Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5665
Revised: May 9, 2013
Accepted: May 18, 2013
Published online: September 14, 2013
Processing time: 203 Days and 19.1 Hours
AIM: To compare the mucosal concentrations of 5-aminosalicylic acid (5-ASA) resulting from different pharmaceutical formulations and analyse the influence of inflammation on the mucosal concentrations.
METHODS: The study included 130 inflammatory bowel disease (IBD) patients receiving 5-ASA as pH-dependent-release formulations (73 patients), time-dependent-release formulations (11 patients), or pro-drugs (18 patients). In addition, 28 patients were receiving topical treatment (2-4 g/d) with pH-dependent-release formulations. Endoscopic biopsies were obtained from the sigmoid region during the colonoscopy. The 5-ASA concentrations (ng/mg) were measured in tissue homogenates using high-pressure liquid chromatography with electrochemical detection. The t test and Mann-Whitney test, when appropriate, were used for statistical analysis.
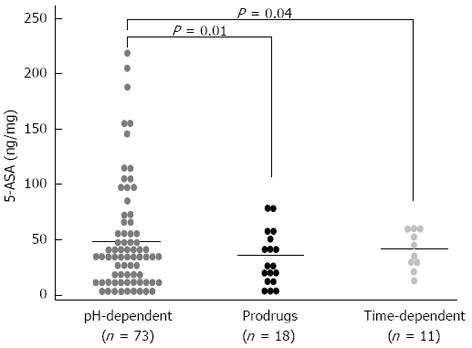
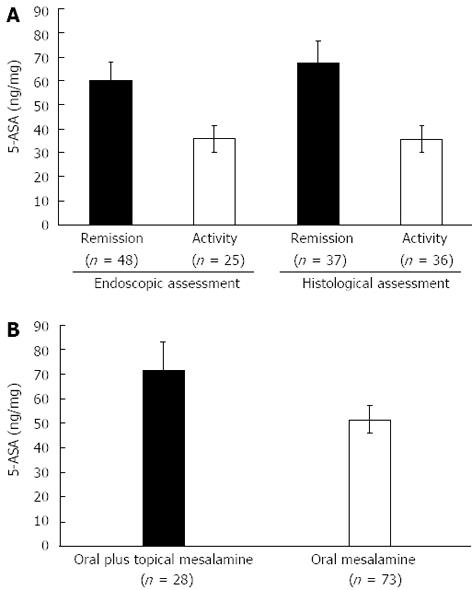
RESULTS: Patients receiving pH-dependent-release formulations showed significantly higher mucosal concentrations of 5-ASA (51.75 ± 5.72 ng/mg) compared with patients receiving pro-drugs (33.35 ± 5.78 ng/mg, P = 0.01) or time-dependent-release formulations (38.24 ± 5.53 ng/mg, P = 0.04). Patients with endoscopic remission had significantly higher mucosal concentrations of 5-ASA than patients with active disease (60.14 ± 7.95 ng/mg vs 35.66 ± 5.68 ng/mg, P = 0.02). Similar results were obtained when we compared patients with the histological appearance of remission and patients with active histological inflammation (67.53 ± 9.22 ng/mg vs 35.53 ± 5.63 ng/mg, P < 0.001). Significantly higher mucosal concentrations of 5-ASA were detected in patients treated with both oral and topical treatments in combination compared with patients who received oral treatment with pH-dependent-release formulations alone (72.33 ± 11.23 ng/mg vs 51.75 ± 5.72 ng/mg, P = 0.03).
CONCLUSION: IBD patients showed significant variability in mucosal 5-ASA concentrations depending on the type of formulation, and the highest mean concentration was achieved using pH-dependent-release formulations.
Core tip: We report on the concentrations of 5-aminosalicylic acid in the colonic mucosa of ulcerative colitis patients. Significant variations in concentration were observed that were dependent on the type of pharmaceutical formulation and the presence of active disease. Combined oral and topical therapy yielded higher tissue mesalamine concentrations. These differences should be taken into account in treatment strategies, especially in view of the fact that mesalamine can induce mucosal healing in ulcerative colitis.
- Citation: D’Incà R, Paccagnella M, Cardin R, Pathak S, Baldo V, Giron MC, Sturniolo GC. 5-ASA colonic mucosal concentrations resulting from different pharmaceutical formulations in ulcerative colitis. World J Gastroenterol 2013; 19(34): 5665-5670
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5665
Mesalamine[5-aminosalicylic acid (5-ASA)]-containing formulations represent the first-line therapy for the treatment of mild to moderate active ulcerative colitis and the prevention of recurrence[1]. When 5-ASA is administered and is absorbed by the colonic epithelium, N-acetyltransferase 1 metabolises a large amount of the 5-ASA to N-Ac-5-ASA, an inactive metabolite that is secreted back into the intestinal lumen and excreted in the faeces[2]. Sulphasalazine (Salazopyrin EN) is a pro-drug composed of sulphapyridine and 5-ASA connected by an azo-bond. Salazopyrin is metabolised to sulphapyridine and 5-ASA by the bacterial azoreductases of the intestinal microbiota. Sulphapyridine is excreted in the urine after most of it is absorbed from the colon, acetylated in the liver, and conjugated with glucuronic acid. The main action of sulphapyridine is to carry the 5-ASA moiety to the colon while preventing its proximal absorption. Absorption through the colon is necessary for the efficacy of 5-ASA[3]. Side effects, such as nausea, heartburn, headache, anaemia, skin rashes, reversible abnormalities of sperm number and morphology, and, rarely, hepatitis and nephritis, occur primarily due to high plasma sulphapyridine concentrations, which can generally be detected in patients taking higher doses of sulphasalazine or in genetically predisposed individuals (slow acetylators)[4]. Alternative preparations include modified-release formulations (which are supplied with pharmacological coatings that dissolve at a given pH or in a time-dependent manner) and pro-drugs. In pro-drugs such as sulphasalazine, an azo-bond links 5-ASA molecules to a carrier molecule. Similar compounds include olsalazine and balsalazide. Olsalazine was the first formulation, and it contains two 5-ASA molecules linked by an azo-bond. Approximately 12%-16% of patients being treated with olsalazine may suffer from secretory diarrhoea[5-7]. Balsalazide consists of 5-ASA linked via an azo-bond to an internal carrier (4-aminobenzoyl-β-alanine). This formulation is not systemically absorbed. Modified-release formulations include delayed-release formulations (which release 5-ASA along a pH gradient) and sustained-release formulations (which release 5-ASA over a specified time interval) that are targeted to release 5-ASA in the lower small intestine and right colon. The pH-sensitive acrylic resin coat of Eudragit dissolves when the luminal pH rises above a critical value. Pentasa is a sustained-release formulation that is gradually released based on a time-controlled mechanism. It consists of ethylcellulose-coated microgranules from which mesalazine is released into the small and large intestine. Its ethylcellulose coating is a semi-permeable membrane that dissolves when hydrated[8]. Combination therapy with oral and topical mesalazine administration can achieve higher mucosal concentrations than oral treatment alone[9]. Several in vitro studies have established a direct dose-effect relationship between 5-ASA and most of its immuno-inflammatory targets[10]. Furthermore, in vivo studies have demonstrated that there are inverse relationships between mucosal 5-ASA concentrations and the endoscopic and histological scores and mucosal levels of sIL-2R (a marker of mucosal inflammation). Higher drug mucosal concentrations lead to lower disease activity[11]. It follows that inadequate mucosal concentrations will result in inadequate disease management, particularly in patients with Crohn’s disease and especially for the prevention of post-operative recurrence[12]. As a result, we can state that the therapeutic efficacy of 5-ASA is directly related to its mucosal concentration. Nevertheless, a large degree of individual variability in mucosal mesalamine concentrations exists, which is possibly due to differences in intestinal behaviour, dosage, route of administration, and the severity of the colonic inflammation[13-16].
In this study, we focused on different pharmaceutical formulations of 5-ASA.
The study included 130 consecutive ulcerative colitis patients (mean age 47.76 years, range 23-84 years; 81 men and 49 women) who were referred to the Department of Surgical, Oncological and Gastroenterological Sciences, Gastroenterology Unit on continuous oral 5-ASA treatment. The general characteristics of the patients are shown in Table 1. All of the patients were receiving treatment with oral 5-ASA three times per day in one of three different pharmaceutical formulations: pH-dependent delayed-release formulations (73 patients at a dose of 2.4 g daily; Asacol Giuliani-Bracco Italy, Pentacol Sofar Italy), mesalamine pro-drug (18 patients at a dose of 3 g daily; Salazopyrin EN, Pfizer, Italy), and time-dependent sustained-release formulations (11 patients at a dose of 3 g daily; Pentasa, Ferring, Italy). There were 28 patients who received both oral and topical (2-4 g/d by enema) pH-dependent-release formulations. The patients receiving combined treatment (mean age 46.5 years, range 23-79 years; 64.2% male) were comparable with respect to age and gender distribution to patients receiving oral therapy alone (mean age 47.24 years, range 25-84 years; 67.1% male). No concomitant immunological, renal, or hepatic disorders were reported by any of the patients. Moreover, none of the patients were taking steroids, immunosuppressive agents, antibiotics, H2-receptor antagonists, or proton pump inhibitors. Colonoscopy was performed for surveillance or to detect symptom re-exacerbation. Bowel cleansing was achieved using a polyethylene glycol oral solution, 3-5 L, on the day before the colonoscopy. After the patients provided their informed consent, the time at which they took their last pill/enema was recorded, and two biopsies were taken from the sigmoid region at 25 cm from the anal verge. The observation of inflammatory changes in the colonic mucosa on endoscopy was considered endoscopic activity following the Baron classification, while the absence of mucosal inflammatory changes was considered endoscopic remission[17,18]. The histological activity of the disease was examined according to a semi-quantitative score that took into consideration the extent of lymphocytic and polymorphonuclear leukocyte infiltration, mucus depletion, crypt distortion, the presence of crypt abscesses, and lymphoid follicle activation. Histological remission was defined as the absence of inflammatory changes in the mucosa[19].
| Characteristics | pH-dependent delayed release (n= 73) | Pro-drugs (n= 18) | Time-dependent sustained release (n= 11) |
| Age (mean ± SE) (yr) | 47.24 ± 1.61 | 51.38 ± 2.39 | 47.54 ± 4.97 |
| Gender (M/F) | 49/24 | 8/10 | 6/5 |
| Extent of disease | |||
| Proctosigmoiditis | 22% | 22% | 0% |
| Left colitis | 10% | 11% | 0% |
| Pancolitis | 68% | 67% | 100% |
| Age at diagnosis (mean ± SE) | 34.88 ± 1.61 | 32.72 ± 2.30 | 31.09 ± 3.30 |
| Duration of disease (yr) | 11.56 ± 0.81 | 17.22 ± 4.21 | 15.00 ± 2.62 |
| Time since last 5-ASA administration (h) | 21.43 ± 1.22 | 23.88 ± 4.21 | 20.63 ± 2.52 |
Purified 5-ASA was obtained from Acros (NJ, United States). Purified water and methanol were used for the high-pressure liquid chromatography (HPLC) analysis. The use of these products was important to reduce background current and noise within the HPLC-electrochemical detection system.
A stock solution of 5-ASA was prepared at 1 mg/mL in 0.2 mol/L protocatechuic acid (PCA), 100 μmol/L ethylenediaminetetraacetic acid (EDTA), and 100 μmol/L sodium metabisulfite and stored at 4 °C.
The specimens were homogenised in 0.2 mol/L PCA, 100 μmol/L EDTA, and 100 μmol/L sodium metabisulfite at 4 °C and then centrifuged (1800 g) for 10 min. The supernatants were collected and filtered with 0.2-μm cellulose acetate filters and stored at -80 °C. A sample volume of 20 μL was used throughout this study.
A sensitive HPLC method capable of measuring the mucosal 5-ASA concentrations was used. Briefly, analyses were performed on a chromatographic apparatus (Alliance Waters, United States) that consisted of a model 2695 solvent-delivery system and an electrochemical detector, Coulochem (ESA, United States) Model 5100A, that was integrated with Empower Software (Waters, United States).
Separation of the analytes was achieved using a reversed-phase DHBA-250 column (5 μm, 250 × 3.0 mm), and the analytes were detected on a high-sensitivity analytical cell model 5011, with the oxidation potentials of electrodes 1 and 2 adjusted to +750 mV to oxidise the 5-ASA.
The mobile phase consisted of 50 mmol/L sodium acetate, 50 mmol/L sodium citrate, 8% methanol, and 2% 2-propanol. The pH of the mobile phase was adjusted to 2.5 with phosphoric acid after the addition of the organic modifiers. The mobile phase was passed through the system at 0.5 mL/min.
The standard curve for 5-ASA was linear in the selected range (r2 = 0.99) with an inter-assay coefficient of variation < 4%.
The mucosal 5-ASA concentrations in patients being treated with any pharmaceutical 5-ASA formulation and in patients with different endoscopic and histological degrees of activity were compared using an unpaired Student’s t test and the Mann-Whitney test where appropriate. A P value of 0.05 or less was consideredsignificant. The data are presented as the mean ± SE. The 5-ASA concentrations are expressed as ng/mg tissue. The statistical analyses were performed using the statistical software package SPSS for Windows, version 13.0 (SPSS, Chicago, IL, United States).
The demographic and clinical characteristics of the patients being treated with different pharmaceutical formulations were similar (Table 1). Figure 1 shows the distribution of the mucosal concentrations of mesalamine in the sigmoid region for the three groups studied. The mean mucosal mesalamine concentration was significantly higher in patients being treated with pH dependent-release formulations than in patients being treated with pro-drugs (51.75 ± 5.72 ng/mg vs 33.35 ± 5.78 ng/mg, P = 0.01). Similarly, the concentration of mesalamine was significantly higher in patients being treated with pH-dependent-release formulations than in patients being treated with time-dependent-release formulations (51.75 ± 5.72 ng/mg vs 38.24 ± 5.53 ng/mg, P = 0.04). Furthermore, the absolute mucosal 5-ASA concentrations were significantly higher in patients being treated with pH-dependent-release formulations; specifically, 28% of the patients were found to have mucosal 5-ASA concentrations above 70 ng/mg of tissue, which was the highest concentration achieved with any of the other formulations. Figure 2A shows the distribution of the mucosal mesalamine concentrations in patients receiving pH-dependent-release formulations according to endoscopic and histological activity or disease remission.
Twenty-five patients showed active disease in the sigmoid colon, while the remaining 48 patients presented an endoscopic appearance of remission or a normal assessment. The histological grade of the mucosal inflammation in the sigmoid colon was “active” in 36 patients, while the remaining 37 patients presented a “normal” histological assessment or the appearance of remission. Patients with a normal endoscopic assessment or an endoscopic appearance of remission showed significantly higher concentrations of mucosal 5-ASA than patients with active endoscopic inflammation (60.14 ± 7.95 ng/mg vs 35.66 ± 5.68 ng/mg, P = 0.02). Similarly, significant differences were found in the mucosal 5-ASA concentrations between patients with a normal histological assessment or the histological appearance of remission and patients with active histological inflammation (67.53 ± 9.22 ng/mg vs 35.53 ± 5.63 ng/mg, P < 0.001).
Twenty-eight patients received both pH-dependent mesalamine orally at a dose of 2.4 g/d and rectal mesalamine at a dose of 4 g/d. Figure 2B shows the distribution of mucosal 5-ASA concentrations in patients receiving combination treatment compared with those in patients receiving oral treatment alone. The mean mucosal 5-ASA concentration was significantly higher in patients receiving combined oral and topical treatment than in patients taking oral mesalamine only (72.33 ± 11.23 ng/mg vs 51.75 ± 5.72 ng/mg, P = 0.03).
IBD patients usually receive chronic treatment with 5-ASA to both control the active disease and reduce the frequency and severity of clinical relapses. Although this drug has been used for the last 50 years, the precise mechanism of action of 5-ASA, with the exception of its topical efficacy, is unknown[20]. However, its topical activity implies the necessity of its delivery to the inflamed tissue. Therefore, appropriate dosages and targeted delivery of the drug are needed. Many studies have demonstrated marked variability in 5-ASA metabolism and distribution following oral dosing[14,15]. Thus, the clinical course of the disease, which encompasses periods of prolonged remission and frequent episodes of relapse, could derive from variable availability of the drug. We know from several in vitro studies that there is a direct relationship between the 5-ASA concentration and its therapeutic efficacy[21,22]. Previous in vivo studies have reported that the therapeutic efficacy is dose-dependent. In fact, high mucosal mesalamine concentrations have been shown to be associated with endoscopic and histological scores of disease remission or mild activity rather than moderate or severe disease in ulcerative colitis. They have also been shown to be associated with a reduced risk of severe post-operative recurrences in Crohn’s disease[11,12]. However, the appropriate mucosal concentration is unknown, and it is therefore not easy to provide guidance. There is wide inter-individual variability in mucosal concentrations, and the factors governing tissue drug concentrations are largely unknown because increased mucosal concentrations do not always derive from increased oral doses[23].
Adherence to therapy may be another important factor that influences mucosal concentrations. We did not test adherence specifically; however, samples were obtained only from patients who reported that they had taken their last pill within 24 h of endoscopy. Moreover, the 5-ASA concentration can be variable along the entire length of the colon. Oral administration of 5-ASA ensures a higher drug concentration in the right colon than in the rectum, where the amount often becomes negligible; however, the rectum is the preferential site of the disease and is almost invariably affected. Because the oral dose is not strictly related to the 5-ASA concentration, the tissue absorption, drug metabolism and excretion, and pharmaceutical variables, such as the route of administration and formulation type, need to be investigated. Several clinical studies have confirmed that the highest therapeutic efficacy is reached when patients are treated with oral and topical treatments[24,25]. Patients with active distal disease in whom oral treatment is frequently inadequate respond to mesalazine enemas[9]. Rectal formulations are also successful in maintaining remission in patients suffering from frequent relapses. According to Frieri et al[26], patients being treated with oral and topical treatments show similar mucosal mesalamine concentrations in the rectum and in the descending colon, while oral treatment alone results in a higher drug concentration in the descending colon than in the rectum. Indeed, we found higher mucosal concentrations in the sigmoid mucosa in patients receiving both topical and oral 5-ASA than in patients receiving oral mesalamine alone. It is possible that the level of adherence may have been higher in patients experiencing active disease who therefore were receiving combined therapy.
To date, few and discordant reports have investigated the relationship between the colonic mucosal concentration of 5-ASA and its different pharmaceutical formulations[27,28]. We demonstrated that the sigmoid mucosal concentration of 5-ASA was significantly higher in IBD patients receiving pH-dependent delayed-release formulations compared with patients receiving preparations dependent on bacterial degradation (pro-drugs). Similarly, the mucosal concentration of 5-ASA in the sigmoid mucosa of the pH-dependent delayed-release formulations group was higher than that of the time-dependent sustained-release formulations. Moreover, we found that the absolute mucosal 5-ASA concentrations were significantly higher in patients being treated with pH-dependent-release formulations than in patients being treated with pro-drugs. In fact, in 28% of the patients receiving pH-dependent-release formulations, the mucosal 5-ASA concentration was above 70 ng/mg of tissue, which was the highest value achieved from any of the formulations. Because of the dose-related anti-inflammatory effect of 5-ASA, we should expect the highest efficacy when the highest mucosal tissue concentration of 5-ASA is achieved. As reported by Hussain et al[23], rectal mucosal concentrations of aminosalicylates are lower during relapses. As previously demonstrated by Frieri et al[11], we confirmed that the colonic mucosal concentrations of 5-ASA were inversely related to disease activity as measured by both endoscopic and histological evaluation. Oedema occurring during the active phase of the disease may account for a possible dilution effect on the biopsy specimens. Alternatively, the faster rate of tissue renewal in the presence of inflammation could produce a washout effect on the drug and contribute to a reduced mucosal mesalamine concentration.
In conclusion, because we have reinforced the relationship between tissue mesalamine levels and disease activity, additional studies are needed to determine how to reach optimal mucosal concentrations of 5-ASA. We have demonstrated that different pharmaceutical preparations achieve different mucosal concentrations and that disease activity lowers the drug mucosal concentration. Higher dosages could therefore be justified during active disease to obtain better clinical results.
The use of mesalamine represents the first-line treatment strategy in patients with ulcerative colitis. Many formulations are available, and they are often used interchangeably because it is assumed that they are all equally effective.
The mucosal concentration of 5-aminosalicylic acid (5-ASA) was measured in the colon of ulcerative colitis patients and at the anastomotic site of Crohn’s disease patients in the post-operative setting. In this study, the authors demonstrated that tissue concentrations could be measured by high-pressure liquid chromatography and that the type of 5-ASA formulation influenced the mucosal concentration.
Mesalamine is poorly absorbed; therefore, blood monitoring is not helpful. The tissue concentration may represent a better tool for tailoring therapy in ulcerative colitis patients.
The response to treatment with mesalamine in ulcerative colitis patients can be optimised by administering the proper formulation of the drug at the right dose to the patient.
The authors examined the colonic mucosal concentrations of mesalamine in patients with ulcerative colitis who were being treated with different formulations of mesalamine. The results are interesting and may guide clinicians in tailoring therapies to their patients.
P- Reviewers de Barreiro-de Acosta MB, Tsujikawa T S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 372] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 2. | Vree TB, Dammers E, Exler PS, Sörgel F, Bondesen S, Maes RA. Liver and gut mucosa acetylation of mesalazine in healthy volunteers. Int J Clin Pharmacol Ther. 2000;38:514-522. [PubMed] |
| 3. | Azadkhan AK, Truelove SC, Aronson JK. The disposition and metabolism of sulphasalazine (salicylazosulphapyridine) in man. Br J Clin Pharmacol. 1982;13:523-528. [PubMed] |
| 4. | Chen M, Xia B, Chen B, Guo Q, Li J, Ye M, Hu Z. N-acetyltransferase 2 slow acetylator genotype associated with adverse effects of sulphasalazine in the treatment of inflammatory bowel disease. Can J Gastroenterol. 2007;21:155-158. [PubMed] |
| 5. | Kruis W, Brandes JW, Schreiber S, Theuer D, Krakamp B, Schütz E, Otto P, Lorenz-Mayer H, Ewe K, Judmaier G. Olsalazine versus mesalazine in the treatment of mild to moderate ulcerative colitis. Aliment Pharmacol Ther. 1998;12:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Wright JP, O’Keefe EA, Cuming L, Jaskiewicz K. Olsalazine in maintenance of clinical remission in patients with ulcerative colitis. Dig Dis Sci. 1993;38:1837-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Kruis W, Judmaier G, Kayasseh L, Stolte M, Theuer D, Scheurlen C, Hentschel E, Kratochvil P. Double-blind dose-finding study of olsalazine versus sulphasalazine as maintenance therapy for ulcerative colitis. Eur J Gastroenterol Hepatol. 1995;7:391-396. [PubMed] |
| 8. | Clemett D, Markham A. Prolonged-release mesalazine: a review of its therapeutic potential in ulcerative colitis and Crohn’s disease. Drugs. 2000;59:929-956. [PubMed] |
| 9. | Frieri G, Pimpo MT, Palumbo GC, Onori L, Viscido A, Latella G, Galletti B, Pantaleoni GC, Caprilli R. Rectal and colonic mesalazine concentration in ulcerative colitis: oral vs. oral plus topical treatment. Aliment Pharmacol Ther. 1999;13:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 10. | Pullman WE, Doe WF. IL-2 production by intestinal lamina propria cells in normal inflamed and cancer-bearing colons. Clin Exp Immunol. 1992;88:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Frieri G, Giacomelli R, Pimpo M, Palumbo G, Passacantando A, Pantaleoni G, Caprilli R. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut. 2000;47:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Frieri G, Pimpo MT, Andreoli A, Annese V, Comberlato M, Corrao G, Palumbo G, Sturniolo GC, Tonelli F, Caprilli R. Prevention of post-operative recurrence of Crohn’s disease requires adequate mucosal concentration of mesalazine. Gruppo Italiano per lo Studio del Colon e del Retto. Aliment Pharmacol Ther. 1999;13:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Klotz U, Maier KE. Pharmacology and pharmacokinetics of 5-aminosalicylic acid. Dig Dis Sci. 1987;32:46S-50S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Brunner M, Lackner E, Exler PS, Fluiter HC, Kletter K, Tschurlovits M, Dudczak R, Eichler HG, Müller M. 5-aminosalicylic acid release from a new controlled-release mesalazine formulation during gastrointestinal transit in healthy volunteers. Aliment Pharmacol Ther. 2006;23:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003;17:29-42. [PubMed] |
| 16. | Schwab M, Klotz U. Pharmacokinetic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2001;40:723-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Ardizzone S, Bianchi Porro G. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252:475-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Hirai F, Matsui T, Aoyagi K, Inoue N, Hibi T, Oshitani N, Fujii H, Kobayashi K, Suzuki Y, Tanaka S. Validity of activity indices in ulcerative colitis: comparison of clinical and endoscopic indices. Dig Endosc. 2010;22:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Jenkins D, Balsitis M, Gallivan S, Dixon MF, Gilmour HM, Shepherd NA, Theodossi A, Williams GT. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol. 1997;50:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Brogden RN, Sorkin EM. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 1989;38:500-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Stevens C, Lipman M, Fabry S, Moscovitch-Lopatin M, Almawi W, Keresztes S, Peppercorn MA, Strom TB. 5-Aminosalicylic acid abrogates T-cell proliferation by blocking interleukin-2 production in peripheral blood mononuclear cells. J Pharmacol Exp Ther. 1995;272:399-406. [PubMed] |
| 22. | Mahida YR, Lamming CE, Gallagher A, Hawthorne AB, Hawkey CJ. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991;32:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Hussain FN, Ajjan RA, Riley SA. Dose loading with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters. Br J Clin Pharmacol. 2000;49:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Harris MS, Lichtenstein GR. Review article: delivery and efficacy of topical 5-aminosalicylic acid (mesalazine) therapy in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2011;33:996-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Ford AC, Khan KJ, Achkar JP, Moayyedi P. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in Ulcerative Colitis: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:167-176; author reply 177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Frieri G, Pimpo M, Galletti B, Palumbo G, Corrao G, Latella G, Chiaramonte M, Caprilli R. Long-term oral plus topical mesalazine in frequently relapsing ulcerative colitis. Dig Liver Dis. 2005;37:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Forbes A, Cartwright A, Marchant S, McIntyre P, Newton M. Review article: Oral, modified-release mesalazine formulations--proprietary versus generic. Aliment Pharmacol Ther. 2003;17:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Naganuma M, Iwao Y, Ogata H, Inoue N, Funakoshi S, Yamamoto S, Nakamura Y, Ishii H, Hibi T. Measurement of colonic mucosal concentrations of 5-aminosalicylic acid is useful for estimating its therapeutic efficacy in distal ulcerative colitis: comparison of orally administered mesalamine and sulfasalazine. Inflamm Bowel Dis. 2001;7:221-225. [PubMed] |