INTRODUCTION
Duplications of gastrointestinal tract are infrequent in general population with an incidence of two or three cases per year in referred pediatric center[1]. The most frequent localization is the ileum 30% and the ileocecal valve 30%, followed by the jejunum with 8%, the colon 6%-7% and the rectum 5%[2,3]. Since 1950, less than 50 colonic duplication have been reported in literature[4] and they were more often discovered in the first two years of life (80%)[5]. If no associated malformation is present, they remain frequently hidden for several years until a complication occurs[6]. The symptoms are unspecific and depend on the type of duplication and on the associated abnormality. More often they are abdominal mass, chronic pain, constipation, occlusion, and less frequently volvulus[7] intussusception, bleeding[8] or perforation (especially in sigmoid colon mimicking diverticulitis). Also the arising of carcinoma is observed: the most frequent is adenocarcinoma[9-13] followed by squamous carcinoma and carcinoid tumors[14-16]. The carcinoembryonic antigen expression can be observed in association with the arising of adenocarcinoma[17], but the presence of the carbohydrate antigen 19-9 is not well defined yet.
CASE REPORT
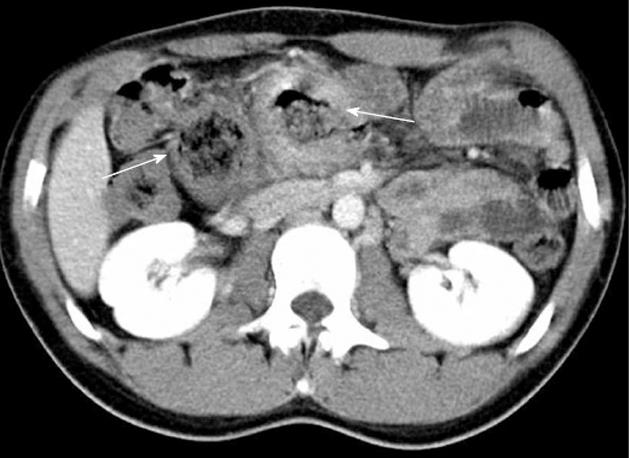
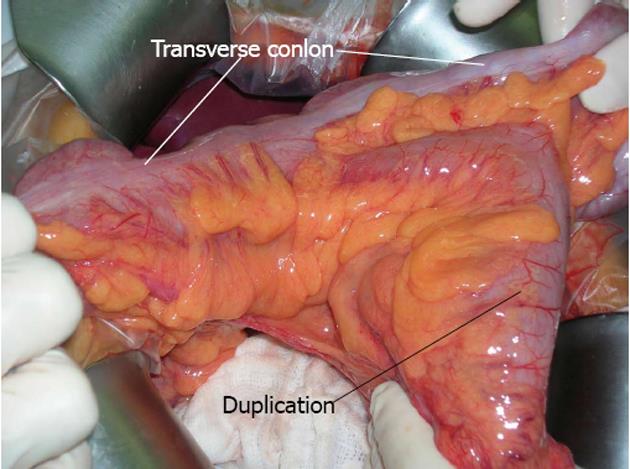
A 21-year-old man with a history of chronic constipation was referred to our department for abdominal pain with retrosternal irradiation. During childhood he was hospitalized for the same symptoms without a clear diagnosis: alimentary intolerance or celiac disease were excluded, and all the abdominal ultrasounds were always negative. The mother had no particular problems during pregnancy and labour, she had no exposition to pollution, but the patient was an eight months premature birth. At the hospitalization the blood samples showed mild leukocytosis and the abdominal Rx plane was normal. Abdominal sonography was performed but it was not diagnostic, because of air hiding; an abdominal computed tomography (CT) scan (Figure 1) revealed an oval mass with wall enhancement and similar stool material inside, suggestive for intestinal duplication. A small-bowel contrast study was performed but no alteration could be demonstrated. The patient was treated with antibiotics and was discharged asymptomatic with a planned intervention for the following week. At the explorative laparotomy a transverse colon Y-type duplication was shown (Figure 2); the duplication was bent down at the origin of the transverse mesocolon and an own mesocolon was seen. After dissection from the mesocolic stamp, the duplication was unbent revealing that the blood supply origin from a distal division of the middle colic artery with a trifurcation for right and left transverse colon and one branch for the duplication. The upper part of the common mesocolon presented a crabbed fusion of small vessels. To preserve the left marginal arcade, we dissected the artery for the duplication with conservation of the two branches for the native transverse colon and with resection of the duplicated bowel and 5 cm of the native transverse colon. A colo-colic anastomosis was performed. The post operative period was uneventful and the patient was discharged; at the moment he lies symptoms free. Histopathology confirmed the tubular duplication with multiple mucosal ulceration.
Figure 1 Computed tomography scan.
The arrows indicate the duplication.
Figure 2 Intraoperative dissection.
DISCUSSION
Digestive duplication is defined by the three Rowling’s criteria[18]: (1) the wall of the duplication is in continuity with one of the duplicated organ; (2) the cyst is surrounded by a smooth muscular layer; and (3) a layer of digestive mucosa is present, more often typical or heterotopic as gastric mucosa, colonic mucosa, bronchial or pancreatic structure[19,20]. Smith[21] well defined the term of duplication as “these formations, either tubular or spherical, which lie in contiguity with normal bowel and which share with it a common blood supply and usually a common muscle coat. There may or may not be a communication between the normal and anomalous lumina and the dividing septum may be muscular or merely a double layer of epithelium”. Gross et al[22] described four variations in the shape of duplications: (1) a tubular structure that branches out from the intestine and extends for some distance between the mesenteric leaves; (2) a double-barreled structure communicating with the intestinal lumen at one or both ends; (3) a cystic structure lying in the peritoneal cavity, attached by a mesenteric stalk; and (4) a spherical lesion contiguous with some part of the bowel, particularly along the ileum. Cystic types are the most frequent with 90%-95% of the cases and the tubular type 5%-10%; the latter can be distinguished in double barreled (80%) or Y-shaped form (20%)[23-26]. For colonic duplication McPherson makes another classification as type I (simple cysts), type II (diverticula) or the most common type III (tubular colonic duplication)[27]. Several theories have been proposed to describe this abnormality in which environmental factors, such as trauma or hypoxia, can play a role[28]. The aberrant lumen recanalization theory exposed by Bremer[29] seems to be the most comprehensive. As described by Johnson[30] in 1910 the gut grows and obliterates the lumen at the sixth week with subsequent internal vacuolization and coalescence of vacuoles with the formation of the single lumen. The aberrant vacuolization well explains that vacuoles can remain separated and create one or more duplication that can be separated or communicating with the original gut. This theory can be easily associated to the double barrel duplication. Another theory is the diverticular one, described by Lewis et al[31], criticized by some authors[1] because it doesn’t explain the forms of entire duplication of the colon (for example) with the presence of circular and longitudinal muscle coats. Blinder describes more simply that the tubular forms can be caused by an alteration of the lateral closure of the embryonal disk at the stage 9 of Steeter for an abnormality of the longitudinal line, instead of cystic forms that originate from diverticulation later in the evolution[32,33]. Another explanation, well described by Smith[21], is the dorsal protrusion of the yolk-sac caused by its herniation or adhesion to the ectoderm in same phase of presomite development taking to a “dorsal remnants” structure, that can be divided into: congenital dorsal enteric fistula, congenital dorsal enteric sinus, congenital dorsal enteric cyst, congenital dorsal enteric divericulum[34]. This last theory can also explain the association with other abnormalities, especially the vertebral alteration. The association of other malformations, as vertebral or genitourinary alterations, can be classified in the notochordodysraphies: duplication or alteration of the hindgut is associated with genitourinary problem in 60% of cases[35,36] and high malformation with vertebral problems[37-40]. In particular the hindgut duplication can be explained by an insult to the caudal cell mass at the days 23-25 resulting in the “split notochord syndrome[41,42]. Until 2011 only 18 cases of transverse colon duplication have been reported. The particular localisation sometimes can give problems in diagnosis, like mismatching with pancreatic cyst[5,43]. In tubular type, CT scan, contrast enema and colonoscopy, could be the best diagnostic tools: in fact the presence of air and stool can facilitate the diagnosis; in ultrasonography the presence of air doesn’t actually allow a good vision. Probably sonography, instead of CT scan, can be more performant in the cystic type, where the diagnosis is more difficult as described above. In fact in the cystic type of the transverse colon, the lesion have fluid inside and it could be adherent to the mesocolon or the pancreas, mimicking other alterations. The small-bowel contrast study, as in our case, is not so performant also in tubular type. The management of colonic duplication consists in the resection of the duplicated bowel with an extension of 2 cm in the normal bowel, due to the common blood supply at the two organs, the presence of fibrosis in the conjunction area[6,44] and the probability of arising tumor in the duplication. Our report shows that the symptomatology could be hidden for a long time, especially in absence of other anatomical alterations; probably for the presence of a big communication with the native bowel. The presence of stool inside the duplication helped us in the diagnosis, but it remains not easy to do, as reported by some authors[43]. In conclusion the colon duplication is not frequent especially in adulthood; the differential diagnosis is not easy; it must be resected even if asymptomatic, because of the risk of arising tumor.