Published online Oct 14, 2013. doi: 10.3748/wjg.v19.i38.6329
Revised: August 11, 2013
Accepted: September 16, 2013
Published online: October 14, 2013
Processing time: 148 Days and 10.3 Hours
Recently the European Federation of Societies for Ultrasound in Medicine and Biology Guidelines and Recommendations have been published assessing the clinical use of ultrasound elastography. The document is intended to form a reference and to guide clinical users in a practical way. They give practical advice for the use and interpretation. Liver disease forms the largest section, reflecting published experience to date including evidence from meta-analyses with shear wave and strain elastography. In this review comments and illustrations on the guidelines are given.
Core tip: The presented paper is intended to comment the “European Federation of Societies for Ultrasound in Medicine and Biology Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography” and discuss the multivariate factors that have an influence on liver stiffness, and the current techniques of ultrasound elastography as well as magnetic resonance elastography.
- Citation: Cui XW, Friedrich-Rust M, Molo CD, Ignee A, Schreiber-Dietrich D, Dietrich CF. Liver elastography, comments on EFSUMB elastography guidelines 2013. World J Gastroenterol 2013; 19(38): 6329-6347
- URL: https://www.wjgnet.com/1007-9327/full/v19/i38/6329.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i38.6329
Non-invasive methods for liver stiffness (LS) assessment have been researched over decades, often mirroring the development of new drugs in the treatment of chronic liver disease. So far, two main forms of elastography have become established in clinical practice. The first is known as quasi-static or strain elastography (SE). Imaging of strain and elastic modulus distributions in soft tissues based on external tissue compression, with subsequent computation of the strain profile along the transducer axis, was first described by Ophir et al[1,2]. Strain imaging can be applied to the liver by inducing probe pressure[3]. The temporal derivative of strain, i.e., the strain rate, is a measure of the rate of deformation[4]. Strain Rate Imaging is a Doppler-based method that can be used to measure strain of moving tissue[5,6]. The second form is shear wave elastography (SWE). Shear waves are generated in the tissues when a directional force is applied to the tissue which causes shear deformation. Shear waves are rapidly attenuated by tissue, they travel much more slowly (between 1 and 10 m/s) and they are not supported by liquids of low viscosity[7].
The use of different ultrasound methods to estimate liver fibrosis have been published, such as transient elastography (TE) (FibroScan™)[8-10], strain elastography (e.g., Hitachi Aloka Medical)[11-14] and SWE using acoustic radiation force impulse (ARFI) (Siemens et al)[14-16]. Other techniques including 2D-SWE (Supersonic, Siemens) and 3D-SWE (Supersonic) have since been introduced[17-19].
Recently the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines and Recommendations have been published assessing the clinical use of ultrasound elastography[7,20]. The document is intended to form a reference and to guide clinical users in a practical way. The guidelines also give practical advice on its use and interpretation. Liver disease forms the largest section, reflecting the published experience to date, including evidence from meta-analyses with shear wave and strain elastography. This article comments on the EFSUMB elastography guidelines, discusses the multivariate factors that have an influence on LS, and the current techniques of ultrasound elastography as well as magnetic resonance elastography (MRE).
LS (elasticity) is a dynamic and multifunctional process. This means that factors influencing the stiffness and elasticity of a healthy liver are different to the factors in advanced fibrosis. However, many studies have examined the grade of liver fibrosis as the sole indicator of LS[21-25] (Table 1); a few others have evaluated more factors[26-29] (Table 1).
| Title | Comment | Ref. | |
| Univariate approach | |||
| Elastographic assessment of liver fibrosis in children: A prospective single center experience | Pearson’s correlation | [21] | |
| Is it better to use two elastographic methods for liver fibrosis assessment? | Spearman rank correlation | [22] | |
| Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis? | Spearman rank correlation | [23] | |
| Factors that influence the correlation of acoustic radiation force impulse, elastography with liver fibrosis | Spearman rank correlation | [24] | |
| Liver stiffness measurement using acoustic radiation force impulse elastography and effect of necroinflammation | Pearson product-moment correlation | [25] | |
| Multivariate approach | |||
| Liver stiffness measurements in patients with different stages of non-alcoholic fatty liver disease: Diagnostic performance and clinicopathological correlation | Spearman’s correlation (no attention paid to Bonferroni or alpha correction) | [26] | |
| 6 factors (higher age, serum albumin, serum AST, serum cholesterol, diabetes mellitus, LSM), LSM is the only independent predictor of advanced fibrosis (odds ratio = 1.47, 95%CI: 1.23-1.77, P < 0.001) | |||
| Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease | Spearman’s correlation (with Bonferroni test). In multivariate analysis including fibrosis, HAH, and steatosis, fibrosis was the only histological parameter significantly correlated with LSM | [27] | |
| FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis | Pearson correlation (no attention paid to Bonferroni or alpha correction) | [28] | |
| 12 factors. Multivariate analysis showed that LSM positively correlates with hepatic fibrosis, necro-inflammatory activity and ultrasound scores | |||
| Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis | Spearman’s correlation (no attention paid to Bonferroni or alpha correction) | [29] | |
| 4 factors (fibrosis, ballooning, Lobular inflammation, steatosis). Multivariate analysis found fibrosis as the only factor influencing independently liver stiffness in NASH patients | |||
In patients with chronic liver disease, the assessment of the patient should include age, liver-related comorbidity, aetiology and duration of the liver disease, grading (inflammation), fatty infiltration, risk of malignant transformation, fibrosis, general comorbidity and many other factors. Such factors are important as they guide management and indicate prognosis. Therefore, the assessment of liver fibrosis is only one of many other important factors to determine before treatment. However, the focus on the assessment of liver fibrosis seems to be overstated and many studies lack the design of multivariate analysis.
Factors influencing liver elasticity in healthy subjects depend mainly on blood volume and perfusion parameters that are reported by surgeons during daily routine. Studies have reported a positive correlation of LS with central venous pressure[30], therefore knowledge of co-existing cardiac and pulmonary disease is necessary for interpretation of results.
In addition, it is also reported that food intake could significantly increase the LS in adults[31,32], children[33] and the patients with chronic or resolved hepatitis C virus (HCV) infection[34], therefore, elastography should be performed in fasting conditions. However, there is controversy on the influence of respiration on LS. Yun et al[35] reported that LS was significantly elevated during expiration especially in patients without liver cirrhosis while Goertz et al[32] did not find differences on the LS in deep inspiration, deep expiration and during Valsalva maneuver.
In liver cirrhosis, the degree and architecture of fibrosis is presumed to be the most important factor influencing LS (elasticity). The factors influencing liver elasticity in intermediate (significant) fibrosis are still not known in detail.
The factors influencing liver elasticity in patients with inflammatory disease (at least to some degree), independent of fibrosis, are acute hepatitis, any flare of transaminase values, acute-on chronic hepatitis[36,37], cholestasis[38] and acute liver failure[39]. In a recent study of 104 patients with chronic hepatitis B (CHB) and 453 patients within chronic hepatitis C (CHC), histological necro-inflammatory activity was found to be an independent risk factor for the overestimation of LS in HCV and hepatitis B virus (HBV), while histological steatosis was a risk factor in HCV patients only[40].
Other factors influencing liver elasticity in patients with fatty liver (hepatic steatosis) with or without inflammatory activity, with or without fibrosis, have also been described[41-47].
The multivariate intercorrelation of factors influencing liver elasticity under different circumstances is not known. Since multiple factors have shown to influence LS measurements, interpretation of results has to be performed taking into account all these risk factors.
Liver biopsy (LB) has been considered the “gold-standard” for grading and follow-up of necro-inflammatory activity and staging of fibrosis for more than fifty years[48,49].
However, substantial limitations are obvious. Firstly, it is an invasive method with a significant complication rate[50]. A review of the literature regarding possible complications has recently been published[51]. Secondly, LB has shown some sampling variability[52]. The specimen obtained by LB represents a very small part of the liver (about 1/50000) but inflammatory and fibrotic activity is known to be patchy within the liver. The sampling variability can be reduced by mini-laparoscopic guided biopsy with the ability to evaluate the liver surface[53-57], however, it has been shown that the sampling error using mini-laparoscopic guided biopsy is still about 30%[58]. LB has also shown some intra- and inter-observer variability[58,59]. Thirdly, there is a high inter-observer variability during microscopic evaluation[58].
Therefore, one difficulty for the evaluation of non-invasive markers of fibrosis is the use of LB as a reference method. Taking into account the limitations of LB, a perfect non-invasive method cannot be distinguished from an unacceptable fibrosis marker. Thus a new reference marker is needed. Studies have shown that non-invasive tests for liver fibrosis with FibroTest, enhanced liver fibrosis (ELF) and TE can predict 5-10 year survival of patients with CHC[60-64]. However, more studies using liver related mortality as the endpoint are still awaited to identify the best non-invasive methods[3].
One important non-invasive method for assessment of the severity of fibrosis includes serum markers[65-68]. So far, many serum biomarkers, both direct and indirect, have been evaluated for their ability to stage liver fibrosis[69-71]. Direct serum markers, reflecting either the deposition or the removal of extracellular matrix in the liver, include: (1) collagens such as type IV collagen, procollagen III N-peptide, collagenases; (2) inhibitors of collagens such as matrix metalloproteases and tissue inhibitory metalloprotease-1; and (3) glycoproteins such as serum hyaluronate, laminin, and YKL-40. So-called indirect markers include factors that can be measured from routine blood tests, such as platelet count, prothrombin index, and aspartate aminotransferase/alanine aminotransferase (AST/ALT), which indicate alterations in hepatic function. The usefulness of these markers has been assessed mostly in patients with CHC[70-72] and hyaluronate has been the most extensively studied direct marker[73,74]. These direct and indirect markers, when used individually, are useful for the diagnosis or the exclusion of cirrhosis but have limited accuracy for the diagnosis of clinically significant fibrosis[75]. Therefore, more sophisticated algorithms or indices combining the results of groups of markers have been developed to improve the diagnostic accuracy. The FibroTest™ (proprietary formula; Biopredictive, Paris, France) was the first algorithm that combined these data[76]. Thereafter, several other indices, such as Fibrosure™ in the United States (LabCorp, Burlington, NC, United States), the Fibrometers™ (BioLiveScale, Angers, France), the FibroSpect II™ (Promotheus Laboratory Inc., San Diego, CA, United States), the ELF™ (Enhanced Liver Fibrosis Test, iQur Ltd, Southampton, United Kingdom) and the Hepascore™ (PathWest, University of Western Australia, Australia), have been developed. They are mainly for patients with CHC[77-80], but can also be used in patients with hepatitis B[81,82] and human immunodeficiency virus (HIV)-HCV co-infection[83,84]. Among these indices, Fibrotest has been the one most extensively studied[69].
In a prospective cohort of 537 HCV-infected patients, Fibrotest had a 5 year prognostic value (HCV-related complications and death) similar to that of LB[61]. In a meta-analysis[85] which included 6378 subjects with both FibroTest and biopsy (3501 HCV and 1457 HBV), the mean standardized area under the receiver operator curve (AUROC) for diagnosing significant fibrosis was 0.84 (95%CI: 0.83-0.86), without differences between HCV, 0.85 (95%CI: 0.82-0.87) and HBV, 0.80 (95%CI: 0.77-0.84). ELF has been evaluated in a recently published study[86] that included 196 patients. The ELF panel had an AUROC of 0.90 for distinguishing severe fibrosis, 0.82 for moderate fibrosis, and 0.76 for no fibrosis, and it was improved to 0.98, 0.93 and 0.84, respectively, by the addition of simple markers. The clinical utility model showed that 82% and 88% of liver biopsies could potentially be avoided for the diagnosis of severe fibrosis using ELF and the combined panel, respectively[62,64].
The practical advantages of analysing serum biomarkers to measure fibrosis include their high applicability and high inter-laboratory reproducibility[87,88]. However, the direct markers of liver fibrosis are not routinely available in most hospital settings, and none of the serum markers are liver specific-their results can be influenced by comorbidity. For example, FibroTest and Hepascore produce false-positive results in patients with Gilbert’s syndrome or haemolysis as these patients have hyperbilirubinaemia[89]. Similarly, acute hepatitis can produce false-positive results in the marker measuring the level of aminotransferases, such as aspartate-to-platelet ratio index (APRI), Forns index, FIB-4, or Fibrometer tests.
In recent years, magnetic resonance elastography (MRE) has been developed as a non-invasive functional magnetic resonance imaging (MRI) method for assessing and staging liver fibrosis, using a modified phase-contrast method to image the propagation characteristics of shear waves in the liver[90,91]. Elasticity is quantified by MRE and expressed in kilopascals (kPa) using a formula that determines the shear modulus, equivalent to one-third of the Young’s modulus which is estimated with TE[72,92]. So far, there is only limited data on the accuracy of MRE. Several studies[92-96] have evaluated the usefulness of MRE for the assessment of LS among patients with chronic liver disease and have shown that increased shear stiffness measured on MRE is associated with increased severity of the fibrotic process. In addition, MRE has relatively high sensitivity and specificity for predicting the stage of hepatic fibrosis. It has shown at least equivalent diagnostic performance in fibrosis staging compared with TE with fewer limitations regarding its application in patients with a large amount of ascites or who are obese[92-95]. Yin et al[95] reported sensitivity of 86% and 78%, and specificity of 85% and 96%, with cut-off values of 4.89 and 6.47 kPa, respectively. Huwart et al[93] showed similarly high sensitivity of 98% and 95%, and specificity of 100% and 100%, for discrimination, but lower cut-off values of 2.5 and 3.1 kPa were used. The reason for the difference in cut-off values obtained in the two studies may potentially be explained by the differently manufactured scanners used for MRE acquisition, case mixes, imaging protocols, and post-processing procedures. A meta-analysis has been recently published[97].
Compared with TE, dynamic MRE has the potential to assess larger volumes (almost the entire liver) and to provide full three-dimensional information about the viscoelastic properties of tissues[98], moreover, due to the theoretical advantages, MRE is capable of application to patients with obesity or ascites. However, MRE cannot be performed on the liver of patients with iron overload because of signal-to-noise limitations and it is too costly and time-consuming to use in routine practice[72].
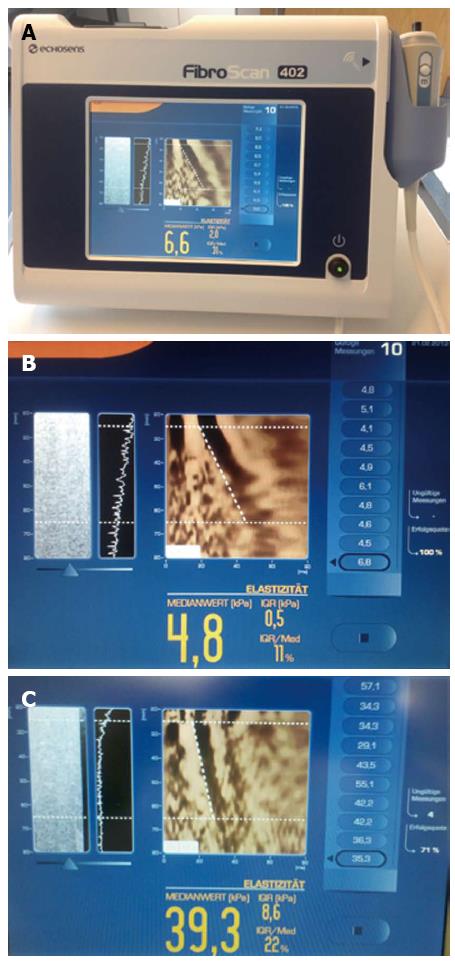
TE (FibroScan®) was the first tool introduced for routine clinical use (Echosens, Paris, France) (Figure 1). TE does not display a conventional ultrasound image. TE has been mainly evaluated in patients with chronic viral hepatitis C and also in a few patients with HIV/viral hepatitis C co-infection and some other liver diseases (see below)[99].
Basic principles: TE is an ultrasound-based non-invasive method. It is characterized by the material’s strain response to external stress according to the principle of Hooke’s law[9]. Briefly, an ultrasound transducer probe is mounted on the axis of a vibrator. Vibrations of mild amplitude and low frequency (50 Hz) are transmitted by the transducer from a right intercostal space, inducing an elastic shear wave that propagates through the liver. Pulse-echo ultrasound acquisition is used to follow the propagation of the shear wave and to measure its speed. This speed is proportional to the tissue stiffness, with faster wave progression occurring through stiffer material. The elastic modulus E is expressed as E = 3qV2, where V is the shear wave speed and q is the material density (assumed constant for tissues): the stiffer the tissue, the faster the shear wave propagates[100]. TE measures LS in a cylindrical volume approximately 10 mm wide and 40 mm long, between 25 and 65 mm below the skin surface with the standard M-probe, and between 35 and 75 mm for the recently developed XL probe, recommended for obese patients[101,102]. This volume is at least 100 times bigger than a biopsy sample and it has been suggested, therefore, that the results compared to LB are more representative of the hepatic parenchyma. However, TE does not work for the left liver lobe or from a subcostal approach and the measurement is only feasible via a few intercostal spaces. Therefore, the technique is limited. Inter- and intra-observer variability depend on the intercostal space used, the presence of ascites, musculoskeletal habitus, depth of subcutaneous tissue, position of the patient, and many other factors[47,103].
How to perform? The measurements with FibroScan® are taken from the right liver lobe via an intercostal space, while the patient lies flat on his/her back, with the right arm tucked behind the head to facilitate access to the liver parenchyma. The tip of the probe is covered with coupling gel and placed on the skin between the ribs at the level of the right lobe where LB would be performed. Once the measurement area has been located, the operator presses the probe button (shot) to start an acquisition. When a shot is unsuccessful, the machine does not give a reading. Measurement of LS is measured in kilopascals (kPa) (range is between 2.5 and 75 kPa)[100].
Advantages: TE with FibroScan® is a rapid procedure (less than 5 min), painless, and easy to perform even in the outpatient clinic or at the bedside. The results are immediately available[103]. The examination can be performed by a nurse after a short learning curve (about 100 examinations)[104]. In addition, TE analysis has excellent inter- and intra-observer agreement, which makes it suitable for widespread application in clinical practice[103,105,106].
Limitations: TE provides only a regional elasticity measurement (determined by the width of the ultrasound beam and depth of the shear wave penetration), but no anatomical images or elastograms. Other drawbacks include limited depth (several cm), the inability of the shear wave to propagate beyond fluid collections (ascites) and difficulty in obtaining sufficient signal in obese patients. Recently, a new probe (XL probe; Echosens, Paris, France) has been proposed for overweight and obese patients[107], and a so-called S-probe has been developed for patients with narrow intercostal spaces, especially children[108]. However, it remains impossible to obtain TE results from patients with ascites[105].
The validity of the TE result also depends on two important parameters: (1) the success rate (the ratio of the number of successful measurements to the total number of acquisitions) should be at least 60%; and (2) the interquartile range (IQR), which reflects the variability of the validated measurements, should not exceed 30% of the median value[109] (Figure 1). Both the feasibility and reproducibility of the TE measurement may be affected by high body mass index (BMI). In a study with 13369 TE measurements, a failure rate of 3.1% was reported. Unreliable results were reported in 15.8% of measurements and were associated with a BMI > 30 kg/m2, age > 52 years, female sex, operator experience and type 2 diabetes[47].
The clinical interpretation of TE results should always be made by an expert clinician and with reference to the patient’s history, disease aetiology and essential laboratory parameters Castera et al[110].
Intra- and inter-observer variability: Several studies[103,105,106] have shown that the intra- and inter-observer reproducibility of TE measurements are good, at least in non-obese subjects. In the study by Sandrin et al[105] intra- and inter-observer variation in TE was investigated in 15 patients and was around 3%, but with a wide variation (2%-18%). The sample size of this study was small, and therefore inadequate to draw firm conclusions on host- and disease-related co-variates that may interfere with TE performance. Another study by Fraquelli et al[103] with a larger sample obtained similar results; 800 TE examinations were performed by two operators in 200 patients with various chronic liver diseases. Both inter- and intra-observer agreement was high and TE reproducibility was excellent, with an intraclass correlation coefficient (ICC) of 0.98. However, inter-observer agreement was significantly reduced in patients with mild hepatic fibrosis, and hepatic steatosis.
The probe location during the TE measurement may affect its reproducibility. In a recent study[111] TE was performed on 625 consecutive patients with chronic liver disease at three different sampling sites. Sampling variability according to probe location was seen in approximately 30% of patients and it was suggested that TE should be performed from various sites to minimize the sampling error.
Chronic viral hepatitis: For patients with CHC, LS values > 6.8-7.6 kPa are indicative of significant fibrosis (F ≥ 2) using the gold standard of LB, and the cut-off values for predicting complete cirrhosis (F = 4) range between 11.0 and 13.6 kPa[20,112,113]. TE is able to distinguish mild fibrosis from advanced liver fibrosis and cirrhosis, which is important for decision making[114]. In contrast, TE does not allow differentiation between the contiguous stages of liver fibrosis. In a meta-analysis including 40 studies[114], the pooled sensitivity and specificity of TE was 79% and 78% for the diagnosis of significant fibrosis; 82% and 86% for diagnosing severe fibrosis; and 83% and 89% for the diagnosis of liver cirrhosis.
It might be of interest to remember that conventional ultrasound techniques can also distinguish between liver cirrhosis and early liver disease in approximately 70% of patients with high specificity but low sensitivity[115-124]. However, TE had an acceptable diagnostic accuracy for detecting early compensated cirrhosis in patients with CHB who did not fulfil the clinical and ultrasound criteria for cirrhosis[125]. Conventional ultrasound techniques are helpful in the detection of complications of liver cirrhosis including portal hypertension[126,127] and can also give important information about fatty infiltration[128-132] and inflammation[133-136]. In a study with 90 patients with suspected liver cirrhosis, liver surface nodularity on conventional ultrasound and TE showed comparable results for diagnosis and exclusion of liver cirrhosis, with the best results when both methods were combined. Liver surface nodularity was better for the diagnosis of liver cirrhosis, while TE was better at ruling out cirrhosis[137].
The performances of TE, when compared, have been shown to be similar between patients with HBV and HCV[138]. Several studies have investigated the performance of TE in an Asian population with CHB[125,139-144] and concluded that TE is a promising and accurate tool for the early detection of cirrhosis. It is demonstrated that the optimal cut-off values for diagnosing HBV-related cirrhosis were between 9.0 and 10.1 kPa in the Asian population[125,140,142,145], which is lower than that in patients with CHC[146,147]. Since there is an increasing number of evidence on the usefulness of TE in patients with CHB, especially in the Asian population, TE should also be recommended in patients with CHB, though the evidence is more limited compared to CHC. Future and updated guidelines have to include this recommendation.
It would be interesting to know in what percentage of patients TE can give important additional information which is of relevance to the treatment, over and above sophisticated ultrasound technology in the hand of an expert hepatologist[43,148].
TE can be used to assess the severity of liver fibrosis in patients with chronic viral hepatitis, provided that confounding factors are taken into account, and especially to distinguish patients with nil/mild fibrosis from those with significant fibrosis, and to identify those with cirrhosis. TE is useful for assessement of liver fibrosis in patients with non-alcoholic fatty liver disease (NAFLD), alcoholic liver diseases, and in patients co-infected with HIV and HCV. Other types of chronic liver disease might also have been investigated, but the evidence is more limited. TE is useful for assessement of liver fibrosis in patients with post-transplant recurrence of CHC. TE has some value for predicting the occurrence of complications of liver cirrhosis, portal hypertension, hepatocellular carcinoma (HCC) and liver-associated mortality. It cannot replace upper gastrointestinal endoscopy for identifying patient with varices[20].
Point shear wave elastography (pSWE) has been introduced by different companies, each currently at different stages of development[7,20]. Acoustic radiation force impulse (ARFI) was the second method to be introduced as a tool for liver fibrosis assessment in a clinical setting. ARFI has a significant advantage over TE in that it simultaneously displays a conventional ultrasound image. The accuracy of both methods has been shown to be similar in the differentiation of normal liver parenchyma from liver cirrhosis[15,149,150]. ARFI has been mainly evaluated in patients with chronic viral hepatitis C and in a few other liver diseases.
Basic principles: ARFI quantification has been developed by two companies (Siemens and Philips) according to the guidelines[7,20], almost all reported studies were done with a conventional high-end ultrasound machine (Siemens S2000). It uses a region of interest (ROI) cursor to interrogate the elastic properties of a specific anatomic region, while real-time B-mode imaging of the abdomen being performed. Short-duration acoustic pulses with a fixed transmit frequency of 2.67 MHz, are generated in the vicinity of the ROI and the subsequent mechanical excitation of the tissues results in tissue displacement and the formation of shear waves that propagate away from the region of excitation. Ultrasound tracking beams laterally adjacent to the single push-beam are used to estimate the shear wave speed in the tissue by the measurement of the time to peak displacement at each lateral location[151]. The shear wave speed is estimated in the central window 5 mm long by 4 mm wide within a graphically displayed ROI of size 10 mm long by 6 mm wide. The results are expressed in meters per second (m/s) (range: 0.5-4.4 m/s with ± 20% accuracy over the range), the shear wave propagation speed being proportional to the square root of the tissue elasticity[152,153]. The ARFI imaging examination takes approximately 5 min. Unlike FibroScan®, ARFI can be utilized in patients with ascites. No limitations concerning measurement are known[154].
Tips and tricks: When scanning the right lobe (especially segment VIII), an optimal window should be used. To reduce the variance of the measurement, it is recommended to apply minimal scan pressure and for the patient to minimize breathing, the influence of cardiac motion should also be avoided. In general, the best and most consistent results will occur when the “normal” state of the liver is measured. When scanning intercostally, no pressure should be applied to the liver and the patient should be asked to just stop breathing for a moment (instead of deep inspiration and breath hold).
In difficult patients, several measurement attempts are needed to “average” out the readings, and data that varies significantly should be excluded. It is recommended to put the patient in a left lateral decubitus position with right arm behind the head in order to get better access to the liver without excessive pushing or the need for breath holds[155]. However, it may still not be possible to get reliable readings in 5.3 % of patients[156].
Intra- and inter-observer variability: Reproducibility of ARFI is also an important pre-requisite for its widespread application in clinical practice. Good inter-observer variability has been reported[157]. Since ARFI allows different measurement sites, comparison of measurements in the right and left liver lobes have been made, and have shown a trend toward higher values in the left lobe[157-159]. However, results in the right lobe revealed higher diagnostic accuracy compared to the left (AUROC: for diagnosis of F1, F2, F3, F4, right lobe: 0.92; 0.83; 0.86; 0.80; left lobe: 0.77; 0.71; 0.78; 0.84; sensitivity, specificity, positive predictive value and negative predictive value of right lobe: 0.88; 0.81; 0.74; 0.92; left lobe: 0.80; 0.75; 0.87; 0.68)[159] (Table 2).
| Ref. | n | Subjects | Left lobe (m/s) | Right lobe (m/s) |
| Karlas et al[158] | 50 | Healthy individuals | 1.28 ± 0.19 | 1.15 ± 0.17 |
| Karlas et al[158] | 23 | Patients with F1, F2 fibrosis | 2.1 ± 0.73 | 1.75 ± 0.89 |
| Toshima et al[159] | 103 | 24 healthy volunteers, 79 patients with chronic liver disease | 1.90 ± 0.68 | 1.61 ± 0.51 |
| Piscaglia et al[157] | 14 | Healthy individuals | 1.29 (1.00-1.60) | 1.15 (0.80-1.74) |
| Piscaglia et al[157] | 114 | Patients with chronic liver disease | 1.79 (0.80-4.00) | 1.67 (0.45-3.76) |
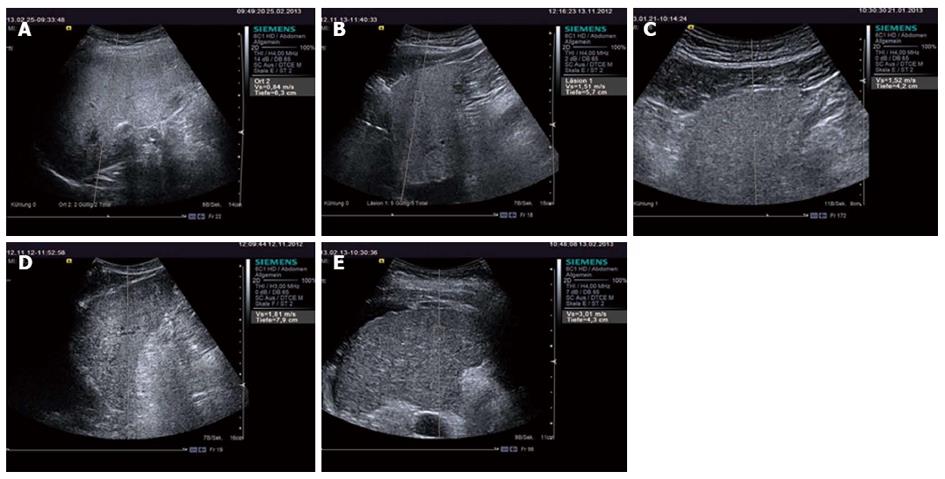
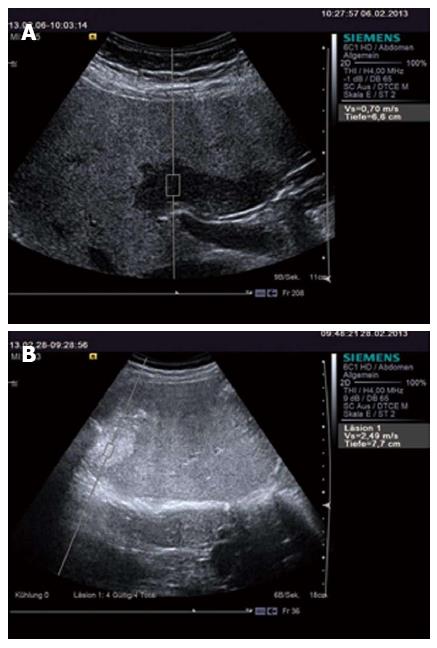
Chronic viral hepatitis: In patients with significant fibrosis (F ≥ 2) the ARFI cut-off values published have been between 1.21-1.34 m/s (AUROCs 0.85-0.89)[23,149,150] and in patients with cirrhosis, 1.55-2 m/s (AUROC’s 0.89-0.93)[15,23,149,160] (Figure 2). Similar to TE, SWE has not proved accurate enough to distinguish between contiguous stages of fibrosis[20].
Other liver diseases: ARFI has also been evaluated in patients with NAFLD and NASH[161,162] and in patients after liver transplantation[163].
Meta-analysis: Friedrich-Rust et al[154] published a meta-analysis which included 9 studies with a combined total of 518 patients with chronic liver disease and evaluated the diagnostic performance of ARFI imaging for the staging of liver fibrosis. The diagnostic accuracy of ARFI quantified by the AUROC was 87% for predicting significant fibrosis (F ≥ 2), 91% for the diagnosis of severe fibrosis (F ≥ 3) and 93% for the diagnosis of liver cirrhosis. The meta-analysis revealed good diagnostic accuracy for ARFI in the diagnosis of significant liver fibrosis and excellent diagnostic accuracy for the diagnosis of liver cirrhosis.
It was also shown that a comparison of ARFI with TE in the four studies that included 312 patients, resulted in comparable diagnostic accuracies for both methods in the diagnosis of severe fibrosis, and slightly, but significantly, higher diagnostic accuracies of TE for the diagnosis of significant fibrosis and liver cirrhosis. However, a recent study showed superior results for ARFI elastography[150]. Future multicentre studies are necessary to compare the different methods before any conclusions can be drawn.
In contrast to TE, ARFI has been shown to be less influenced by obesity and ascites[151]. One study showed that valid LS measurement (LSM) were obtained in all 23 patients with morbid obesity (mean BMI was higher than 44 kg/m2)[164]. In addition, it can be easily added to a commercial ultrasound machine.
However, in contrast to TE values, ARFI values have a narrow measurement range (0.5-4.4 m/s), which limits the definition of cut-off values required for decisions on patient management. In addition, inflammatory activity and elevated aminotransferase levels may lead to overestimation of ARFI-LS values[15,25] as has been shown for TE. Moreover, since this is a new technique, the quality criteria are not yet well-defined.
SWE [Aixplorer®, SuperSonic Imagine (SSI), France] has been introduced as a 2D and also 3D-technique. So far, only 2D-SWE has been evaluated in studies on the liver. The studies of 3D-SWE have mainly focused on the breast[165,166].
This technique is based on the combination of a radiation force induced in the tissues by focused ultrasonic beams and very high frame rate (up to 5000 f/s) ultrasound imaging capable of catching, in real time, the transient propagation of the resulting shear waves[167,168]. The local shear wave speed is recovered using a dedicated time-of-flight estimation technique and enables the 2-D quantitative mapping of elasticity. This imaging modality can be performed using a conventional ultrasound probe, during a standard intercostal ultrasound examination. Three supersonic shear wave imaging sequences are applied successively to the left, middle and right parts of the 2-D ultrasound image. The resulting elasticity images in the three regions are concatenated to provide the final image covering the entire region-of-interest. The ability of the SWE technique to provide a quantitative and local estimation of liver shear modulus with a millimetric resolution has been proven in a pilot study in 15 healthy volunteers[18]. Liver moduli extracted from in vivo data from healthy volunteers, were consistent with those reported in the literature (Young’s modulus ranging from 4 to 7.5 kPa). Moreover, LSM using the SWE mode was fast (less than one second), repeatable (5.7% standard deviation) and reproducible (6.7% standard deviation)[3].
Intra- and inter-observer variability: To date, there has only been one study[169] aimed at assessing the intra- and inter-observer precision of 2D-SWE measurements in the evaluation of liver elasticity. It was reported that the reproducibility was good with high intra- and inter-observer agreement. In this study, 2D-SWE was performed on 60 volunteers (42 cases with 10 consecutive measurements, 18 cases with 2 measurements) on 2 different days by 2 operators (one expert and one novice). The intra-observer agreement between measurements performed in the same subject on the same day (day 1 or day 2) showed intraclass correlation coefficient (ICC) values of 0.95 and 0.93 for the expert operator and novice, respectively, and the ICC values for intra-observer agreement between measurements performed in the same subject on different days were 0.84 and 0.65, respectively. The inter-observer agreement was 0.88. Therefore, real-time 2D-SWE has been shown to be a reproducible method to measure liver elasticity, but the novice operator showed lower measurement reproducibility over time than the expert operator.
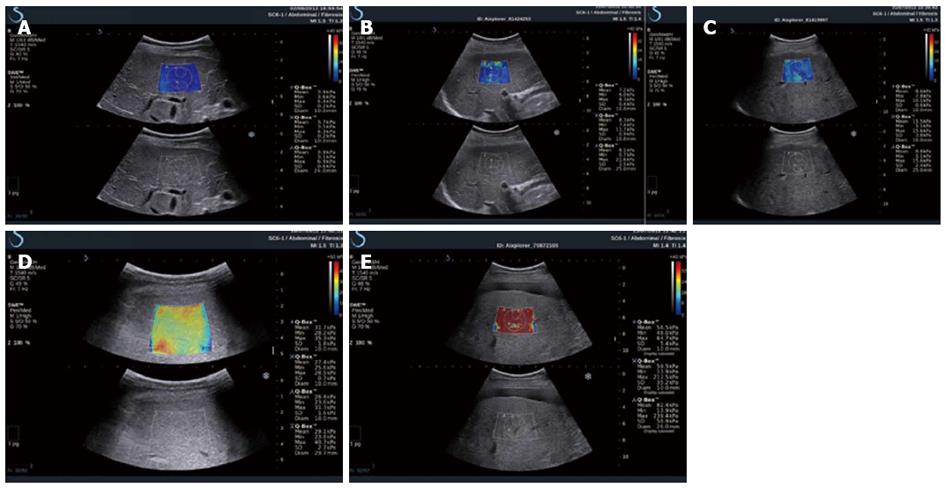
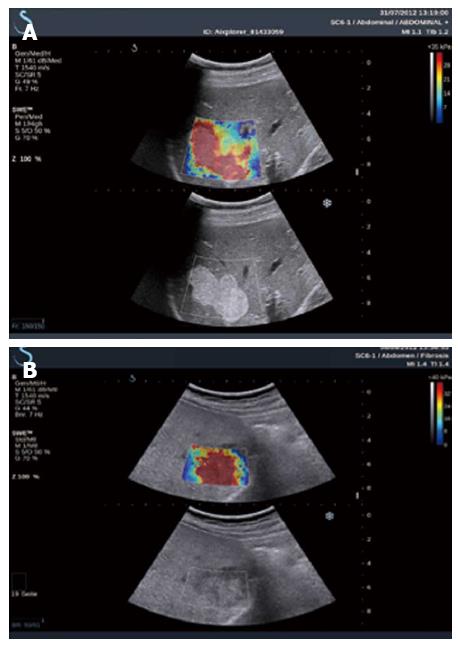
CHC: 2D-SWE might be used in assessing liver fibrosis for patients with CHC, as has been proved in two large studies. Ferraioli et al[170] assessed the accuracy of 2D-SWE in comparison with transient elastography in 121 patients with CHC using LB as the reference standard, and found that LS values increased in parallel with the degree of liver fibrosis both with 2D-SWE and TE. The AUROC was 0.92 for 2D-SWE and 0.84 for TE (P = 0.002); 0.98 for 2D-SWE and 0.96 for TE (P = 0.14); 0.98 for 2D-SWE and 0.96 for TE (P = 0.48), when comparing F0-F1 vs F2-F4, F0-F2 vs F3-F4, and F0-F3 vs F4, respectively (Figure 3). Therefore, the real-time 2D-SWE was more accurate than TE in assessing significant fibrosis (≥ F2). In the other study which included 113 hepatitis C virus patients, a good agreement was shown between 2D-SWE and TE, the AUROC for elasticity values assessed by 2D-SWE were 0.948, 0.962 and 0.968 for patients with predicted fibrosis levels F ≥ 2, F ≥ 3 and F = 4, respectively. However, LB was only available in 39 patients[17].
Strain elastography (SE), also termed as quasi-static strain imaging, has been developed by several manufacturers, however, only Hitachi ultrasound system has been evaluated for use in liver.
SE is based upon the fact that soft tissue can be more easily compressed than hard tissue. When subtle compression is applied with probe, SE shows the relative degree of tissue strain, but not demonstrates the physical elasticity directly. SE calculates the strain response of the tissue to stress (relative tissue elasticity) and displays it as a colour overlay [ranges from red (soft) to blue (hard)] on the B-mode image[171]. The echo signals could be captured in real-time by incorporating a high speed algorithm, in addition, both the B-mode image and corresponding tissue elasticity image could be simultaneous displayed[172]. Semi-quantitative elastography techniques are based on quantification of the strain distribution within a defined ROI.
Because the pressure generated by the operator’s compression may influence both the image of elasticity and the resulting elasticity score, Hitachi medical system has recently developed an elastography method that did not require extra external stress. The required liver distortion for future analysis would be achieved from the rhythmic pulsations of the abdominal aorta or the heart.
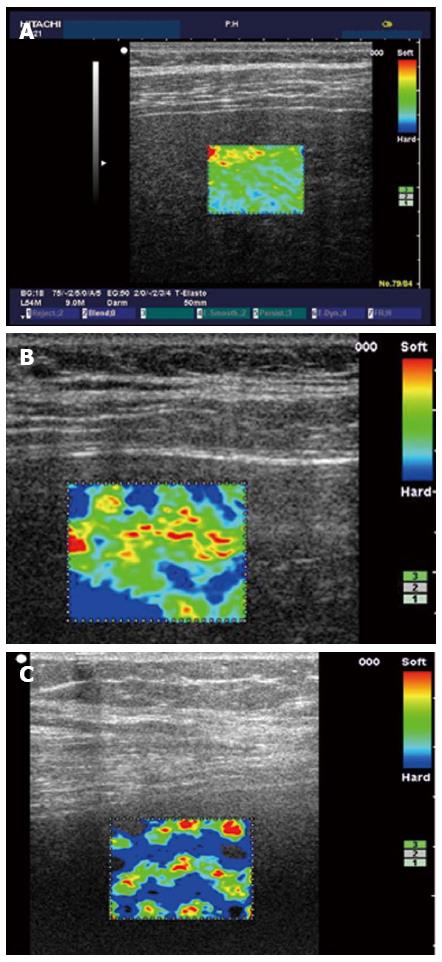
In 2007, Frederich-Rust et al[11] reported the clinical application of SE in the liver. They developed an elasticity score by assessing the colour-coded strain image using the computer program Matlab. The diagnostic accuracy for F2, F3 and F4 were 0.75, 0.73 and 0.69, respectively. In 2009, the same group[173] compared SE with TE (Fibroscan) and serum fibrosis marker (Fibrotest), and concluded that SE in its evaluated format could not replace TE for non-invasive assessment of liver fibrosis at the time of the study. After the software for elastography was developed by Hitachi medical systems, good results were published by several studies. Morikawa et al[174] transferred the pixel data in the ROI into a histogram and a binary image for semi quantification with a devised system, and found that the mean value on the histogram and the percentage of hard tissue may directly represent liver elasticity. The diagnostic accuracy of SE for liver fibrosis was also compared with TE, the author felt SE compared favourably with TE and suggested SE could potentially be used as a routine imaging tool to evaluate liver fibrosis. A Chinese group[175] utilized a new Hitachi ultrasound system (HI VISION Preius) and concluded that there was a strong positive correlation (r = 0.81) between the elasticity index and fibrosis stage. Diagnostic accuracies of SE for the diagnosis of F1, F2, F3, F4 were 0.93, 0.92, 0.84 and 0.66, respectively. Koizumi et al[176] performed a semi-quantitative analysis using the elastic ratio method (ratio of strain distribution in two selected ROI) on 70 patients with CHC and with the hepatic vein as the internal control and found that the AUROC curves for elastic ratio were superior to serum fibrosis markers and scores of fibrotic change based on blood results (Figure 4).
More studies including meta-analysis about the use of SE for the evaluation of liver fibrosis are required to establish a protocol for accurate imaging and to standardize analysis.
The main advantage of this technique is the relatively large region of interest that can be interrogated in the right liver lobe, plus the quantification method that can measure the change from the diffuse soft uniform architecture of the liver to a patchy hard pattern as hepatic fibrosis progresses.
The intra-observer variability and intra-observer agreement of SE for the assessment of liver fibrosis have been criticized in several studies[173,177,178]. In a more recent study, a Japanese group[176] used a semi-quantitative method (elastic ratio) and found that the measurements obtained from four separate locations had no observed variation between the two operators (K = 0.835, ICC = 0.966).
Advantages and disadvantages of currently available non-invasive methods in patients with chronic viral hepatitis C are summarized in Table 3.
| Parameters | Transient elastography | ARFI | 2D-SWE | MR Elastography | Serum biomarkers |
| Advantages | High and rapid performance | High and rapid performance | High and rapid performance | High performance (applicability) | Availability |
| Reproducibility | Reproducibility | Reproducibility | Reproducibility | Reproducibility | |
| Easy to learn | Easy to learn | Easy to learn, large ROI | Examination of the whole liver | Low cost | |
| Combined with conventional ultrasound | Combined with conventional ultrasound | Combined with conventional MRI | |||
| Obesity and ascites are not limiting | Ascites are not limiting | obesity and ascites are not limiting | |||
| Disadvantages | Technical requirements (equipment) without additional use | Technical requirements (ultrasound equipment) | Technical requirements (ultrasound equipment) | Technical requirements (MRI equipment) | Non-specific (hyperbilirubinemia, hemolysis, inflammation, others) |
| Intermediate cost | Intermediate cost | Intermediate cost | Extremely high cost, time consuming | Relatively high cost, limited availability (patent) | |
| Limited recognition of intermediate stages of fibrosis | Limited recognition of intermediate stages of fibrosis | Limited recognition of intermediate stages of fibrosis | Limited recognition of intermediate stages of fibrosis | Limited recognition of intermediate stages of fibrosis | |
| Blind selection of region of interest | Not applicable in case of iron deposition | Results not immediately available | |||
| Restricted value in obese patients and ascites | Narrow range of values, small ROI | ||||
| False positive values in patients with acute hepatitis, cholestasis, and heart failure | Quality criteria not well defined | Quality criteria not well defined |
Elastography methods have been also applied for detection and characterisation of focal liver lesions (FLL). Although the method so far cannot be applied to all segments and the limited depth of penetration is so far disappointing, several studies have evaluated the performance of ARFI to differentiate FLL, and the results are encouraging. ARFI has shown a high accuracy for the identification of malignant FLL. In a meta-analysis by Ying et al[179] including 590 lesions in eight studies, the summary sensitivity and specificity for identification of malignant liver lesions were 0.86 and 0.89, respectively. The hierarchical summary receiver operating characteristic (HSROC) was 0.94. However, one paper showed that ARFI did not permit differentiation between benign and malignant FLL because high ARFI values occur in benign as well as in malignant lesions[180]. In another study by Gallotti A, the mean shear wave speed of HCC, haemangioma, adenoma, metastasis, FNH was 2.17, 2.30, 1.25, 2.87, 2.75 m/s, respectively. Adenoma showed similar stiffness to the surrounding liver, and was significantly softer than the other four types of lesion. FNH showed different stiffness to HCC and metastasis, however, haemangioma showed no difference to HCC, metastasis and FNH[181] (Figures 5 and 6). The performance of ARFI is summarized in Table 4.
| No. of FLL | Rate of malignancy | Reference standard | Lesion types | ARFI cut-off (m/s) | QUADAS score | Ref. |
| 105 | 64.8% | Biopsy, imaging | Haemangioma,FNH, focal fatty sparing, focal fat deposits adenomas, HCC, metastasis | 2.7 | 11 | [182] |
| 60 | 71.7% | Biopsy, CT/MRI | haemangioma, HCC, CCC, metastasis | 2 | 10 | [183] |
| 128 | 53.1% | Biopsy, surgery, imaging | Haemangioma, FNH, focal fatty change, abscess, adenoma, solitary necrotic nodule, HCC, metastasis, CCC | 2.2 | 10 | [184] |
| 42 | 64.3% | Biopsy | Haemangioma, lymphoma, FNH, sarcoid, abscess, focal fatty sparing, HCC, metastasis | 2.5 | 12 | [185] |
| 45 | 22.2% | Biopsy, CT/MRI | Haemangioma, metastasis | 2.5 | 8 | [186] |
Although promising results have been reported, more research is needed, especially in comparison to CEUS, before recommendations on its use in clinical practice can be made. So far, elastography cannot be recommended for the differential diagnosis of benign from malignant liver lesions[7,20].
P- Reviewers Badea R, Kim SU S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111-134. [PubMed] |
| 2. | Ophir J, Garra B, Kallel F, Konofagou E, Krouskop T, Righetti R, Varghese T. Elastographic imaging. Ultrasound Med Biol. 2000;26 Suppl 1:S23-S29. [PubMed] |
| 3. | Sporea I, Friedrich-Rust M, Gilja OH. Estimation of liver stiffness using ultrasound waves. EFSUMB European Course Book. London: European Federation of Societies for Ultrasound in Medicine and Biology 2012; 91-108. |
| 4. | Dietrich CF. Real-time tissue elastography. Multiple clinical applications. Multiple clinical solutions. Endoskopie Heute. 2011;24:177-212. |
| 5. | Matre K, Moen CA, Fanneløp T, Dahle GO, Grong K. Multilayer radial systolic strain can identify subendocardial ischemia: an experimental tissue Doppler imaging study of the porcine left ventricular wall. Eur J Echocardiogr. 2007;8:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Gilja OH, Heimdal A, Hausken T, Gregersen H, Matre K, Berstad A, Ødegaard S. Strain during gastric contractions can be measured using Doppler ultrasonography. Ultrasound Med Biol. 2002;28:1457-1465. [PubMed] |
| 7. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 771] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 8. | Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 10. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 11. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Tatsumi C, Kudo M, Ueshima K, Kitai S, Takahashi S, Inoue T, Minami Y, Chung H, Maekawa K, Fujimoto K. Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology. 2008;51 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Havre RF, Elde E, Gilja OH, Odegaard S, Eide GE, Matre K, Nesje LB. Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol. 2008;34:1638-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Sporea I, Sirli R, Bota S, Popescu A, Sendroiu M, Jurchis A. Comparative study concerning the value of acoustic radiation force impulse elastography (ARFI) in comparison with transient elastography (TE) for the assessment of liver fibrosis in patients with chronic hepatitis B and C. Ultrasound Med Biol. 2012;38:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112-4118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 16. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 17. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 18. | Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol. 2009;35:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 623] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 21. | Marginean CO, Marginean C. Elastographic assessment of liver fibrosis in children: A prospective single center experience. Eur J Radiol. 2012;81:e870-e874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Sporea I, Şirli R, Popescu A, Bota S, Badea R, Lupşor M, Focşa M, Dănilă M. Is it better to use two elastographic methods for liver fibrosis assessment? World J Gastroenterol. 2011;17:3824-3829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Sporea I, Sirli R, Bota S, Fierbinţeanu-Braticevici C, Petrişor A, Badea R, Lupşor M, Popescu A, Dănilă M. Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis? World J Radiol. 2011;3:188-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Bota S, Sporea I, Sirli R, Popescu A, Dănilă M, Sendroiu M. Factors that influence the correlation of acoustic radiation force impulse (ARFI), elastography with liver fibrosis. Med Ultrason. 2011;13:135-140. [PubMed] |
| 25. | Yoon KT, Lim SM, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Cho M, Lee JW, Kim SU. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig Dis Sci. 2012;57:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Kumar R, Rastogi A, Sharma MK, Bhatia V, Tyagi P, Sharma P, Garg H, Chandan Kumar KN, Bihari C, Sarin SK. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig Dis Sci. 2013;58:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Lédinghen V, Douvin C, Marcellin P, Ganne-Carrié N, Trinchet JC, Beaugrand M. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008;49:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Wang JH, Changchien CS, Hung CH, Eng HL, Tung WC, Kee KM, Chen CH, Hu TH, Lee CM, Lu SN. FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis. J Gastroenterol. 2009;44:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Lupsor M, Badea R, Stefanescu H, Grigorescu M, Serban A, Radu C, Crişan D, Sparchez Z, Iancu S, Maniu A. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2010;19:53-60. [PubMed] |
| 30. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 31. | Popescu A, Bota S, Sporea I, Sirli R, Danila M, Racean S, Suseanu D, Gradinaru O, Ivascu Siegfried C. The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography-preliminary results. Ultrasound Med Biol. 2013;39:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Goertz RS, Egger C, Neurath MF, Strobel D. Impact of food intake, ultrasound transducer, breathing maneuvers and body position on acoustic radiation force impulse (ARFI) elastometry of the liver. Ultraschall Med. 2012;33:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Goldschmidt I, Streckenbach C, Dingemann C, Pfister ED, di Nanni A, Zapf A, Baumann U. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr. 2013;57:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Yun MH, Seo YS, Kang HS, Lee KG, Kim JH, An H, Yim HJ, Keum B, Jeen YT, Lee HS. The effect of the respiratory cycle on liver stiffness values as measured by transient elastography. J Viral Hepat. 2011;18:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 37. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 38. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 39. | Kuroda H, Takikawa Y, Onodera M, Kakisaka K, Yoshida Y, Kataoka K, Sawara K, Miyamoto Y, Oikawa K, Endo R. Serial changes of liver stiffness measured by acoustic radiation force impulse imaging in acute liver failure: a case report. J Clin Ultrasound. 2012;40:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, Rumi M, Lampertico P, Colombo M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol. 2011;54:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, Merrouche W, Chu SH, Pesque S. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 42. | de Lédinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, Le Bail B, Choi PC, Chermak F, Yiu KK. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 43. | de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 44. | de Lédinghen V, Vergniol J, Gonzalez C, Foucher J, Maury E, Chemineau L, Villars S, Gin H, Rigalleau V. Screening for liver fibrosis by using FibroScan(®) and FibroTest in patients with diabetes. Dig Liver Dis. 2012;44:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 46. | Myers RP, Crotty P, Pomier-Layrargues G, Ma M, Urbanski SJ, Elkashab M. Prevalence, risk factors and causes of discordance in fibrosis staging by transient elastography and liver biopsy. Liver Int. 2010;30:1471-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 48. | Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, Sulkowski M, Bass EB. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | McHutchison J, Poynard T, Afdhal N. Fibrosis as an end point for clinical trials in liver disease: a report of the international fibrosis group. Clin Gastroenterol Hepatol. 2006;4:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 51. | Jenssen C, Dietrich CF. Kontraindikationen, Komplikationen, Komplikationsmanagement. Interventioneller Ultraschall. Lehrbuch und Atlas für die interventionelle Sonographie. Stuttgart: Georg Thieme Verlag 2011; 127-160. |
| 52. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [PubMed] |
| 53. | Denzer U, Arnoldy A, Kanzler S, Galle PR, Dienes HP, Lohse AW. Prospective randomized comparison of minilaparoscopy and percutaneous liver biopsy: diagnosis of cirrhosis and complications. J Clin Gastroenterol. 2007;41:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Helmreich-Becker I, Schirmacher P, Denzer U, Hensel A, Meyer zum Büschenfelde KH, Lohse AW. Minilaparoscopy in the diagnosis of cirrhosis: superiority in patients with Child-Pugh A and macronodular disease. Endoscopy. 2003;35:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Manns MP, Schneider A, Meier PN. Minilaparoscopy for early diagnosis of cirrhosis: is the endoscopist’s eye better than the histopathologist’s? Endoscopy. 2003;35:74-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Orlando R, Lirussi F. Laparoscopy and guided liver biopsy in the diagnosis of cirrhosis: conventional technique vs. minilaparoscopy. Endoscopy. 2003;35:1079-180; author reply 1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Schneider AR, Riemann JF, Arnold JC. [Value of minilaparoscopy in comparison with conventional laparoscopy in diagnosis of liver diseases--intermediate term results of a prospective, randomized study]. Z Gastroenterol. 2001;39:15-18. [PubMed] |
| 58. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 59. | Persico M, Palmentieri B, Vecchione R, Torella R, de SI. Diagnosis of chronic liver disease: reproducibility and validation of liver biopsy. Am J Gastroenterol. 2002;97:491-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-199, 1970-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 61. | Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, Alexander G, Ramage J, Dusheiko G. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 63. | Parkes J, Guha IN, Harris S, Rosenberg WM, Roderick PJ. Systematic review of the diagnostic performance of serum markers of liver fibrosis in alcoholic liver disease. Comp Hepatol. 2012;11:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, Irving W, Zaitoun A, Wheatley M, Ryder S. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] |
| 66. | Poynard T, Zoulim F, Ratziu V, Degos F, Imbert-Bismut F, Deny P, Landais P, El Hasnaoui A, Slama A, Blin P. Longitudinal assessment of histology surrogate markers (FibroTest-ActiTest) during lamivudine therapy in patients with chronic hepatitis B infection. Am J Gastroenterol. 2005;100:1970-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 68. | Lainé F, Bendavid C, Moirand R, Tessier S, Perrin M, Guillygomarc’h A, Guyader D, Calon E, Renault A, Brissot P. Prediction of liver fibrosis in patients with features of the metabolic syndrome regardless of alcohol consumption. Hepatology. 2004;39:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Castera L. Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat. 2009;16:300-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 72. | Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 73. | Mehta P, Ploutz-Snyder R, Nandi J, Rawlins SR, Sanderson SO, Levine RA. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2008;103:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Halfon P, Bourlière M, Pénaranda G, Deydier R, Renou C, Botta-Fridlund D, Tran A, Portal I, Allemand I, Rosenthal-Allieri A. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol. 2005;4:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 76. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1037] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 77. | Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 78. | Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, Lin R, Samarasinghe D, Liddle C, McCaughan GW. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology. 2004;39:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Hui AY, Chan HL, Wong VW, Liew CT, Chim AM, Chan FK, Sung JJ. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol. 2005;100:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, Chen CW, Wang JY, Cai X, Gao CF. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, Moore RE, Afdhal NH. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 84. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3556] [Article Influence: 187.2] [Reference Citation Analysis (0)] |
| 85. | Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 86. | Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 548] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 87. | Calès P, Veillon P, Konaté A, Mathieu E, Ternisien C, Chevailler A, Godon A, Gallois Y, Joubaud F, Hubert-Fouchard I. Reproducibility of blood tests of liver fibrosis in clinical practice. Clin Biochem. 2008;41:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Imbert-Bismut F, Messous D, Thibault V, Myers RB, Piton A, Thabut D, Devers L, Hainque B, Mercadier A, Poynard T. Intra-laboratory analytical variability of biochemical markers of fibrosis (Fibrotest) and activity (Actitest) and reference ranges in healthy blood donors. Clin Chem Lab Med. 2004;42:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 90. | Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854-1857. [PubMed] |
| 91. | Wang Y, Ganger DR, Levitsky J, Sternick LA, McCarthy RJ, Chen ZE, Fasanati CW, Bolster B, Shah S, Zuehlsdorff S. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:553-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 92. | Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, Peeters F, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 94. | Rouvière O, Yin M, Dresner MA, Rossman PJ, Burgart LJ, Fidler JL, Ehman RL. MR elastography of the liver: preliminary results. Radiology. 2006;240:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 95. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 714] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 96. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 539] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 97. | Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 98. | Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, Felmlee JP, Greenleaf JF, Ehman RL. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5:237-254. [PubMed] |
| 99. | Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol. 2010;25:1726-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 101. | Wong GL. Transient elastography: Kill two birds with one stone? World J Hepatol. 2013;5:264-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Wong GL, Vergniol J, Lo P, Wai-Sun Wong V, Foucher J, Le Bail B, Choi PC, Chermak F, Leung KS, Merrouche W. Non-invasive assessment of liver fibrosis with transient elastography (FibroScan®): applying the cut-offs of M probe to XL probe. Ann Hepatol. 2013;12:570-580. [PubMed] |
| 103. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 104. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 105. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 106. | Saito H, Tada S, Nakamoto N, Kitamura K, Horikawa H, Kurita S, Saito Y, Iwai H, Ishii H. Efficacy of non-invasive elastometry on staging of hepatic fibrosis. Hepatol Res. 2004;29:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 108. | Engelmann G, Gebhardt C, Wenning D, Wühl E, Hoffmann GF, Selmi B, Grulich-Henn J, Schenk JP, Teufel U. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. 2012;171:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 109. | Lucidarme D, Foucher J, Le Bail B, Vergniol J, Castera L, Duburque C, Forzy G, Filoche B, Couzigou P, de Lédinghen V. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 110. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1070] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 111. | Zelber-Sagi S, Yeshua H, Shlomai A, Blendis L, Leshno M, Levit S, Halpern Z, Oren R. Sampling variability of transient elastography according to probe location. Eur J Gastroenterol Hepatol. 2011;23:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 113. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Lissandrin R, Filice G, Filice C, Above E, Barbarini G, Brunetti E. Performance of liver stiffness measurements by transient elastography in chronic hepatitis. World J Gastroenterol. 2013;19:49-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 114. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 115. | Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology. 1980;135:273-283. [PubMed] |
| 116. | Dietrich CF, Wehrmann T, Zeuzem S, Braden B, Caspary WF, Lembcke B. [Analysis of hepatic echo patterns in chronic hepatitis C]. Ultraschall Med. 1999;20:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Dietrich CF, Lee JH, Gottschalk R, Herrmann G, Sarrazin C, Caspary WF, Zeuzem S. Hepatic and portal vein flow pattern in correlation with intrahepatic fat deposition and liver histology in patients with chronic hepatitis C. AJR Am J Roentgenol. 1998;171:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472-478. [PubMed] |
| 119. | Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D’Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979-985. [PubMed] |
| 120. | Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 121. | Honda H, Onitsuka H, Masuda K, Nishitani H, Nakata H, Watanabe K. Chronic liver disease: value of volumetry of liver and spleen with computed tomography. Radiat Med. 1990;8:222-226. [PubMed] |
| 122. | Hess CF, Schmiedl U, Koelbel G, Knecht R, Kurtz B. Diagnosis of liver cirrhosis with US: receiver-operating characteristic analysis of multidimensional caudate lobe indexes. Radiology. 1989;171:349-351. [PubMed] |
| 123. | Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383-387. [PubMed] |
| 124. | Schalm SW. The diagnosis of cirrhosis: clinical relevance and methodology. J Hepatol. 1997;27:1118-1119. [PubMed] |
| 125. | Kim do Y, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, Yoon KT, Paik YH, Lee KS, Chon CY. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci. 2009;54:1758-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 126. | Berzigotti A, Piscaglia F. Ultrasound in portal hypertension--part 2--and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012;33:8-32; quiz 30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Nuernberg D. Diagnostiche und therapeutische Parazentese freier abdomineller und thorakaler Flüssigkeit. Interventioneller Ultraschall. Lehrbuch und Atlas für die interventionelle Sonographie. Stuttgart: Georg Thieme Verlag 2011; 184-198. |
| 128. | Dietrich CF, Jenssen C. [Focal liver lesion, incidental finding]. Dtsch Med Wochenschr. 2012;137:2099-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 129. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 130. | Dietrich CF. Liver tumor characterization--comments and illustrations regarding guidelines. Ultraschall Med. 2012;33 Suppl 1:S22-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 131. | Dietrich CF, Cui XW, Schreiber-Dietrich DG, Ignee A. EFSUMB guidelines 2011: comments and illustrations. Ultraschall Med. 2012;33 Suppl 1:S11-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 132. | Hirche TO, Ignee A, Hirche H, Schneider A, Dietrich CF. Evaluation of hepatic steatosis by ultrasound in patients with chronic hepatitis C virus infection. Liver Int. 2007;27:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Dietrich CF, Lee JH, Herrmann G, Teuber G, Roth WK, Caspary WF, Zeuzem S. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Dietrich CF, Stryjek-Kaminska D, Teuber G, Lee JH, Caspary WF, Zeuzem S. Perihepatic lymph nodes as a marker of antiviral response in patients with chronic hepatitis C infection. AJR Am J Roentgenol. 2000;174:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Dietrich CF, Leuschner MS, Zeuzem S, Herrmann G, Sarrazin C, Caspary WF, Leuschner UF. Peri-hepatic lymphadenopathy in primary biliary cirrhosis reflects progression of the disease. Eur J Gastroenterol Hepatol. 1999;11:747-753. [PubMed] |
| 136. | Braden B, Faust D, Ignee A, Schreiber D, Hirche T, Dietrich CF. Clinical relevance of perihepatic lymphadenopathy in acute and chronic liver disease. J Clin Gastroenterol. 2008;42:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 137. | Berzigotti A, Abraldes JG, Tandon P, Erice E, Gilabert R, García-Pagan JC, Bosch J. Ultrasonographic evaluation of liver surface and transient elastography in clinically doubtful cirrhosis. J Hepatol. 2010;52:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | Cardoso AC, Carvalho-Filho RJ, Stern C, Dipumpo A, Giuily N, Ripault MP, Asselah T, Boyer N, Lada O, Castelnau C. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 139. | Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2010;31:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 140. | Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 141. | Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, Lau KK, Chim AM, Yiu KK, Chan FK. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 142. | Kim SU, Ahn SH, Park JY, Kang W, Kim do Y, Park YN, Chon CY, Han KH. Liver stiffness measurement in combination with noninvasive markers for the improved diagnosis of B-viral liver cirrhosis. J Clin Gastroenterol. 2009;43:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 143. | Goyal R, Mallick SR, Mahanta M, Kedia S, Shalimar , Dhingra R, Sharma H, Das P, Gupta SD, Panda S. Fibroscan can avoid liver biopsy in Indian patients with Chronic Hepatitis B. J Gastroenterol Hepatol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Fung J, Lai CL, Wong DK, Seto WK, Hung I, Yuen MF. Significant changes in liver stiffness measurements in patients with chronic hepatitis B: 3-year follow-up study. J Viral Hepat. 2011;18:e200-e205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 145. | Kim SU, Han KH, Ahn SH. Transient elastography in chronic hepatitis B: an Asian perspective. World J Gastroenterol. 2010;16:5173-5180. [PubMed] |
| 146. | Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 147. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 953] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 148. | Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 149. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 150. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 151. | Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 152. | Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715-1723. [PubMed] |
| 153. | Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419-1435. [PubMed] |
| 154. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 155. | Dietrich CF, Serra C, Jedrzejczyk K. Ultrasound of the liver. EFSUMB European Course Book. London: European Federation of Societies for Ultrasound in Medicine and Biology 2012; 31-90. |
| 156. | Sporea I, Sirli RL, Deleanu A, Iulia R, Tudora A, Dan I, Popescu A. What did we learn from the first 3,459 cases of liver stiffness measurement by transient elastography (FibroScan®)? Ultraschall Med. 2011;32:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 157. | Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 158. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 159. | Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 160. | Kircheis G, Sagir A, Vogt C, Vom Dahl S, Kubitz R, Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol. 2012;18:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 161. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 162. | Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325-e331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 163. | Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, Martínez SM, Gilabert R, Forns X, Jiménez W, Navasa M. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 164. | Guzmán-Aroca F, Frutos-Bernal MD, Bas A, Luján-Mompeán JA, Reus M, Berná-Serna Jde D, Parrilla P. Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging. Eur Radiol. 2012;22:2525-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 165. | Youk JH, Gweon HM, Son EJ, Chung J, Kim JA, Kim EK. Three-dimensional shear-wave elastography for differentiating benign and malignant breast lesions: comparison with two-dimensional shear-wave elastography. Eur Radiol. 2013;23:1519-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 166. | Lee SH, Chang JM, Kim WH, Bae MS, Cho N, Yi A, Koo HR, Kim SJ, Kim JY, Moon WK. Differentiation of benign from malignant solid breast masses: comparison of two-dimensional and three-dimensional shear-wave elastography. Eur Radiol. 2013;23:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 167. | Bercoff J, Pernot M, Tanter M, Fink M. Monitoring thermally-induced lesions with supersonic shear imaging. Ultrason Imaging. 2004;26:71-84. [PubMed] |
| 168. | Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396-409. [PubMed] |
| 169. | Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, Filice C. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012;81:3102-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 170. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 171. | Frey H. [Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity]. Radiologe. 2003;43:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 172. | Jaffer OS, Lung PF, Bosanac D, Shah A, Sidh Ps. Is ultrasound elastography of the liver ready to replace biopsy? A critical review of the current techniques. Ultrasound. 2012;20:24-32. |
| 173. | Friedrich-Rust M, Schwarz A, Ong M, Dries V, Schirmacher P, Herrmann E, Samaras P, Bojunga J, Bohle RM, Zeuzem S. Real-time tissue elastography versus FibroScan for noninvasive assessment of liver fibrosis in chronic liver disease. Ultraschall Med. 2009;30:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 174. | Morikawa H, Fukuda K, Kobayashi S, Fujii H, Iwai S, Enomoto M, Tamori A, Sakaguchi H, Kawada N. Real-time tissue elastography as a tool for the noninvasive assessment of liver stiffness in patients with chronic hepatitis C. J Gastroenterol. 2011;46:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 175. | Wang J, Guo L, Shi X, Pan W, Bai Y, Ai H. Real-time elastography with a novel quantitative technology for assessment of liver fibrosis in chronic hepatitis B. Eur J Radiol. 2012;81:e31-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 176. | Koizumi Y, Hirooka M, Kisaka Y, Konishi I, Abe M, Murakami H, Matsuura B, Hiasa Y, Onji M. Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography--establishment of the method for measurement. Radiology. 2011;258:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 177. | Sãftoiu A, Gheonea DI, Ciurea T. Hue histogram analysis of real-time elastography images for noninvasive assessment of liver fibrosis. AJR Am J Roentgenol. 2007;189:W232-W233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 178. | Ferraioli G, Gulizia R, Filice C. Real-time elastography in the assessment of liver fibrosis. AJR Am J Roentgenol. 2007;189:W170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 179. | Ying L, Lin X, Xie ZL, Tang FY, Hu YP, Shi KQ. Clinical utility of acoustic radiation force impulse imaging for identification of malignant liver lesions: a meta-analysis. Eur Radiol. 2012;22:2798-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 180. | Heide R, Strobel D, Bernatik T, Goertz RS. Characterization of focal liver lesions (FLL) with acoustic radiation force impulse (ARFI) elastometry. Ultraschall Med. 2010;31:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 181. | Gallotti A, D’Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012;81:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 182. | Yu H, Wilson SR. Differentiation of benign from malignant liver masses with Acoustic Radiation Force Impulse technique. Ultrasound Q. 2011;27:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 183. | Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 184. | Shuang-Ming T, Ping Z, Ying Q, Li-Rong C, Ping Z, Rui-Zhen L. Usefulness of acoustic radiation force impulse imaging in the differential diagnosis of benign and malignant liver lesions. Acad Radiol. 2011;18:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 185. | Kapoor A, Kapoor A, Mahajan G, Sidhu BS, Lakhanpal VP. Real-time elastography in differentiating metastatic from nonmetastatic liver nodules. Ultrasound Med Biol. 2011;37:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 186. | Davies G, Koenen M. Acoustic radiation force impulse elastography in distinguishing hepatic haemangiomata from metastases: preliminary observations. Br J Radiol. 2011;84:939-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |