Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7586
Revised: September 5, 2013
Accepted: September 16, 2013
Published online: November 21, 2013
Processing time: 128 Days and 16.3 Hours
Intraperitoneal carcinomatosis (PC) may occur with several tumor entities. The prognosis of patients suffering from PC is usually poor. Present treatment depends on the cancer entity and includes systemic chemotherapy, radiation therapy, hormonal therapy and surgical resection. Only few patients may also benefit from hyperthermic intraperitoneal chemotherapy with a complete tumor remission. These therapies are often accompanied by severe systemic side-effects. One approach to reduce side effects is to target chemotherapeutic agents to the tumor with carrier devices. Promising experimental results have been achieved using drug-eluting beads (DEBs). A series of in vitro and in vitro experiments has been conducted to determine the suitability of their extravascular use. These encapsulation devices were able to harbor CYP2B1 producing cells and to shield them from the hosts immune system when injected intratumorally. In this way ifosfamide - which is transformed into its active metabolites by CYP2B1 - could be successfully targeted into pancreatic tumor growths. Furthermore DEBs can be used to target chemotherapeutics into the abdominal cavity for treatment of PC. If CYP2B1 producing cells are proven to be save for usage in man and if local toxic effects of chemotherapeutics can be controlled, DEBs will become promising tools in compartment-based anticancer treatment.
Core tip: Intraperitoneal carcinomatosis occurs with several tumor entities and prognosis is usually poor. Besides standard therapy, only few patients may benefit from hyperthermic intraperitoneal chemotherapy. The treatment may cause severe systemic side-effects. One different approach to target chemotherapeutic agents to the tumor employs carrier devices. Contemplable carriers are drug-eluting beads (DEBs). DEBs can be used to transfer drugs or pro-drug converting enzymes directly to the tumor. Furthermore, DEBs can successfully target chemotherapeutics into the abdominal cavity for ip treatment. When local toxic effects are controlled, DEBs are effective tools in compartment-based therapy.
- Citation: Binder S, Lewis AL, Löhr JM, Keese M. Extravascular use of drug-eluting beads: A promising approach in compartment-based tumor therapy. World J Gastroenterol 2013; 19(43): 7586-7593
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7586
Peritoneal carcinomatosis (PC) is a disseminated tumor stage, which is observed in patients with ovarian, pancreatic, gastric and colorectal cancer. With median survival rates of 3.1 mo for gastric cancer and 5.2 mo for colorectal cancer, respectively[1], the prognosis is usually poor[2].
Survival is prolonged by new agents used in palliative chemotherapy. With the availability of oxaliplatin, irinotecan, bevacizumab, and cetuximab the 5-year survival has significantly increased over the last decade[3-7].
Although some patients seem to benefit from these drugs, the physical and psychological strain for patients suffering from PC remains high. In addition to the commonly known side effects of chemotherapy[8], patients show a variety of symptoms originating from PC itself, ranging from abdominal pain, nausea and obstipation up to bowel obstruction and obstructive uropathy[9].
The treatment of peritoneal carcinomatosis requires an interdisciplinary and multimodal approach. Modern therapy combines cytoreductive surgery (CRS), radiation therapy and systemic chemotherapy, depending on the origin of the tumor[10-12]. Unfortunately survival rates remain low[1,13] at the cost of frequently observed dose limiting side-effects[8].
Since tumor spread into the abdominal cavity may also be considered as an early step of dissemination - comparable to liver metastasis in colorectal carcinoma - and not as a state of generalized systemic disease[14-16], one approach may be to resect all detectable tumor nodules and target drugs directly to the peritoneal cavity[17].
The surgical procedure removes all macroscopic tumor manifestations by combination of different peritonectomy procedures, including greater omentectomy, splenectomy, left upper quadrant peritonectomy, right upper quadrant peritonectomy, lesser omentectomy, cholecystectomy with stripping of the omental bursa, pelvic peritonectomy with sleeve resection of the sigmoid colon, and antrectomy[18-21], as well as parietal peritonectomy[22,23]. After the resection, the dissolved chemotherapeutic agent and carrier solution are heated up to 42 °C and pumped through the abdominal cavity for 40-90 min[24]. Since the abdomen remains opened, it is possible for the surgeon to support the circulation in the abdominal cavity manually[25]. This procedure is followed by thorough lavage, anastomosis of resected bowel segments and closure of the abdominal wall[24].
A survival benefit using CRS and hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown[26]. Verwaal et al[27] reported a 3-year survival of 38% in their patients. A more recent follow up of the same cohort shows similar survival rates[28]. Median progression-free survival was 7.7 mo in the control arm and 12.6 mo in the HIPEC arm. A 5-year survival of 45% was found in patients, in which R1 resection could be achieved. This indicates that CRS combined with HIPEC is superior to systemic chemotherapy alone. Nevertheless the findings of Franko et al[29] suggest, that CRS combined with HIPEC as well as systemic chemotherapy alone have their roles in the multidisciplinary approach treating peritoneally disseminated cancer.
In selected patients even a long term survival may be possible, with CRS and HIPEC being a curative approach in disseminated colorectal carcinoma[28,30].
The HIPEC procedure itself is demanding for most of the patients. Even though the median survival rates increased, the 30-d mortality rate of 4.8% and a morbidity rate reaching up to 55% are high[31]. The surgery itself and severe systemic side-effects may lead to deterioration of health or death[32,33]. Given that, the inclusion criteria to receive CRS and HIPEC remain strict. The peritoneal surface has to be the only site of disease dissemination[27] and the preoperative assessment[34] should suggest a high likelihood of achieving complete cytoreduction (CC-0)[35]. Therefore only patients with medium-sized intraperitoneal tumor nodules and a limited distribution within the abdomen are selected[36]. Patients have to be physically fit to endure this extensive procedure. Considering that peritoneal carcinomatosis only becomes symptomatic in advanced stages, where CC-0 or CC-1 can rarely be achieved, only few highly selected patients have access to this approach[37]. Excluded patients are left with systemic chemotherapy.
Alternative techniques have been investigated to target chemotherapeutic agents to the body cavities without the strain of surgery. These are promising approaches to circumvent both the systemic side effects and the hazard of an extensive surgical procedure.
Promising carriers for contemplable agents such as doxorubicin, irinotecan or mitoxantrone are drug-eluting beads (DEB).
By far the most commonly used product in clinic is DC Bead™, which are microspheres comprised of a sulphonate-modified polyvinyl-alcohol hydrogel. They are available in sizes from 70-700 μm[38] and can be loaded with doxorubicin (DOX), irinotecan (IRI) or mitoxantrone (MTX)[39]. When drug-loaded, the product provides an accurate dosage of drug per unit volume of beads in vitro[38], which they release via ion exchange constantly over weeks[40,41]. In vitro, the beads are robust and maintain their size and shape after drug loading[42]. This is a prerequisite for DEBs, since damage of the beads may cause rapid liberation and significant systemic distribution of the encapsulated drug or adverse effects by the debris itself.
The surface of the DEBs itself is inert and did not cause any immune reaction in control groups treated with unloaded beads[39,43]. Furthermore the biomechanical engineered material is able to shield its content from the immune system[44,45].
DEBs are used in clinical practice for trans-arterial chemoembolisation (TACE) of hypervascularized tumors[46], such as hepatocellular carcinoma (HCC) and liver metastasis. By administering them selectively into the tumor-feeding vessels, the route for essential nutrients is obstructed and high levels of antineoplastic drugs can be reached within the tumor[47].
As the procedure itself can be carried out under local anesthesia, morbidity and complication rates are low[48], TACE has become the standard palliative approach in patients with unresectable HCC[49-51]. The objective response rates range from 70%-75%[52,53] at a low rate of complications[53]. This suggests a good risk-benefit ratio.
For both associated side effects[54] and progression free survival[55] as well as overall disease control[54,55], doxorubicin-loaded DC beads (DOXDEB™) produced the most promising results.
Since DEBs are able to liberate agents continuously in vitro[56] they can also serve as drug carriers for extravascular application if the beads are directly instilled into the compartments.
The median survival of rats with experimental glioblastoma multiforme (GBM) could be successfully prolonged using doxorubicin polymers[57]. This demonstrated a superior effect of chemotherapeutic carriers as compared to iv administration of the free drug. The most efficient drug - namely doxorubicin - caused the most severe side effects. Intracerebral hemorrhage and edema as well as hemiparesis were observed[57]. A significantly longer median survival could be achieved in patients with GBM using carmustine warfers[58], but they did not affect the recurrence-free survival times[59]. These findings justified the idea of compartment-based therapy, but also called for new delivery systems and alternative antineoplastic drugs in return.
Baltes et al[60] showed that the intracerebral administration of DEBs is safe for use depending on the loaded drug. Both doxorubicin- and irinotecan-loaded DEBs significantly improved survival time in a rat BT4Ca GBM model. Doxorubicin again caused severe side effects[57] whereas irinotecan seemed to selectively affect only the cancer cells and not healthy brain tissue[61,62]. These findings could be confirmed in follow-up experiments where alginate was used as a viscosity modifier to secure the administration of the beads into the tissue[63].
The efficacy of irinotecan- and topotecan-loaded DEBs have been evaluated by use of a modified MTS assay and in a PSN-1 mouse xenograft model of pancreatic cancer by direct injection at the tumor site. Topotecan was shown to be more potent than irinotecan in the in vitro cell assay, had reasonable efficacy and tolerability at 0.2-0.4 mg doses but was lethal at doses of 0.83-1.2 mg. Irinotecan however, was well tolerated even with repeated injections of doses from 3.3-6.6 mg and displayed good efficacy[64]. A similar study evaluated combinations of doxorubicin, irinotecan, topotecan and rapamycin DEBs and demonstrated synergistic activity for certain drug combinations, in particular doxorubicin and rapamycin[65].
Feasibility for the clinical application of the direct intratumoral delivery of a compartment-based therapy was first demonstrated by delivery of a reservoir of a thermosensitive gel containing paclitaxel (Oncogel®) into the pancreas by use of ultrasound-guided endoscopic needle injection[66,67]. This approach has been subsequently adapted for the administration of irinotecan-loaded DEBs suspended in alginate into the tail of the pancreas of a healthy pig. The therapy was well tolerated up to doses of 300 mg of irinotecan, with only localized pancreatic tissue reactions on histopathologic review[68].
An elegant approach to target drugs to a tumor is to administer them as pro-drugs and activate them intratumorally. The active metabolites are formed by enzymes which are selectively injected into the tumor.
Routinely, ifosfamide has been used via iv application in pancreatic cancer treatment[69,70]. After administration cytochrome P450 2B1 (CYP2B1) produced by hepatocytes, transforms ifosfamide into 4-OH-ifosfamide, which results in the active compounds phosphoramide mustard and acrolein[71]. In vitro preparation and direct administration of the active compounds are limited due to their short half life (45 min)[72].
Löhr et al[73] used encapsulated feline kidney cells, engineered to produce CYP2B1[74], to target activated ifosfamide into pancreatic carcinoma[73]. Therefore, cells were encapsulated in cellulose sulphate[75] for immobilization and to protect them from the immune system when injected into the tumor. To model a pancreatic cell-like carcinoma PaCa-44 human pancreatic tumor cells were injected subcutaneously into nude mice. All mice received ifosfamide iv, one group received intra-tumorous injection of encapsulated CYP2B1 producing cells and one group received nonencapsulated cells. Tumor growth was impaired in all mice receiving ifosfamide. However, the most significant tumor reduction was detectable in the group that had received encapsulated cells. Complete macro- and microscopic tumor remission could be achieved in 20% of the animals. Although the same dosages of ifosfamide were used in both groups, the apoptotic rate of tumor cells was three times higher in the group receiving encapsulated cells. Furthermore these animals appeared healthier than the ones receiving nonencapsulated cells. Müller et al[43] were able to reproduce these results using a CYP2B1 producing cell line of human origin. This cell line did not produce potentially harmful retroviruses[76] and is immune resistant[77].
The approach worked for other tumor entities as well. Samel et al[78] showed, that similar results could be achieved in Balb/c mice carrying peritoneal tumor nodules, induced by syngenic C-26 cells injected into the abdominal cavity. This cell line is highly malignant and rapidly forms tumor nodules on the peritoneum. Again, in some animals a complete response was achieved. One major drawback of this approach is the use of genetically engineered cells. These cells may maintain a malignant potential. It remains to be shown if they can be safely applied to patients.
Therefore, an easier approach directly employs encapsulated chemo agents. In vitro tests with wild-type C-26 murine colon-carcinoma cells showed potent tumor toxicity for free DOX, IRI and MTX and the encapsulated drugs when combinations of the chemotherapeutic agents and DEBs were tested[39]. For free IRI and MTX the inhibition of cell growth was superior to their encapsulated forms. The proportion of apoptotic cells was significantly higher for free DOX as well as for DOXDEB™ when compared to the other two agents. Both DOX and MTX showed a dose-depending induction of apoptosis, whereas IRI did not show any significant effect.
In vivo tests followed after determining appropriate concentration levels[39]. For better detection of micrometastases and for tumor load quantification, C-26 cells had been transfected with the marker protein enhanced green florescent protein as described[78,79]. All animals developed disseminated PC. Thereafter, animals were treated with free and encapsulated DOX and MTX. Best tumor reduction was obtained when splitting the DEB application into three sessions. Complete tumor remission could be obtained. Weight loss and mortality of the subjects was significantly higher in the groups which were treated with the corresponding free drugs, suggesting a lower toxicity in the DEB groups.
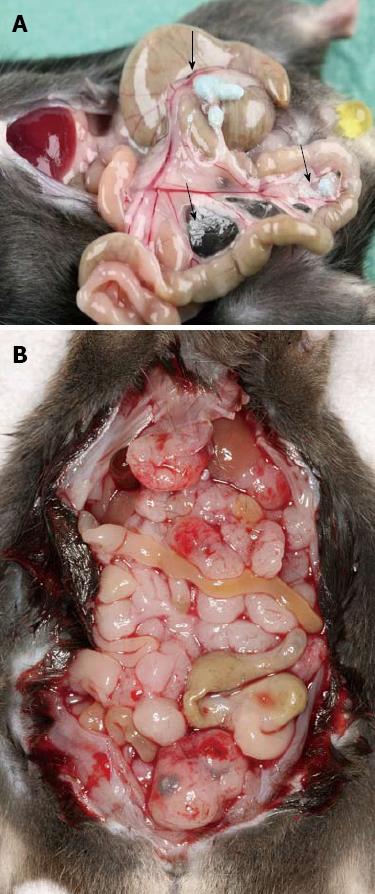
The results obtained in this model of colorectal tumor, could be reproduced for pancreatic carcinoma dissemination (Figure 1). Yagublu et al[80] used a model of peritoneally metastasized panc02 pancreatic carcinoma cells in C57 black6 mice. Treatment was performed with free and encapsulated DOX, IRI and MTX. The free drug was more potent in decreasing tumor cell growth and inducing apoptosis than the encapsulated drugs in vitro. Again, in vivo free drug administration caused more weight loss and significantly higher lethality than the encapsulated drug, while no relevant differences in antitumoral activity could be observed.
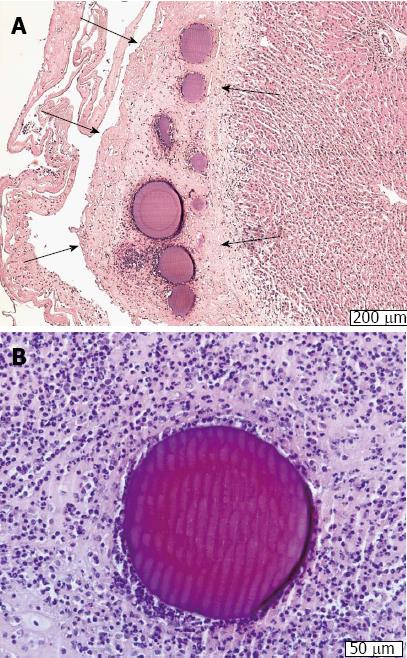
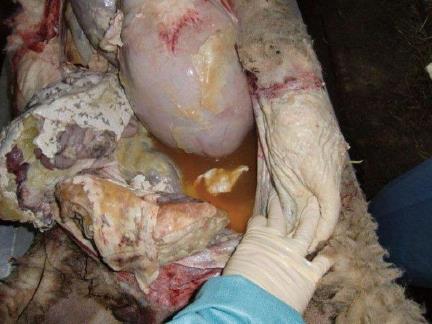
To test the safety of the intraperitoneal injection and therapy using the DOXDEB™ a large animal trail was carried out[81]. Black-headed meat-sheep received an application of DOXDEB™ into the abdominal cavity. Up to 50% of the maximal cumulative dose suggested for male humans were used in one single intraperitoneal injection[82]. DEBs were injected using a verres needle. Upon autopsy, no DEBs were distributed via blood or lymphatic vessels. Beads remained on the peritoneum, immobilized by a fibrin layer (Figure 2A and B). No evidence for organ-related damage or systemic toxicity was observed. This is remarkable, as cardio toxicity[83-87] and myelosuppresion[88-91] are frequently described with the systemic use of doxorubicin, along with less severe side effects such as stomatitis, alopecia, nausea and vomiting[90]. The systemic distribution of DOX followed a three-compartment-model omitting a rapid and high peak, in comparison to iv administration. Serum levels reached a steady-state 360 min after application with a half-life of 615 h. Some sheep did not reach the end point and developed a chemical peritonitis[82,92,93] (Figure 3). By circumventing the systemic administration and its accompanying side effects, local toxicity was the only limiting factor. This underlines the importance of drug choice when it comes to DEB therapy within the intraperitoneal compartment.
There is convincing evidence that drug-eluting beads can be employed in an extravascular environment for a compartment-based therapy. In several tumor models, the carrier devices showed convincing tumor control and side-effects were less likely to occur. Also, encapsulation devices can be used to transform pro-drugs into their active metabolites within or in vicinity of the tumor. Here, drug-eluting beads successfully immobilized transformating-enzyme producing cells and protected them from the host immune system. However, the application of genetically engineered cell lines remains a major safety concern.
Intraperitoneal application of DEBs is a small procedure which can be safely performed under local anesthesia. Within the abdominal cavity DEBs show predictable liberation characteristics, remain inert and do not distribute via blood or lymphatic vessels.
Compartment-based therapy could be considered as a favorable treatment option for palliative patients with a deteriorated general condition, who are not eligible for HIPEC. Local toxicity is a limiting factor. Other drugs - for example irinotecan - have to be tested in a large animal model to further investigate local reactions.
If the adverse effects of the loaded substances are controlled, the extravascular use of drug-eluting beads is a promising future approach in compartment-based tumor therapy.
P- Reviewers: Ding MX, Filep JG, Tan XR S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [PubMed] |
| 2. | Tischoff I, Tannapfel A. Pathologic and anatomic evidence of peritoneal metastases. Chirurg. 2007;78:1085-1086, 1088-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Dy GK, Krook JE, Green EM, Sargent DJ, Delaunoit T, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP. Impact of complete response to chemotherapy on overall survival in advanced colorectal cancer: results from Intergroup N9741. J Clin Oncol. 2007;25:3469-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441-9442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 509] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 6. | Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 8. | Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersall MH. On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol. 1996;7:189-195. [PubMed] |
| 9. | Carraro PG, Segala M, Cesana BM, Tiberio G. Obstructing colonic cancer: failure and survival patterns over a ten-year follow-up after one-stage curative surgery. Dis Colon Rectum. 2001;44:243-250. [PubMed] |
| 10. | Schmiegel W, Reinacher-Schick A, Arnold D, Graeven U, Heinemann V, Porschen R, Riemann J, Rödel C, Sauer R, Wieser M. Update S3-guideline “colorectal cancer” 2008. Z Gastroenterol. 2008;46:799-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Schmalfeldt B, Pfisterer , J . Interdisziplinäre S2k-Leitlinie für die Diagnostik und Therapie maligner Ovarialtumoren. Kommission Ovar der Arbeitsgemeinschaft Gynäkologische Onkologie eV. München, Wien, New York: W. Zuckschwerdt Verlag GmbH 2007; . |
| 12. | Weller M. Interdisziplinäre S 2 – Leitlinie für die Diagnostik und Therapie der Gliome des Erwachsenenalters. 030/099. Germany: AWMF online 2011; . |
| 13. | Affronti ML, Heery CR, Herndon JE, Rich JN, Reardon DA, Desjardins A, Vredenburgh JJ, Friedman AH, Bigner DD, Friedman HS. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115:3501-3511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14:254-261. [PubMed] |
| 15. | Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon’s role. Langenbecks Arch Surg. 1999;384:576-587. [PubMed] |
| 16. | Glehen O, Osinsky D, Beaujard AC, Gilly FN. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg Oncol Clin N Am. 2003;12:729-739, xiii. [PubMed] |
| 17. | Fernández-Trigo V, Stuart OA, Stephens AD, Hoover LD, Sugarbaker PH. Surgically directed chemotherapy: heated intraperitoneal lavage with mitomycin C. Cancer Treat Res. 1996;81:51-61. [PubMed] |
| 19. | Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 1996;82:235-253. [PubMed] |
| 20. | Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12:703-727, xiii. [PubMed] |
| 21. | Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 2007;134:247-264. [PubMed] |
| 22. | Vazquez Vde L, Sugarbaker PH. Total anterior parietal peritonectomy. J Surg Oncol. 2003;83:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Sugarbaker PH. Parietal peritonectomy. Ann Surg Oncol. 2012;19:1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Kerscher A. Die HIPEC - hypertherme intraperitoneale Chemoperfusion. Würzburg: Peritonealkarzinosezentrum Würzburg, Chirurgische Klinik der Universitätsklinik Würzburg 2010; . |
| 25. | Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, Steves MA, Sugarbaker PH. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790-796. [PubMed] |
| 26. | Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124-132. [PubMed] |
| 27. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1512] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 28. | Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 765] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 29. | Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756-3762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Sugarbaker PH. A curative approach to peritoneal carcinomatosis from colorectal cancer. Semin Oncol. 2005;32:S68-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Glockzin G, Ghali N, Lang SA, Agha A, Schlitt HJ, Piso P. Peritoneal carcinomatosis. Surgical treatment, including hyperthermal intraperitoneal chemotherapy. Chirurg. 2007;78:1100, 1102-1106, 1108-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Cavaliere F, Perri P, Rossi CR, Pilati PL, De Simone M, Vaira M, Deraco M, Di Filippo F. Indications for integrated surgical treatment of peritoneal carcinomatosis of colorectal origin: experience of the Italian Society of Locoregional Integrated Therapy in Oncology. Tumori. 2003;89:21-23. [PubMed] |
| 35. | Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Salti GI, Ailabouni L, Undevia S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal sarcomatosis. Ann Surg Oncol. 2012;19:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 38. | Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, Leppard SW, Wolfenden LC, Palmer RR, Stratford PW. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335-342. [PubMed] |
| 39. | Keese M, Gasimova L, Schwenke K, Yagublu V, Shang E, Faissner R, Lewis A, Samel S, Löhr M. Doxorubicin and mitoxantrone drug eluting beads for the treatment of experimental peritoneal carcinomatosis in colorectal cancer. Int J Cancer. 2009;124:2701-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci. 2007;30:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Gonzalez MV, Tang Y, Phillips GJ, Lloyd AW, Hall B, Stratford PW, Lewis AL. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro: in-vivo correlation. J Mater Sci Mater Med. 2008;19:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010;21:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Müller P, Jesnowski R, Karle P, Renz R, Saller R, Stein H, Püschel K, von Rombs K, Nizze H, Liebe S. Injection of encapsulated cells producing an ifosfamide-activating cytochrome P450 for targeted chemotherapy to pancreatic tumors. Ann N Y Acad Sci. 1999;880:337-351. [PubMed] |
| 44. | Löhr M, Hoffmeyer A, Kröger J, Freund M, Hain J, Holle A, Karle P, Knöfel WT, Liebe S, Müller P. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet. 2001;357:1591-1592. [PubMed] |
| 45. | Okada N, Miyamoto H, Yoshioka T, Sakamoto K, Katsume A, Saito H, Nakagawa S, Ohsugi Y, Mayumi T. Immunological studies of SK2 hybridoma cells microencapsulated with alginate-poly(L)lysine-alginate (APA) membrane following allogeneic transplantation. Biochem Biophys Res Commun. 1997;230:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol. 2008;31:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, Martin RC, O’Grady E, Real MI, Vogl TJ. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 48. | Skowasch M, Schneider J, Otto G, Weinmann A, Woerns MA, Dueber C, Pitton MB. Midterm follow-up after DC-BEAD™-TACE of hepatocellular carcinoma (HCC). Eur J Radiol. 2012;81:3857-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 50. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 51. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 52. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 53. | Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Martin R, Geller D, Espat J, Kooby D, Sellars M, Goldstein R, Imagawa D, Scoggins C. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology. 2012;59:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 55. | Song MJ, Park CH, Kim JD, Kim HY, Bae SH, Choi JY, Yoon SK, Chun HJ, Choi BG, Lee HG. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Lewis AL, Gonzalez MV, Leppard SW, Brown JE, Stratford PW, Phillips GJ, Lloyd AW. Doxorubicin eluting beads - 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18:1691-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25:3825-3831. [PubMed] |
| 58. | Salmaggi A, Duri S, Silvani A, Gaviani P, Milanesi I, Casali C, Di Meco F. Loco-regional treatments in first-diagnosis glioblastoma: literature review on association between Stupp protocol and Gliadel. Neurol Sci. 2011;32 Suppl 2:S241-S245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Chemotherapeutic wafers for High Grade Glioma. Cochrane Database Syst Rev. 2008;CD007294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Baltes S, Freund I, Lewis AL, Nolte I, Brinker T. Doxorubicin and irinotecan drug-eluting beads for treatment of glioma: a pilot study in a rat model. J Mater Sci Mater Med. 2010;21:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Noble CO, Krauze MT, Drummond DC, Yamashita Y, Saito R, Berger MS, Kirpotin DB, Bankiewicz KS, Park JW. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Eyol E, Boleij A, Taylor RR, Lewis AL, Berger MR. Chemoembolisation of rat colorectal liver metastases with drug eluting beads loaded with irinotecan or doxorubicin. Clin Exp Metastasis. 2008;25:273-282. [PubMed] |
| 63. | Glage S, Lewis AL, Mertens P, Baltes S, Geigle P, Brinker T. Evaluation of biocompatibility and anti-glioma efficacy of doxorubicin and irinotecan drug-eluting bead suspensions in alginate. Clin Transl Oncol. 2012;14:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Forster RE, Small SA, Tang Y, Heaysman CL, Lloyd AW, Macfarlane W, Phillips GJ, Antonijevic MD, Lewis AL. Comparison of DC Bead-irinotecan and DC Bead-topotecan drug eluting beads for use in locoregional drug delivery to treat pancreatic cancer. J Mater Sci Mater Med. 2010;21:2683-2690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Forster RE, Tang Y, Bowyer C, Lloyd AW, Macfarlane W, Phillips GJ, Lewis AL. Development of a combination drug-eluting bead: towards enhanced efficacy for locoregional tumour therapies. Anticancer Drugs. 2012;23:355-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Linghu E, Matthes K, Mino-Kenudson M, Brugge WR. Feasibility of endoscopic ultrasound-guided OncoGel (ReGel/paclitaxel) injection into the pancreas in pigs. Endoscopy. 2005;37:1140-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Fowers KD, Rathi R, Brugge WR. EUS-guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video). Gastrointest Endosc. 2007;65:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Karaca C, Cizginer S, Konuk Y, Kambadakone A, Turner BG, Mino-Kenudson M, Sahani DV, Macfarlane C, Brugge W. Feasibility of EUS-guided injection of irinotecan-loaded microspheres into the swine pancreas. Gastrointest Endosc. 2011;73:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Cerny T, Martinelli G, Goldhirsch A, Terrier F, Joss R, Fey MF, Brunner KW, Küpfer A. Continuous 5-day infusion of ifosfamide with mesna in inoperable pancreatic cancer patients: a phase II study. J Cancer Res Clin Oncol. 1991;117 Suppl 4:S135-S138. [PubMed] |
| 70. | Keizer HJ, Ouwerkerk J, Welvaart K, van der Velde CJ, Cleton FJ. Ifosfamide treatment as a 10-day continuous intravenous infusion. J Cancer Res Clin Oncol. 1995;121:297-302. [PubMed] |
| 71. | Dirven HA, van Ommen B, van Bladeren PJ. Glutathione conjugation of alkylating cytostatic drugs with a nitrogen mustard group and the role of glutathione S-transferases. Chem Res Toxicol. 1996;9:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Zheng JJ, Chan KK, Muggia F. Preclinical pharmacokinetics and stability of isophosphoramide mustard. Cancer Chemother Pharmacol. 1994;33:391-398. [PubMed] |
| 73. | Löhr M, Müller P, Karle P, Stange J, Mitzner S, Jesnowski R, Nizze H, Nebe B, Liebe S, Salmons B. Targeted chemotherapy by intratumour injection of encapsulated cells engineered to produce CYP2B1, an ifosfamide activating cytochrome P450. Gene Ther. 1998;5:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Crandell RA, Fabricant CG, Nelson-Rees WA. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973;9:176-185. [PubMed] |
| 75. | Stange J, Mitzner S, Dautzenberg H, Ramlow W, Knippel M, Steiner M, Ernst B, Schmidt R, Klinkmann H. Prolonged biochemical and morphological stability of encapsulated liver cells--a new method. Biomater Artif Cells Immobilization Biotechnol. 1993;21:343-352. [PubMed] |
| 76. | Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392-8396. [PubMed] |
| 77. | Takeuchi Y, Porter CD, Strahan KM, Preece AF, Gustafsson K, Cosset FL, Weiss RA, Collins MK. Sensitization of cells and retroviruses to human serum by (alpha 1-3) galactosyltransferase. Nature. 1996;379:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 206] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Samel S, Keese M, Lux A, Jesnowski R, Prosst R, Saller R, Hafner M, Sturm J, Post S, Löhr M. Peritoneal cancer treatment with CYP2B1 transfected, microencapsulated cells and ifosfamide. Cancer Gene Ther. 2006;13:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Sturm JW, Keese MA, Petruch B, Bönninghoff RG, Zhang H, Gretz N, Hafner M, Post S, McCuskey RS. Enhanced green fluorescent protein-transfection of murine colon carcinoma cells: key for early tumor detection and quantification. Clin Exp Metastasis. 2003;20:395-405. [PubMed] |
| 80. | Yagublu V, Caliskan N, Lewis AL, Jesenofsky R, Gasimova L, Löhr JM, Keese M. Treatment of experimental pancreatic cancer by doxorubicin-, mitoxantrone-, and irinotecan-drug eluting beads. Pancreatology. 2013;13:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Binder S, Lewis AL, Jesenofsky R, Neumaier M, Jäger E, Ströbel P, Löhr JM, Keese M. Intraperitoneal use of doxorubicin drug-eluting beads in sheep: a pilot safety study. Anticancer Res. 2012;32:5167-5174. [PubMed] |
| 82. | Ozols RF, Young RC, Speyer JL, Sugarbaker PH, Greene R, Jenkins J, Myers CE. Phase I and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Res. 1982;42:4265-4269. [PubMed] |
| 83. | Abraham R, Basser RL, Green MD. A risk-benefit assessment of anthracycline antibiotics in antineoplastic therapy. Drug Saf. 1996;15:406-429. [PubMed] |
| 84. | Abu-Khalaf MM, Harris L. Anthracycline-induced cardiotoxicity: risk assessment and management. Oncology (Williston Park). 2009;23:239, 244, 252. [PubMed] |
| 85. | Appel JM, Nielsen D, Zerahn B, Jensen BV, Skagen K. Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncol. 2007;46:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Klein HO. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1997;126:827; author reply 827-828. [PubMed] |
| 87. | Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47-58. [PubMed] |
| 88. | Ackland SP, Ratain MJ, Vogelzang NJ, Choi KE, Ruane M, Sinkule JA. Pharmacokinetics and pharmacodynamics of long-term continuous-infusion doxorubicin. Clin Pharmacol Ther. 1989;45:340-347. [PubMed] |
| 89. | Gottlieb JA, Gutterman JU, McCredie KB, Rodriguez V, Frei E. Chemotherapy of malignant lymphoma with adriamycin. Cancer Res. 1973;33:3024-3028. [PubMed] |
| 90. | Gottlieb JA; Pfizer. Adriblastin - Fachinformation. Berlin: Rote Liste Service GmbH 2009; . |
| 91. | Piscitelli SC, Rodvold KA, Rushing DA, Tewksbury DA. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther. 1993;53:555-561. [PubMed] |
| 92. | Litterst CL, Collins JM, Lowe MC, Arnold ST, Powell DM, Guarino AM. Local and systemic toxicity resulting from large-volume ip administration of doxorubicin in the rat. Cancer Treat Rep. 1982;66:157-161. [PubMed] |
| 93. | Schmoll HJ. Kompendium internistische Onkologie. Standards in Diagnostik und Therapie: Springer; 1999; . |