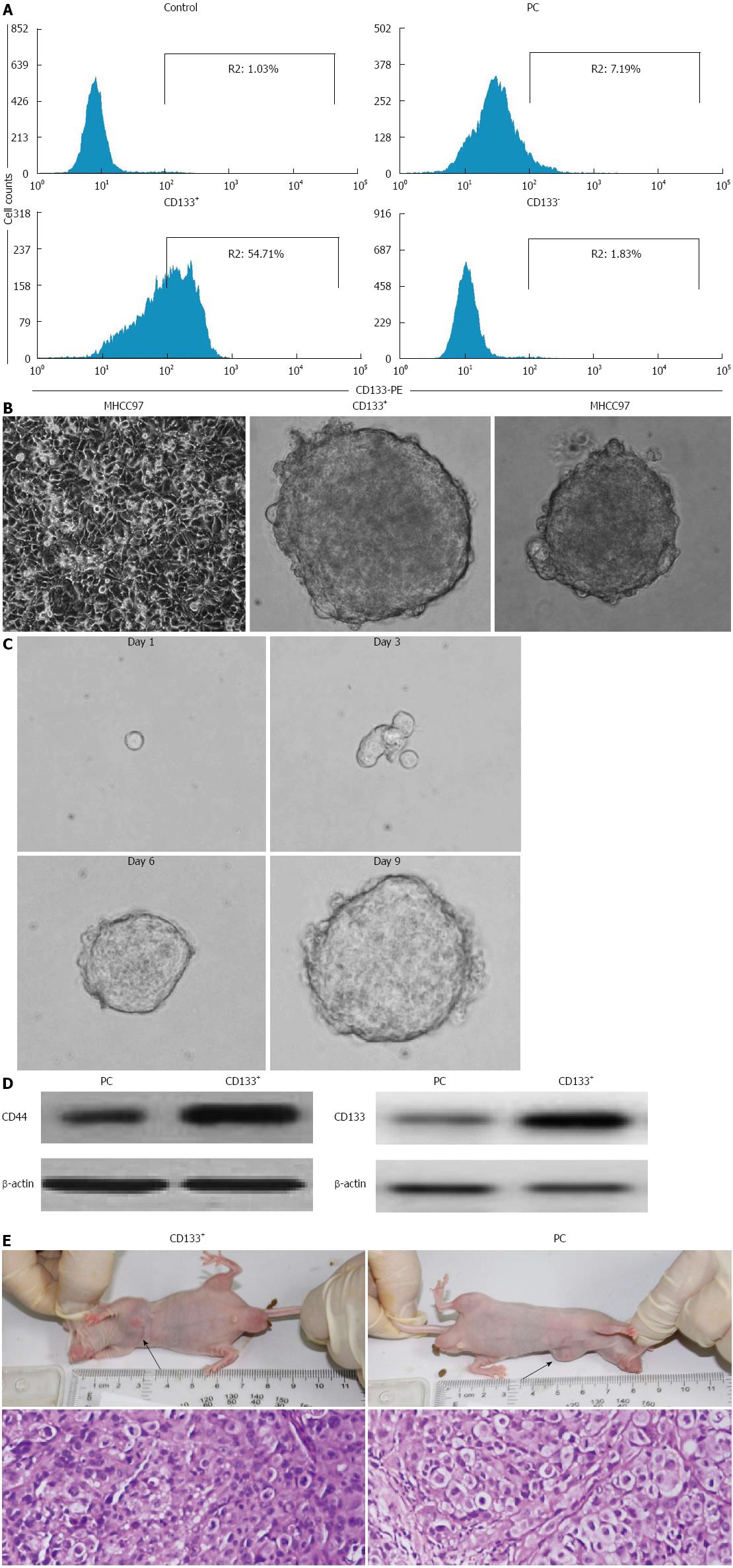
CD133 has previously been classified as a CSC marker in liver cancer. Therefore, we first isolated the CD133+ subpopulation from MHCC97 cells by MACS. Following sorting, we examined the expression of CD133 by flow cytometry. As shown in Figure 1A, the sorted CD133+ cells showed a high purity of 57.29% ± 4.61%, as compared with a purity of 1.02% ± 0.65% for CD133- counterparts and 7.21% ± 1.34% for non-sorted MHCC97 cells. To establish long-term cultures enriched in stem cells from sorted CD133+, we performed the tumorsphere assay by culturing the cells in stem cell-conditioned medium. Within 6 d of culture, we obtained liver cancer spheroids both in CD133+ cells and parental MHCC97 cells (Figure 1B). As shown in Table 1, the CD133+ subpopulation exhibited a 2.7- and 2.5-fold enhancement in tumorsphere formation amount and size, respectively, compared with that of parental cells, whereas, CD133- counterparts could not grow as spheroids in the nonadherent and serum-free conditions.
To further confirm the stem cell properties and functions of the CD133+ SFCs, we evaluated their self-renewal capacity and tumorigenic potential. First, we measured the capacity of single cells obtained from these CD133+ dissociated spheres to form secondary tumorspheres. Within 9 d of culture, we obtained new LCSC spheroids of growing undifferentiated CD133+ cells (Figure 1C). These suggest an in vitro self-renewing capacity of CD133+ SFCs. In addition, CD133+ SFCs also expressed an enhanced level of stem cell markers, CD133 and CD44, compared with their parental cells (Figure 1D). Next, we evaluated the tumorigenic potential of CD133+ SFCs. We investigated the ability of CD133+ SFCs and parental cells to give rise to tumors in Balb/c-nu mice. As many as 1 × 106 parental cells were needed to initiate stable tumor formation 39 d after injection, while, in contrast, as few as 1 × 104 CD133+ SFCs were sufficient to generate visible tumors only 23 d post-injection (Table 2). These data indicate that CD133+ SFCs, namely LCSCs, are more tumorigenic than their parental cells in vivo. Additionally, HE staining was performed and revealed similar histological characteristics in tumor xenografts derived from CD133+ SFCs and their parental cells (Figure 1E).
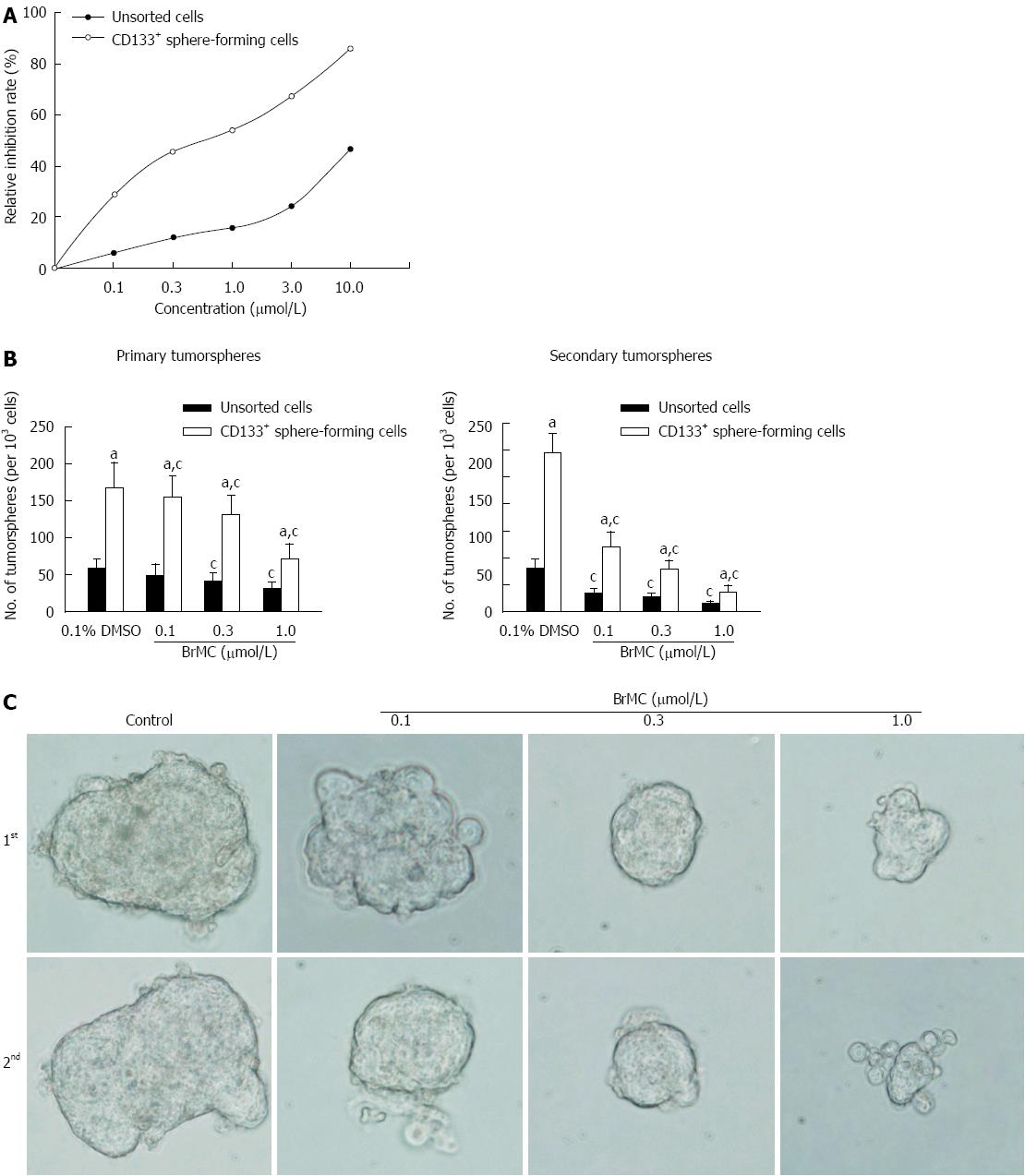
CSCs possess the property of limitless proliferative potential. A number of previous studies have demonstrated that some naturally-occurring polyphenol compounds such as genistein preferentially inhibit proliferation of pancreatic cancer stem cells[26]. In this study, we thus evaluated the anti-proliferative effects of BrMC on LCSCs derived from MHCC97 cell line by MTT assay. As shown in Figure 2A, when cells were treated with different concentrations of BrMC for 48 h, BrMC preferentially inhibited proliferation of LCSCs in a dose-dependent manner, with the IC50 around 0.5 μmol/L for LCSCs and 17.9 μmol/L for parental MHCC97 cells.
In order to evaluate whether BrMC could suppress the self-renewal of LCSCs derived from the MHCC97 cell line in vitro, we treated the primary tumorspheres with varying concentrations of BrMC and then removed the drug and cultured them for another passage to form the secondary spheres. Results showed that BrMC treatment resulted in a decrease both in tumorsphere number and size of LCSCs. Furthermore, a significant decrease in the number and size of the secondary tumorspheres indicated a reduced self-renewal capacity of these LCSCs by BrMC treatment (Figure 2B and C).
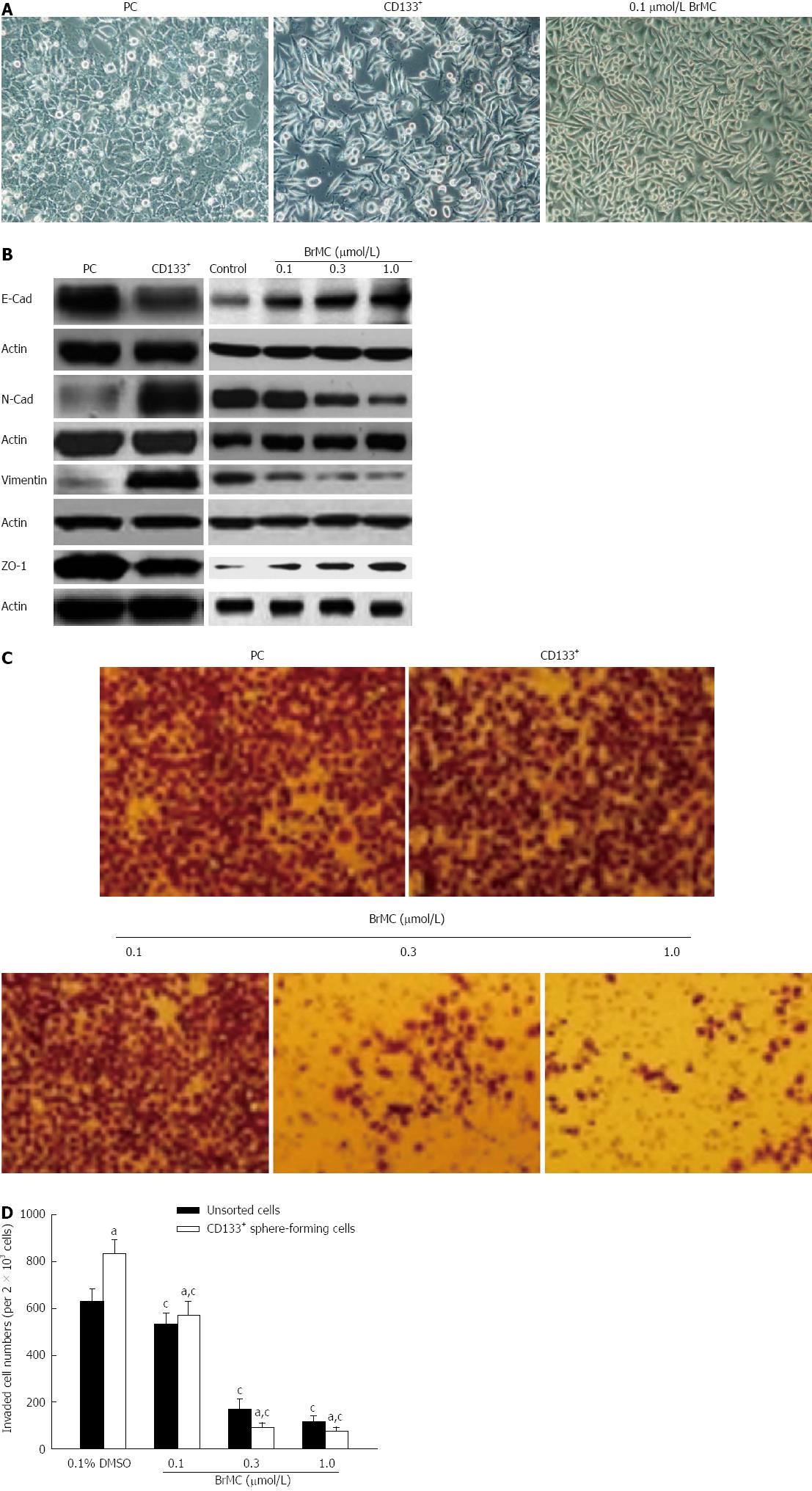
Epithelial-mesenchymal transition (EMT) is an important process during metastasis of LCSCs. Therefore, we sought to examine whether morphological changes existed between LCSCs and parental MHCC97 cells cultured adherently in vitro. As observed in Figure 3A, LCSCs exhibited a spindle-like shape, while parental MHCC97 cells displayed a cobble-stone-like phenotype. However, treatment with 0.1 μmol/L BrMC suppressed EMT in LCSCs as morphological changes from a spindle-like shape to a cobble-stone-like appearance were displayed. Moreover, similar results were further confirmed by western blotting using specific antibodies against EMT-relative markers. Figure 3B shows that LCSCs expressed higher vimentin and N-cadherin protein levels, which are typically associated with mesenchymal cells, and lower expression of epithelium-associated E-cadherin and ZO-1 proteins. However, BrMC induced the upregulation of epithelial markers E-cadherin and ZO-1 and the downregulation of mesenchymal markers N-cadherin and vimentin after treatment for 24 h of LCSCs derived from MHCC97 cell line.
Since EMT has been identified as being associated with increased cancer cell invasion, we next evaluated the effect of BrMC on cell invasion of LCSCs in vitro using a transwell chamber coated with a Matrigel barrier. As shown in Figure 3C and 3D, BrMC significantly reduced the invasiveness capacity of LCSCs in a dose-dependent manner. These results demonstrated that BrMC possesses inhibitory effects on EMT and invasion in LCSCs derived from MHCC97 cell line.
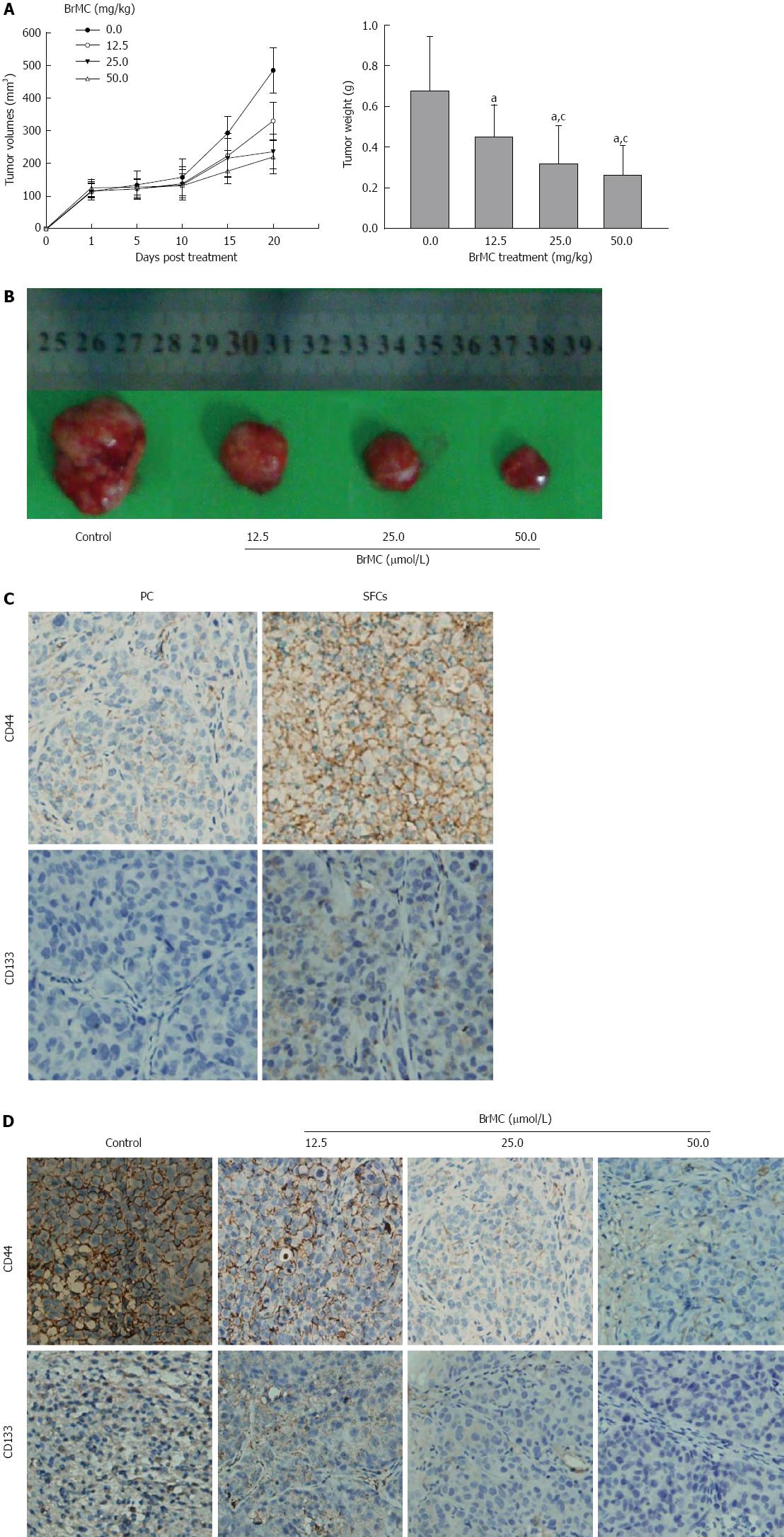
In order to evaluate whether BrMC could target LCSCs in vivo, we utilized the xenograft model of LCSCs from MHCC 97 cells in Balb/c-nu mice. Two weeks after cell inoculation with 5 × 104 LCSCs resuspended in Matrigel, animals underwent daily gastric lavage with various concentrations of BrMC. After 20 d of treatment, tumors in 25 and 50 mg/kg BrMC-treated mice were less than 50% of the size of those in refined olive oil control animals (Figure 4A and B). Immunohistochemical analysis of CD44 and CD133 in LCSC-derived tumors revealed that the LCSC markers CD44 and CD133 were mainly expressed on the cell surface of the cancer cells, and that the tumors derived from CD133+ SFCs showed significantly higher CD44 and CD133 positive rates than that of tumors derived from parental cells (Figure 4C). Furthermore, BrMC treatment can significantly decrease the CD44 and CD133 expression frequency of the tumors derived from LCSCs (Figure 4D).
To further confirm the results, we investigated the growth of secondary tumors in Balb/c-nu mice inoculated with tumor cells dissociated from primary tumor xenografts. In order to avoid possible variations due to heterogeneity, each recipient mouse was inoculated with 5 × 104 cells obtained from 50 mg/kg BrMC-treated tumors and another 5 × 104 cells obtained from control tumors in two opposite sides. Interestingly, we found that tumor cells from control animals exhibited rapid tumor re-growth, reaching a final tumor volume of 567-686 mm3. However, the tumor cells from 50 mg/kg BrMC-treated mice mostly failed to generate any tumors up to 33 d after inoculation (Table 3). These results suggest that BrMC was able to eliminate LCSCs in primary tumor xenografts, thereby inhibiting tumor regrowth in secondary inoculated mice.
| Time (d) | Tumor incidence1 | Volume (mm3) | ||
| Control | BrMC-treated group | Control | BrMC-treated group | |
| 1 | 0/12 | 0/12 | - | - |
| 3 | 0/12 | 0/12 | - | - |
| 6 | 4/12 | 0/12 | 28 ± 12 | - |
| 12 | 9/12 | 0/12 | 134 ± 29 | - |
| 15 | 12/12 | 3/12 | 321 ± 63 | 16 ± 8 |
| 18 | 12/12 | 3/12 | 532 ± 96 | 33 ± 16 |
| 21 | 8/8 | 2/8 | 351 ± 67 | 23 ± 13 |
| 24 | 8/8 | 2/8 | 593 ± 131 | 32 ± 24 |
| 27 | 4/4 | 1/4 | 264 ± 91 | 54 |
| 30 | 4/4 | 1/4 | 387 ± 114 | 67 |
| 33 | 4/4 | 1/4 | 567 ± 126 | 82 |
BrMC inhibits self-renewal in LCSCsthrough modulation ofβ-catenin expression
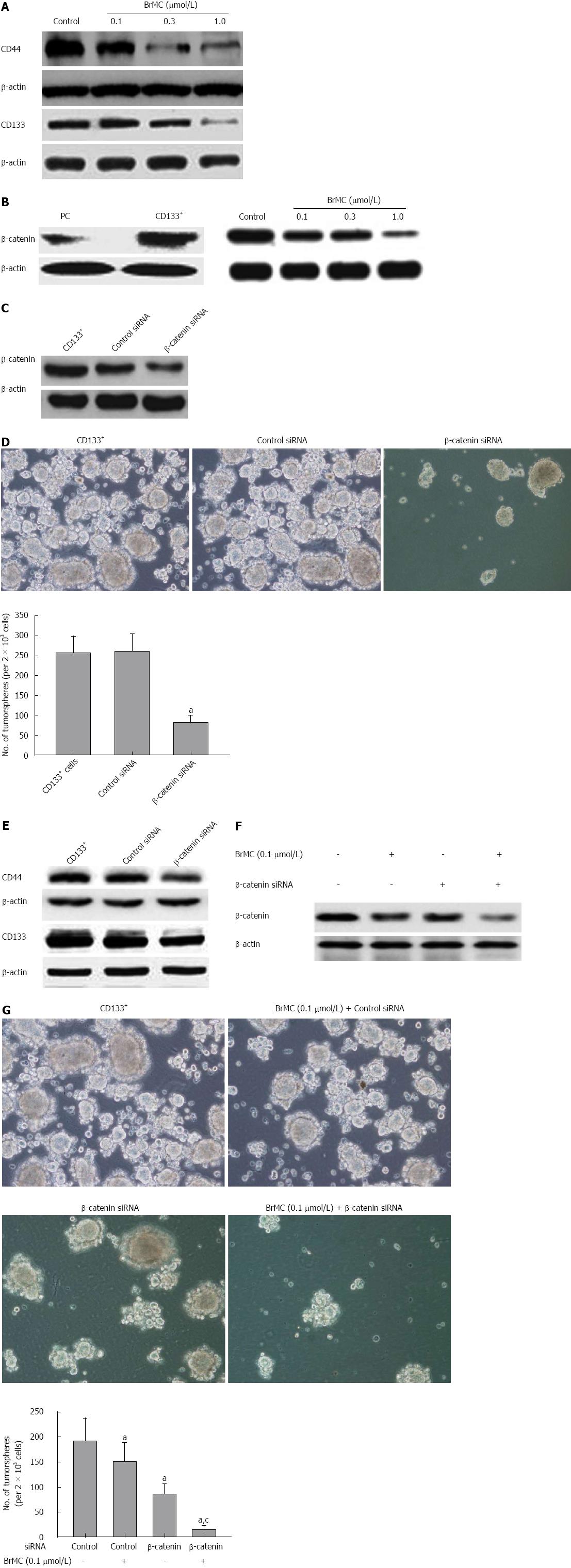
To examine whether BrMC could regulate expression of stem cell markers of LCSCs, we determined the expression of CD44 and CD133 following BrMC treatment by western blotting analysis. Results showed that BrMC downregulated CD44 and CD133 expression in a dose-dependent manner (Figure 5A). This was in accordance with our previous immunohistochemical analysis in LCSC-derived tumors (Figure 4D).
CD44 has been shown to be a downstream target of the β-catenin signaling pathway[27]. Wnt/β-catenin signaling has been implicated in the maintenance of CSCs of liver cancer[28]. Therefore, we measured the expression level of stem cell signal molecule β-catenin in LCSCs and parental MHCC97 cells, and examined whether β-catenin was downregulated by BrMC in LCSCs. Western blotting analysis showed that β-catenin was highly expressed in LCSCs compared with that of parental MHCC97 cells. We also found that BrMC (0.1, 0.3, 1.0 μmol/L) treatment resulted in a significant decrease in β-catenin expression of LCSCs (Figure 5B).
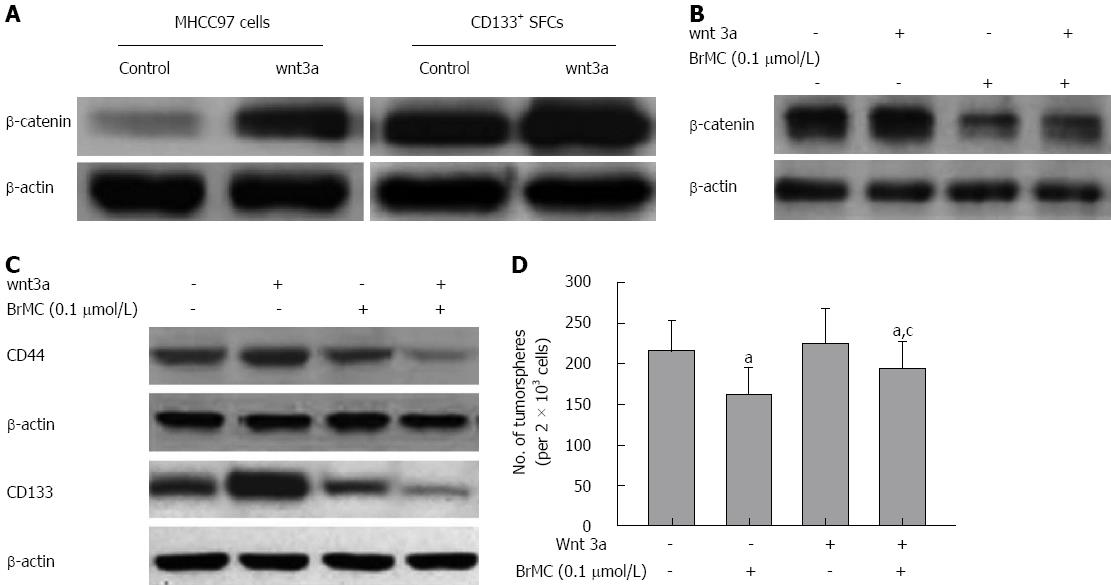
We further determined the role of β-catenin in the maintenance of self-renewal of LCSCs. Silencing of β-catenin by siRNA transfection resulted in less expression of β-catenin protein, as confirmed by Western blotting (Figure 5C). We also found that the downregulation of β-catenin expression significantly decreased the tumorsphere formation ability and inhibited expression of stem cell markers of LCSCs (Figure 5D and 5E). BrMC (0.1 μmol/L) plus β-catenin siRNA inhibited β-catenin expression to a greater degree compared to either alone (Figure 6A). Moreover, β-catenin siRNA potentiated the BrMC-induced decrease in tumorsphere formation of LCSCs (Figure 6B). We also treated LCSCs with Wnt3a, a ligand known to activate the Wnt/β-catenin pathway. As expected, Wnt3a induced β-catenin stabilization and resulted in a corresponding up-regulation of β-catenin in LCSCs (Figure 6C). This upregulation of β-catenin attenuated BrMC-induced downregulation of β-catenin and stem cell markers and antagonized BrMC-induced inhibition of self-renewal of LCSCs (Figure 6D, 6E and 6F). Taken together, these results provide some molecular evidence suggesting that the downregulation of β-catenin expression may contribute to the inhibitory effects of BrMC on LCSCs.
Cancer stem cells are defined as a minor population of tumorigenic cells that are capable of continuous self-renewal and differentiation, and undergo unlimited proliferation, giving rise to new tumors[29,30]. Therefore, finding compound(s) that are capable of inhibiting or killing the CSCs is extremely important to overcome tumor resistance, reduce relapse, and eventually improve overall survival. Our previous study has shown that BrMC possessed promising inhibitory effects on proliferation and apoptosis of colon, gastric and liver cancer cells. In the current study, we first successfully isolated and identified LCSCs from the liver cancer MHCC97 cell line. Further, we showed for the first time that BrMC could preferentially inhibit proliferation and self-renewal, and suppress EMT and invasion of LCSCs. Moreover, BrMC was able to eradicate LCSCs in vivo, as assessed by an in vivo tumorigenicity assay using primary and secondary Balb/c-nu mouse models. Secondly, we found that the inhibitory effects of BrMC on stem cell function and properties of LCSCs were mediated by inhibition of β-catenin pathways: β-catenin siRNA transfection and BrMC were synergistic in inhibiting the self-renewal of LCSCs. Conversely, the inhibition of Wnt3a in LCSCs resulted in an opposite effect.
More recently, CD133 has been used as a surface marker of CSCs in various solid tumors, including liver cancer. However, the function of CD133 is not entirely known yet. Thus, the single phenotypic marker CD133 is not sufficient to identify LCSCs. Tumorsphere culture may provide an alternative approach to identify and enrich LCSCs. Under non-adherent serum-free conditions in vitro, most tumor cells undergo programmed cell death, whereas the rare CSCs divide to generate multicellular 3-dimensional spheres[31,32]. This assay is a powerful tool to enrich CSCs and further assess the functional properties of the isolated CSCs. By employing a combination of this technique and MACS based on the CD133+ surface marker, we have successfully obtained the putative LCSCs, namely CD133+ SFCs, from the MHCC97 cell line. We demonstrated that these CD133+ SFCs possess stem-like properties, including self-renewal, initiation of tumor growth in mice at very low cell numbers and a higher expression level of stem cell marker compared with their parental cells. These data indicated that the method which we used to isolate and indentify LCSCs from liver cancer cell lines may be faster, more economic and effective, compared with methods based on two or more surface markers.
The Wnt/β-catenin pathway is one of the key pathways that modulates stem cell self-renewal[14]. For example, overexpression of β-catenin enhanced self-renewal preferentially and mediated radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line[33]. Hallett et al[34] reported that pharmacological inhibitors of Wnt/β-catenin signaling could inhibit the viability and or self-renewal of breast tumor-initiating cells, and target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. Consistent with these previous reports, we found that downregulation of β-catenin by BrMC resulted in inhibition of CSC function and characteristics of LCSCs, such as significant inhibition of proliferation and self-renewal, suppression of EMT and invasiveness, downregulation of the expression of stem cell markers of LCSCs, and further efficacious promotion of the elimination of LCSCs in vivo.
Previous studies have shown that activated Akt was able to phosphorylate Ser9 on GSK3β, which may decrease the activity of GSK3β, thereby leading to stabilization of β-catenin in the cytoplasm[35]. Chrysin was reported to induce apoptosis through caspase activation and Akt inactivation in leukemia cells[36]. Our previous study also demonstrated that 5,7-dihydroxy-8-nitrochrysin, another synthetic chrysin analogue, could induce activation and nuclear localization of FOXO3a, which was associated with reduced levels of Akt phosphorylation. Therefore, we speculate that the downregulation of β-catenin by BrMC probably occurs via reduced levels of Akt and activation of GSK3β, with the consequent degradation of β-catenin. Wnt3a treatment can induce stabilization of β-catenin, with entry into the nucleus and subsequent activation of the β-catenin pathway. Thus, Wnt3a treatment can antagonize the inhibitory effects of BrMC on self-renewal of LCSCs. On the other hand, Su et al[37] reported that genistein increases levels of membrane E-cadherin and E-cadherin-β-catenin cell adhesion complex, and eventually attenuates β-catenin signaling in mammary epithelial cells. We also found that E-cadherin, an epithelial marker, was upregulated by BrMC in LCSCs. E-cadherin is known to anchor and to sequester β-catenin in the membrane and prevent its activation. Therefore, we suppose that this inactivation of β-catenin by upregulation of E-cadherin can also contribute to the inhibitory effects of LCSCs by BrMC. Interestingly, the inhibition of β-catenin at the protein level was not optimal, as treatment of β-catenin siRNA can further downregulate β-catenin at the transcription level and synergize the inhibition of self-renewal of LCSCs induced by BrMC.
In conclusion, we have presented supportive evidence for the first time that BrMC, a novel synthetic chrysin analogue, can target LCSCs both in vitro and in vivo. Furthermore, our study identified the downregulation of β-catenin expression by BrMC as one of the possible mechanisms for its efficacy. These studies support the use of BrMC for liver cancer chemoprevention or chemotherapy. These findings provide a strong rationale for preclinical and subsequent clinical evaluation of BrMC for liver cancer therapy.
Liver cancer is the fifth most common cancer in the world and the third leading cause of cancer-related death. Recent studies indicated that cancer stem cells (CSCs) may be responsible for tumor recurrence and drug-resistance. Therefore, the identification of a compound that can target liver CSCs (LCSCs) is one of the main steps in improving overall survival of liver cancer patients.
More recently, a number of studies have found that some dietary compounds can directly or indirectly affect CSC self-renewal pathways. 8-bromo-7-methoxychrysin (BrMC) is a synthetic derivative of chrysin, and their previous study have demonstrated the effect of BrMC on the inhibition of proliferation and induction of apoptosis in colon, gastric and liver cancer cells was stronger than that of chrysin. However, the inhibitory effects of BrMC on the characteristics of CSCs have not been reported yet.
The authors firstly showed that BrMC, a novel synthetic chrysin analogue, was able to inhibit cancer stem cell-like properties of LCSCs and eliminate LCSCs in vivo. They also found that BrMC significantly decreased β-catenin expression in LCSCs and knockdown of β-catenin expression could synergize the inhibition of self-renewal of LCSCs induced by BrMC. The downregulation of β-catenin expression appears to contribute to the inhibitory effects of BrMC on the properties of LCSCs.
The present study provided strong evidences for the first time that BrMC was able to target LCSCs both in vitro and in vivo. These studies support the use of BrMC for liver cancer chemoprevention or chemotherapy.
Chrysin (5,7-dihydroxyflavone), a naturally wide distributed flavonoid, has been reported to possess anti-cancer activities. BrMC is a novel synthetic chrysin analogue.
This manuscript concludes that 8-bromo-7-methoxychrysin can inhibit the functions and characteristics of liver cancer stem cells derived from liver cancer MHCC97 cell line through downregulation of β-catenin expression. It is a good research with necessary information.
P- Reviewer: Cong WM S- Editor: Wen LL L- Editor: Cant MR E- Editor: Ma S
| 1. | Dudeck O, Ricke J. Advances in regional chemotherapy of the liver. Expert Opin Drug Deliv. 2011;8:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 3. | Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | Oishi N, Wang XW. Novel therapeutic strategies for targeting liver cancer stem cells. Int J Biol Sci. 2011;7:517-535. [PubMed] |
| 6. | Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, Stanbridge EJ, Lee EY. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243-3250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 448] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 922] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 10. | Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 11. | Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Yu D, Zhang H, He H, Zhang C, Zhao W, Shao RG. CD133(+)EpCAM(+) phenotype possesses more characteristics of tumor initiating cells in hepatocellular carcinoma Huh7 cells. Int J Biol Sci. 2012;8:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, Ng I, Man K, Wong N, To KF. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7:694-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 14. | Li Y, Wicha MS, Schwartz SJ, Sun D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem. 2011;22:799-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Green JM, Alvero AB, Kohen F, Mor G. 7-(O)-Carboxymethyl daidzein conjugated to N-t-Boc-hexylenediamine: a novel compound capable of inducing cell death in epithelial ovarian cancer stem cells. Cancer Biol Ther. 2009;8:1747-1753. [PubMed] |
| 16. | Zhang L, Li L, Jiao M, Wu D, Wu K, Li X, Zhu G, Yang L, Wang X, Hsieh JT. Genistein inhibits the stemness properties of prostate cancer cells through targeting Hedgehog-Gli1 pathway. Cancer Lett. 2012;323:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11:2188-2199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13:881-884. [PubMed] |
| 19. | Xiang HL, ZX , Cao JG. Induction of apoptosis of human gastric carcinoma SGC-790 cell line by 8-bromo-7-mehoxychrysin. Zhongguo Yaolixue Tongbao. 2008;24:1370-1372. |
| 20. | Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ, Qin Y, Liu HQ, Xia H, Cao JG. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5, 7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol. 2007;13:3824-3828. [PubMed] |
| 21. | Yang XH, Zheng X, Cao JG, Xiang HL, Liu F, Lv Y. 8-Bromo-7-methoxychrysin-induced apoptosis of hepatocellular carcinoma cells involves ROS and JNK. World J Gastroenterol. 2010;16:3385-3393. [PubMed] |
| 22. | Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1685] [Cited by in RCA: 1664] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 23. | Ning Y, Li Q, Xiang H, Liu F, Cao J. Apoptosis induced by 7-difluoromethoxyl-5,4’-di-n-octyl genistein via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep. 2012;27:1857-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Choi JN, Kim D, Choi HK, Yoo KM, Kim J, Lee CH. 2’-hydroxylation of genistein enhanced antioxidant and antiproliferative activities in mcf-7 human breast cancer cells. J Microbiol Biotechnol. 2009;19:1348-1354. [PubMed] |
| 25. | Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A, Li Y, Azmi AS, Miele L, Sarkar FH. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296-2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Li J, Zhou BP. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 28. | Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 29. | Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 30. | Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1:552-566. [PubMed] |
| 31. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5562] [Article Influence: 264.9] [Reference Citation Analysis (0)] |
| 32. | Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506-5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1371] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 33. | Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Hallett RM, Kondratyev MK, Giacomelli AO, Nixon AM, Girgis-Gabardo A, Ilieva D, Hassell JA. Small molecule antagonists of the Wnt/β-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS One. 2012;7:e33976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1246] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 36. | Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |