Published online Nov 7, 2013. doi: 10.3748/wjg.v19.i41.7089
Revised: August 10, 2013
Accepted: September 13, 2013
Published online: November 7, 2013
Processing time: 188 Days and 9.3 Hours
AIM: To determine whether magnified observation of short-segment Barrett’s esophagus (BE) is useful for the detection of specialized intestinal metaplasia (SIM).
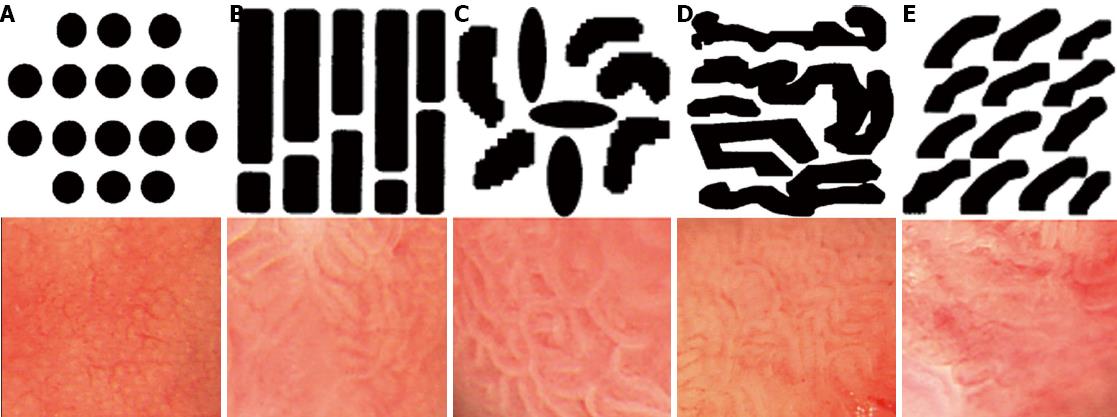
METHODS: Thirty patients with suspected short-segment BE underwent magnifying endoscopy up to × 80. The magnified images were analyzed with respect to their pit-patterns, which were simultaneously classified into five epithelial types [I (small round), II (straight), III (long oval), IV (tubular), V (villous)] by Endo’s classification. Then, a 0.5% solution of methylene blue (MB) was sprayed over columnar mucosa. The patterns of the magnified image and MB staining were analyzed. Biopsies were obtained from the regions previously observed by magnifying endoscopy and MB chromoendoscopy.
RESULTS: Three of five patients with a type V (villous) epithelial pattern had SIM, whereas 21 patients with a non-type V epithelial patterns did not have SIM. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of pit-patterns in detecting SIM were 100%, 91.3%, 92.3%, 60% and 100%, respectively (P = 0.004). Three of the 12 patients with positive MB staining had SIM, whereas 14 patients with negative MB staining did not have SIM. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of MB staining in detecting SIM were 100%, 60.9%, 65.4%, 25% and 100%, respectively (P = 0.085). The specificity and accuracy of pit-pattern evaluation were significantly superior compared with MB staining for detecting SIM by comparison with the exact McNemar’s test (P = 0.0391).
CONCLUSION: The magnified observation of a short-segment BE according to the mucosal pattern and its classification can be predictive of SIM.
Core tip: Various endoscopic approaches and advancements have shown great promise. However, careful endoscopic observation and stepwise four quadrant biopsy still represent the standard for the surveillance of Barrett’s esophagus (BE). In our study, we investigated the usefulness of magnifying endoscopy for the diagnosis of specialized intestinal metaplasia (SIM) in patients with short-segment BE compared with methylene blue chromoendoscopy. We found that the magnified observation of a short-segment BE according to its mucosal pattern and classification can be predictive of SIM.
- Citation: Ham NS, Jang JY, Ryu SW, Kim JH, Park EJ, Lee WC, Shim KY, Jeong SW, Kim HG, Lee TH, Jeon SR, Cho JH, Cho JY, Jin SY, Lee JS. Magnifying endoscopy for the diagnosis of specialized intestinal metaplasia in short-segment Barrett’s esophagus. World J Gastroenterol 2013; 19(41): 7089-7096
- URL: https://www.wjgnet.com/1007-9327/full/v19/i41/7089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i41.7089
Barrett’s esophagus (BE) is important clinically as the link between one of the most common gastrointestinal diseases, gastroesophageal reflux disease (GERD), and the most rapidly increasing cancer of the gastrointestinal (GI) tract, esophageal adenocarcinoma (EAC). For an adenocarcinoma to develop in the esophagus, the squamous epithelium must transition to columnar epithelium and subsequently become dysplastic. This metaplasia-dysplasia-carcinoma sequence is attributed to the repeated injury of the esophagus by gastroesophageal reflux[1-3]. According to the Montreal consensus from 2006, BE is characterized by the replacement of the squamous epithelia in the distal esophagus by columnar epithelia (gastric metaplasia), irrespective of the presence of specialized intestinal metaplasia (SIM)[4]. Controversy exists regarding the absolute requirement of intestinal metaplasia to define BE, primarily because long-term follow-up studies are not available to assess the risk of progression for each histologic subtype. However, cross-sectional and descriptive studies suggest that SIM either coexists with or precedes a significant majority of EAC cases and is likely the precursor lesion[5,6]. Therefore, histologic confirmation of SIM in BE is required. Because of the latent period of transition to high grade dysplasia, EAC is significantly shorter for patients with low grade dysplasia (median of 2.75 years) than for patients without low grade dysplasia (median of 9.88 years)[1].
Patients with SIM are currently recommended to undergo periodic endoscopic surveillance to determine the progression to dysplasia at an early, potentially curable stage[5,7].
Discerning SIM and obtaining satisfactory target biopsies at the region of interest by standard endoscopic observation is difficult[8,9]. Thus, to identify the presence of SIM and dysplasia according to the Seattle protocol, specimens are obtained using a predefined four-quadrant sampling technique[10]. The major disadvantages of this method are the need for multiple biopsies, random choice of biopsy places, and the high cost.
Chromoendoscopy and magnifying endoscopy have been improving mucosal visualization to allow for better differentiation of the SIM and dysplasia from the columnar epithelium during endoscopy[11,12]. These techniques provide more accurate biopsies as well as reduce the number of biopsies[13,14]. Chromoendoscopy involves the use of dyes sprayed over the mucosa. Methylene blue (MB) stains actively absorbing cells, such as the intestinal epithelium and intestinal metaplasia[11]. The sensitivity and specificity of MB staining for SIM detection in BE is still under discussion[15,16]. Magnifying endoscopy, which provides images of fine mucosal detail that correspond to histologic structure, is now widely accepted for the study of GI disorders. After magnification, a characteristic relief called a pit-pattern is visible on the surface of the esophageal epithelium. The most widely known classification of esophageal pit-patterns in relation to histology were described by Endo et al[17]. The usefulness of this classification is its ability to predict the presence of SIM based on the structure of the mucosal surfaces.
BE can be subdivided into long-segment BE (≥ 3 cm) and short-segment BE (< 3 cm)[18]. Just as for long-segment BE, histologic confirmation of SIM in short-segment BE is also needed; not only long-segment BE but also short-segment BE, have been known as major risk factors for the development of EAC[19,20]. Furthermore, small areas of dysplasia can be difficult to diagnose.
The aim of this study was to determine whether the magnified observation of short-segment BE is useful for the detection of SIM and for the prediction of histological diagnosis compared with MB chromoendoscopy.
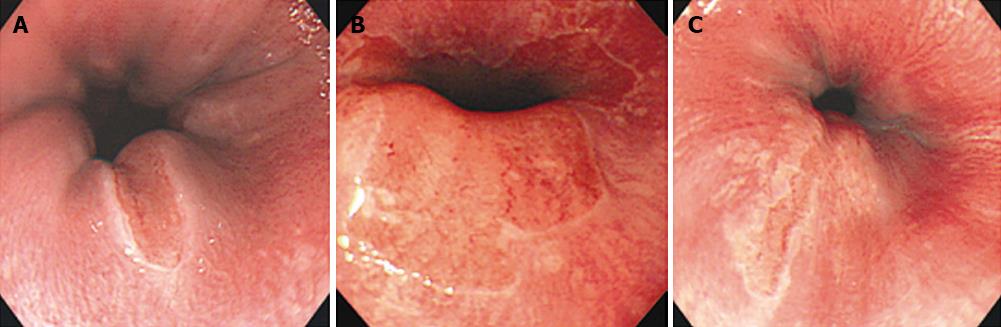
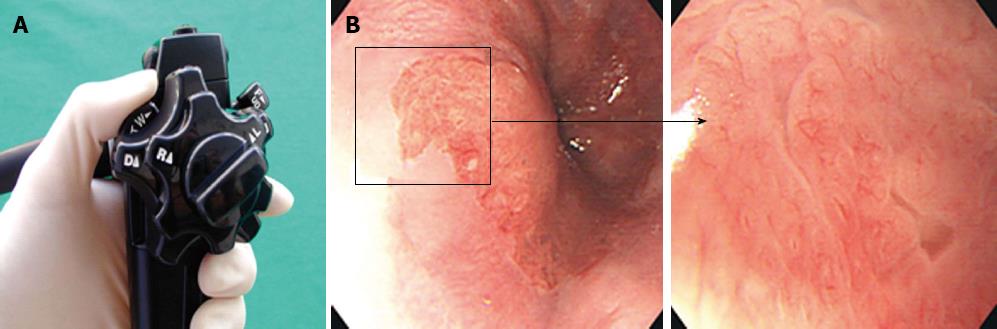
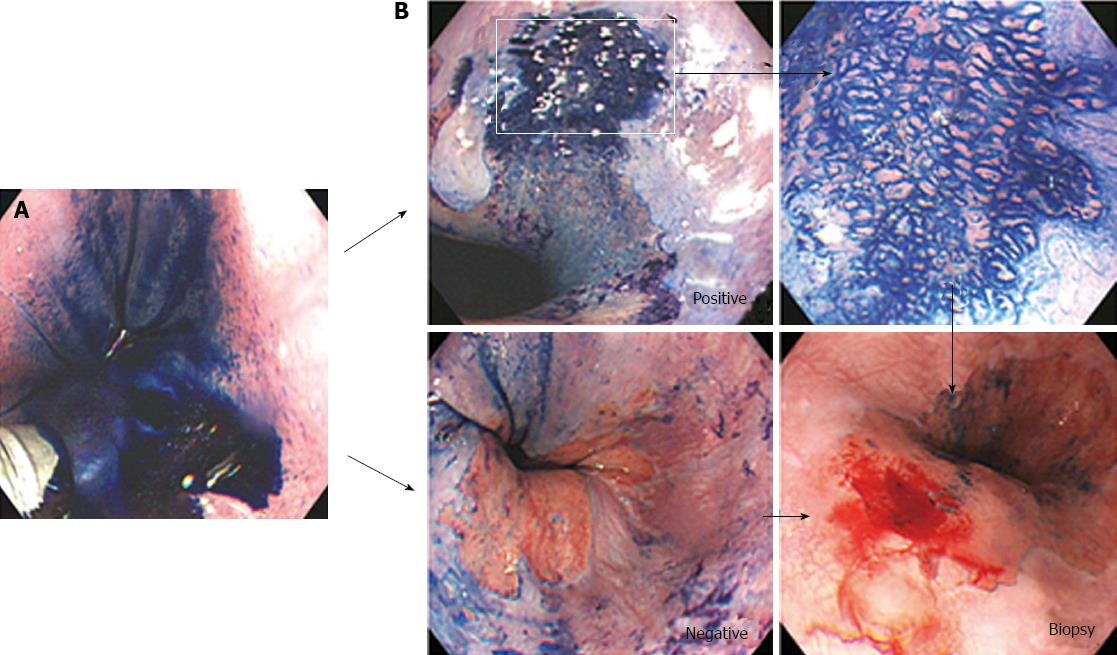
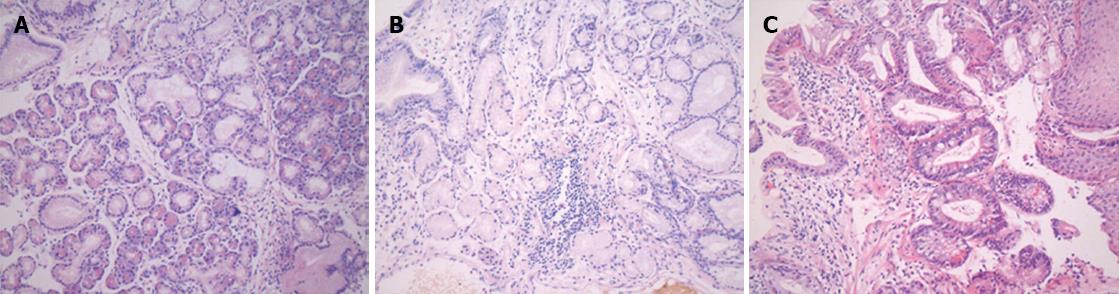
Patients with short-segment BE were prospectively enrolled into this study at Soonchunhyang University Hospital in South Korea between March 2002 and June 2002 (Figure 1). Patients underwent magnifying endoscopy, which could enhance the image up to × 80 (Olympus GIF-Q240Z, Japan) (Figure 2). Mucus was removed by a 10% solution of acetylcysteine instillation. The magnified images were analyzed with respect to pit-patterns, which were simultaneously classified into five epithelial types [I (small round), II (straight), III (long oval), IV (tubular), V (villous)] by Endo’s classification (Figure 3). Then, a 0.5% solution of methylene blue was sprayed over the columnar mucosa. The excess of dye was flushed away with 50 mL of water after 2 min. The patterns of the magnified image and MB staining were analyzed. Biopsies were obtained from the regions previously observed by magnifying endoscopy and MB chromoendoscopy (Figure 4). If the biopsies were unsatisfactory or inaccurately targeted, other biopsies were performed. Every biopsy was classified into three types of epithelium by a pathologist: the fundic type, cardiac type and SIM (Figure 5). The study was performed after receiving approval from the Institutional Review Board of the Soonchunhyang University in Seoul, South Korea.
To analyze the relationships among the variables, Fisher’s exact test was used. We performed an exact McNemar’s test to compare the diagnostic value of MB chromoendoscopy and magnifying endoscopy for detection of SIM. Data analysis was performed using SPSS 14.0. All statistical hypotheses were verified at a significance level of P < 0.05.
Thirty patients, 16 men and 14 women, with an average age of 44.8 years (range 17-75 years), were enrolled into this study. All of the patients had tongue-like columnar epithelium in the tubular esophagus within 3 cm from the Esophagogastric junction, as identified by previous standard endoscopy. No patient had previous histologically proven SIM in the columnar lined epithelium.
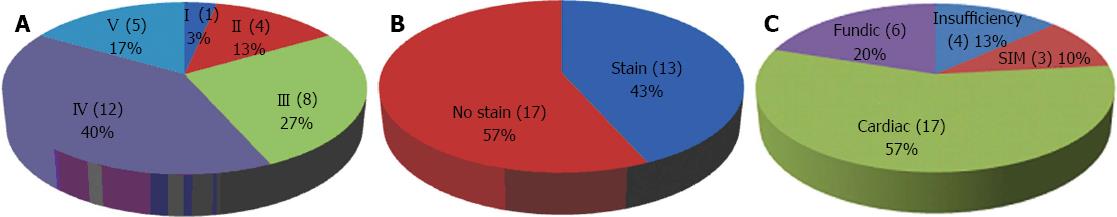
The results for individual patients, including the pit-pattern, MB staining, histologic diagnosis and reflux esophagitis, are listed in Table 1. Distributions of the types of pit-pattern, MB staining, and histologic diagnosis are shown in Figure 6.
| Patient | Type | Stain | Histology | Reflux | Patient | Type | Stain | Histology | Reflux |
| 1 | Villous | Yes | SIM | Yes | 16 | Tubular | No | Insufficiency | Yes |
| 2 | Oval | No | Cardiac | No | 17 | Villous | Yes | SIM | Yes |
| 3 | Oval | No | Cardiac | No | 18 | Oval | No | Fundic | Yes |
| 4 | Straight | No | Fundic | No | 19 | Tubular | No | Insufficiency | No |
| 5 | Straight | Yes | Fundic | No | 20 | Oval | Yes | Fundic | Yes |
| 6 | Tubular | No | Cardiac | No | 21 | Villous | Yes | SIM | No |
| 7 | Oval | No | Cardiac | No | 22 | Tubular | No | Insufficiency | No |
| 8 | Tubular | Yes | Cardiac | No | 23 | Tubular | Yes | Cardiac | Yes |
| 9 | Tubular | No | Cardiac | No | 24 | Villous | Yes | Cardiac | Yes |
| 10 | Small round | Yes | Fundic | No | 25 | Oval | No | Cardiac | Yes |
| 11 | Tubular | No | Cardiac | Yes | 26 | Villous | No | Cardiac | No |
| 12 | Tubular | No | Cardiac | No | 27 | Straight | No | Cardiac | No |
| 13 | Tubular | No | Fundic | No | 28 | Tubular | No | Cardiac | No |
| 14 | Tubular | Yes | Insufficiency | No | 29 | Oval | Yes | Cardiac | Yes |
| 15 | Tubular | Yes | Cardiac | No | 30 | Oval | yes | Cardiac | Yes |
Histologic examination revealed SIM in 3 of 26 patients (11.5%). The remaining four patients could not be diagnosed due to the insufficiency of the specimens for histologic examination. Reflux esophagitis was diagnosed by histologic examination in 11 of 26 patients (42.3%). The patients without RE did not have a history of GERD. SIM in BE was not more common in patients with reflux esophagitis (2 patients, 18.1%) than in those without it (1 patient, 5.2%; P = 0.538, Table 2).
The fine mucosal patterns (pit-pattern) of 30 patients were recorded and classified according to Endo’s classification. The specimens obtained previously from the regions observed by magnification without MB staining underwent histologic examinations to determine the relationship between the type of pit-pattern and SIM by magnifying endoscopy.
Of the 30 patients, one case was type I (small round); four cases were type II (straight); eight cases were type III (long oval); 12 cases were type IV (tubular); and five cases type were V (villous). Type IV (tubular) was the most common epithelial type. As shown in Table 1 and Figure 6, three of five patients with a type V (villous) epithelial pattern had SIM. Twenty-one patients without type V epithelial patterns did not have SIM (P = 0.004). These results suggest that a type V (villous) epithelial pattern is compatible with SIM, and the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of type V pit-pattern in detecting SIM were 100%, 91.3%, 92.3%, 60% and 100%, respectively (Table 2).
Out of 30 patients, 13 patients (43.3%) had positive MB staining, and 17 patients (56.7%) had negative MB staining. One of the 13 patients with positive MB staining and three of the 17 patients with negative MB staining did not receive a histological diagnosis due to insufficient specimens. As shown in Table 1 and Figure 6, three of 12 patients with positive MB staining had SIM, whereas 14 patients with negative MB staining did not have SIM (P = 0.085). The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of MB staining in detecting SIM were 100%, 60.9%, 65.4%, 25% and 100%, respectively (Table 2).
In comparison with MB staining, pit-pattern evaluation according to Endo’s classification had much higher specificity (91.3% vs 60.9%), accuracy (92.3% vs 65.4%), and positive predictive value (60% vs 20%) for the detection of SIM in BE; however, it had a similar sensitivity (both 100%) and negative predictive value (both 100%). The exact McNemar’s test revealed that the specificity and accuracy of pit-pattern evaluation was significantly superior to that of MB staining for detecting SIM by (P = 0.0391; Table 3).
SIM in BE is a risk factor for EAC. A strong relationship has been established between the presence of SIM and the subsequent development of adenocarcinoma[5,6].
Detecting esophageal neoplasias at an earlier stages will allow for the possibility of intervening more quickly and lowering the mortality from EAC. However, the effectiveness of the screening and surveillance of BE has not been studied in randomized, controlled trials. For example, various endoscopic approaches and advancements have shown great promise, yet the confirmation of their utility in high-quality clinical trials has yet to occur[21,22].
Canto et al[11] found that the overall accuracy of MB staining for detecting SIM was 95%. However, the same level of accuracy was not achieved in other studies. Dave et al[16] reported that MB staining was associated with prolonged endoscopy, increased patient discomfort, and potentially serious adverse events; furthermore, it was neither very sensitive nor specific for SIM. According to Horwhat et al[13], chromoendoscopy might decrease the number of biopsies without an improving the overall detection rate of dysplasia compared with a conventional four-quadrant biopsy. Wasielica-Berger et al[14] and Ferguson et al[23] found no convincing data indicating that pit-pattern evaluations may replace multiple biopsies, according to the Seattle recommendations for the detection of SIM in BE. Therefore, the aim of this study was to determine whether the magnified observation of short-segment BE is useful for the detection of SIM or for the prediction of histological diagnosis, compared with MB chromoendoscopy.
Oberg et al[3] showed that a long duration of reflux symptoms (RR = 1.3; 95%CI: 1.2-1.7) were independently associated with an increased risk of developing high-grade dysplasia or esophageal adenocarcinoma. However, SIM in BE was not more common in patients with reflux esophagitis who had a history of GERD compared with those without such a history (P = 0.538).
Endo’s study found that the type IV (tubular) and type V (villous) classifications were characteristic of SIM. Similarly, we found a significant correlation between pit-patterns evaluated according to Endo’s classifications and histology. The differences in the frequency of SIM were related to the particular mucosal pit-pattern types. We frequently found SIM in places with a type V (villous) epithelial pattern (3 of 5 patients). SIM did not coexist in any case with a non-type V epithelial pattern. Therefore, the surface structure of type V (villous) epithelial pattern is compatible with SIM (P = 0.004).
MB is a vital stain that is taken up by actively absorbing tissues, such as the small intestinal and colonic epithelium. In BE, areas of intestinal metaplasia are positively stained, whereas non-absorptive epithelia, such as those found in squamous or gastric mucosa, remain unstained. We found SIM in places with MB-positive stained epithelium (3 of 12 patients). No case of SIM was associated with MB-negative stained epithelium. However, MB-positive staining cannot be considered characteristic of SIM, as the difference was not significant (P = 0.085).
Compared with MB staining, the pit-pattern evaluation by magnifying endoscopy according to Endo’s classification had much higher specificity (91.3% vs 60.9%) and positive predictive value (60% vs 20%) for the detection of SIM in BE, despite similar sensitivity (100% vs 100%) and negative predictive values (100% vs 100%). The specificity and accuracy of pit-pattern evaluations were significantly superior, according to McNemar’s exact test, to those of MB staining for the detection of SIM (P = 0.0391).
There were some limitations to our study. First, we found no sites with dysplasia or cancer cells, which may be attributed to the relatively small number of patients. In addition, the present study enrolled too few patients (3 out of 5 patients with type V pit-pattern). However, this study was very difficult regarding the recruitment of patients due to the refusal of many of the patients and the quite rare prevalence of this condition in Korea[24,25]. Second, long-segment BEs were excluded in our study. The risk of progression to malignancy appears to increase significantly with increasing lengths of BE[26,27]. It would be worth knowing about pit-patterns in long-segment, salmon-colored mucosa and also pit-pattern correlation with histological diagnosis of BE. However, there is conflicting evidence in the literature[28]. Short-segment and long-segment BE are biologically identical and have significant if not equivalent malignant potential. In addition, Kim et al[29] showed that patients with long-segment BE are very rare in South Korea. So, we focused on short-segment BE in this study. Third, we did not address whether the simultaneous use of magnifying endoscopy and MB staining might improve the diagnostic yield. Sharma et al[12] reported that high magnification chromoendoscopy might be a useful clinical tool for the increased detection of patients with intestinal metaplasia. Statistically, there is no doubt that the results are improved when magnifying endoscopy is performed with MB staining simultaneously, if both are characteristics of SIM. In our study, MB-positive staining could not be considered a characteristic of SIM. Therefore, we did not try to demonstrate that the simultaneous performance of magnifying endoscopy and MB staining could improve the results. Fourth, we did not count the total number of biopsies. Thus, we could not show that the magnifying endoscopy might decrease the number of biopsies, generating an overall improvement in the detection rate of dysplasia compared with a conventional, four-quadrant biopsy.
In summary, we identified the usefulness of magnifying endoscopy for the diagnosis of SIM in patients with short-segment BE from preceding studies. However, we were still unable to demonstrate the usefulness of MB chromoendoscopy. Because we did not count the total number of biopsies, we could not confirm that both of the endoscopic examinations decreased the number of biopsies, costs and inspection time. We found that both methods were time-consuming and caused patient discomfort. These are among the disadvantages of the other studies.
Various endoscopic approaches and advancements have shown great promise. Still, careful endoscopic observation and stepwise four quadrant biopsy still represent the standard for the surveillance of BE[21,30]. In our study, the evaluation of mucosal surfaces under magnification has potential to allow the selection of the biopsy site according to the pit-pattern. In conclusion, the magnified observation of short-segment BE according to the mucosal pattern and its classification can be predictive for SIM.
Crosssectional and descriptive studies suggest that specialized intestinal metaplasia (SIM) either coexists with or precedes a significant majority of esophageal adenocarcinoma (EAC) cases and is the likely precursor lesion.
Detecting esophageal neoplasia at an earlier stage will allow for the possibility of intervening more quickly and the lowering mortality due to EAC. However, the effectiveness of screening and surveillance of Barrett’s esophagus (BE) has not been studied in randomized controlled trials. In addition, discerning SIM and obtaining satisfactory target biopsies at the region of interest by standard endoscopic observation is difficult.
The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of pit pattern in detecting SIM were 100%, 91.3%, 92.3%, 60% and 100%, respectively (P = 0.004). The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of methylene blue (MB) staining in detecting SIM were 100%, 60.9%, 65.4%, 25% and 100%, respectively (P = 0.085). The specificity and accuracy of the pit-pattern evaluation were significantly superior compared with MB staining for detecting SIM by comparison of exact McNemar’s test (P = 0.0391).
The study results suggests that the magnified observation of short-segment BE according to the mucosal pattern and its classification can be predictive for SIM.
BE is characterized by the replacement of the squamous epithelia in the distal esophagus by columnar epithelia (gastric metaplasia), irrespective of the presence of specialized intestinal metaplasia.
The paper found that the magnified observation of a short-segment BE according to its mucosal pattern and classification can be predictive of SIM. It’s an informative manuscript, nicely written.
P- Reviewers: Girotra M, Van Rensburg C S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
| 1. | Dulai GS, Shekelle PG, Jensen DM, Spiegel BM, Chen J, Oh D, Kahn KL. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett’s cohort. Am J Gastroenterol. 2005;100:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Gilbert EW, Luna RA, Harrison VL, Hunter JG. Barrett’s esophagus: a review of the literature. J Gastrointest Surg. 2011;15:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Oberg S, Wenner J, Johansson J, Walther B, Willén R. Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann Surg. 2005;242:49-54. [PubMed] |
| 4. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-120; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (2)] |
| 5. | Michalak J, Bansal A, Sharma P. Screening and surveillance of Barrett’s esophagus. Curr Gastroenterol Rep. 2009;11:195-201. [PubMed] |
| 6. | Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, Spechler S, Cameron A, Corley D, Falk G, Goldblum J. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310-330. [PubMed] |
| 7. | Conio M, Blanchi S, Lapertosa G, Ferraris R, Sablich R, Marchi S, D’Onofrio V, Lacchin T, Iaquinto G, Missale G. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Abela JE, Going JJ, Mackenzie JF, McKernan M, O’Mahoney S, Stuart RC. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol. 2008;103:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Harrison R, Perry I, Haddadin W, McDonald S, Bryan R, Abrams K, Sampliner R, Talley NJ, Moayyedi P, Jankowski JA. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol. 2007;102:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Reid BJ, Blount PL, Feng Z, Levine DS. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:3089-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Canto MI, Setrakian S, Petras RE, Blades E, Chak A, Sivak MV. Methylene blue selectively stains intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc. 1996;44:1-7. [PubMed] |
| 12. | Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett’s oesophagus. Gut. 2003;52:24-27. [PubMed] |
| 13. | Horwhat JD, Maydonovitch CL, Ramos F, Colina R, Gaertner E, Lee H, Wong RK. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia and dysplasia in patients with long-segment Barrett’s esophagus. Am J Gastroenterol. 2008;103:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Wasielica-Berger J, Baniukiewicz A, Wroblewski E, Chwiesko A, Dabrowski A. Magnification endoscopy and chromoendoscopy in evaluation of specialized intestinal metaplasia in Barrett’s Esophagus. Dig Dis Sci. 2011;56:1987-1995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Canto MI, Setrakian S, Willis JE, Chak A, Petras RE, Sivak MV. Methylene blue staining of dysplastic and nondysplastic Barrett’s esophagus: an in vivo and ex vivo study. Endoscopy. 2001;33:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Dave U, Shousha S, Westaby D. Methylene blue staining: is it really useful in Barrett’s esophagus? Gastrointest Endosc. 2001;53:333-335. [PubMed] |
| 17. | Endo T, Awakawa T, Takahashi H, Arimura Y, Itoh F, Yamashita K, Sasaki S, Yamamoto H, Tang X, Imai K. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointest Endosc. 2002;55:641-647. [PubMed] |
| 18. | Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus--the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol. 1998;93:1033-1036. [PubMed] |
| 19. | Cameron AJ, Lomboy CT, Pera M, Carpenter HA. Adenocarcinoma of the esophagogastric junction and Barrett’s esophagus. Gastroenterology. 1995;109:1541-1546. [PubMed] |
| 20. | Hamilton SR, Smith RR. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett’s esophagus. Am J Clin Pathol. 1987;87:301-312. [PubMed] |
| 21. | Almond LM, Barr H. Advanced endoscopic imaging in Barrett’s oesophagus. Int J Surg. 2012;10:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kara MA, Peters FP, Rosmolen WD, Krishnadath KK, ten Kate FJ, Fockens P, Bergman JJ. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy. 2005;37:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Ferguson DD, DeVault KR, Krishna M, Loeb DS, Wolfsen HC, Wallace MB. Enhanced magnification-directed biopsies do not increase the detection of intestinal metaplasia in patients with GERD. Am J Gastroenterol. 2006;101:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Shin HR, Jung KW, Won YJ, Park JG. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103-114. [PubMed] |
| 25. | Son JI, Park HJ, Song KS, Kim KJ, Lee CY, Lee SI, Park IS. A single center’s 30 years’ experience of esophageal adenocarcinoma. Korean J Intern Med. 2001;16:250-253. [PubMed] |
| 26. | Martinek J, Benes M, Brandtl P, Hucl T, Vasicek M, Voska L, Lanska V, Nosek V, Spicak J. Low incidence of adenocarcinoma and high-grade intraepithelial neoplasia in patients with Barrett’s esophagus: a prospective cohort study. Endoscopy. 2008;40:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Weston AP, Sharma P, Mathur S, Banerjee S, Jafri AK, Cherian R, McGregor D, Hassanein RS, Hall M. Risk stratification of Barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol. 2004;99:1657-1666. [PubMed] |
| 28. | Rudolph RE, Vaughan TL, Storer BE, Haggitt RC, Rabinovitch PS, Levine DS, Reid BJ. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med. 2000;132:612-620. [PubMed] |
| 29. | Kim JY, Kim YS, Jung MK, Park JJ, Kang DH, Kim JS, Song CW, Lee SW, Bak YT. Prevalence of Barrett’s esophagus in Korea. J Gastroenterol Hepatol. 2005;20:633-636. [PubMed] |