Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5347
Revised: June 3, 2013
Accepted: July 18, 2013
Published online: August 28, 2013
Processing time: 168 Days and 5.7 Hours
AIM: To determine the efficacy profiles of different concentrations of Lactobacillus acidophilus (L. acidophilus) for treating colitis using an experimental murine model.
METHODS: Colitis was established in 64 BALB/c mice by adding 5% dextran sodium sulfate (DSS) to the drinking water and allowing ad libitum access for 7 d. The mice were then randomly divided into the following control and experimental model groups (n = 8 each; day 0): untreated model control; negative-treatment model control (administered gavage of 1 mL/10 g normal saline); experimental-treatment models C4-C8 (administered gavage of 104, 105, 106, 107, or 108 CFU/10 g L. acidophilus, respectively); positive-treatment model control (administration of the anti-inflammatory agent prednisone acetate at 45 μg/10 g). Eight mice given regular water (no DSS) and no subsequent treatments served as the normal control group. Body weight, fecal traits, and presence of fecal occult blood were assessed daily. All animals were sacrificed on post-treatment day 7 to measure colonic length, perform histological scoring, and quantify the major bacteria in the proximal and distal colon. Intergroup differences were determined by one-way ANOVA and post-hoc Student-Newman-Keuls comparison.
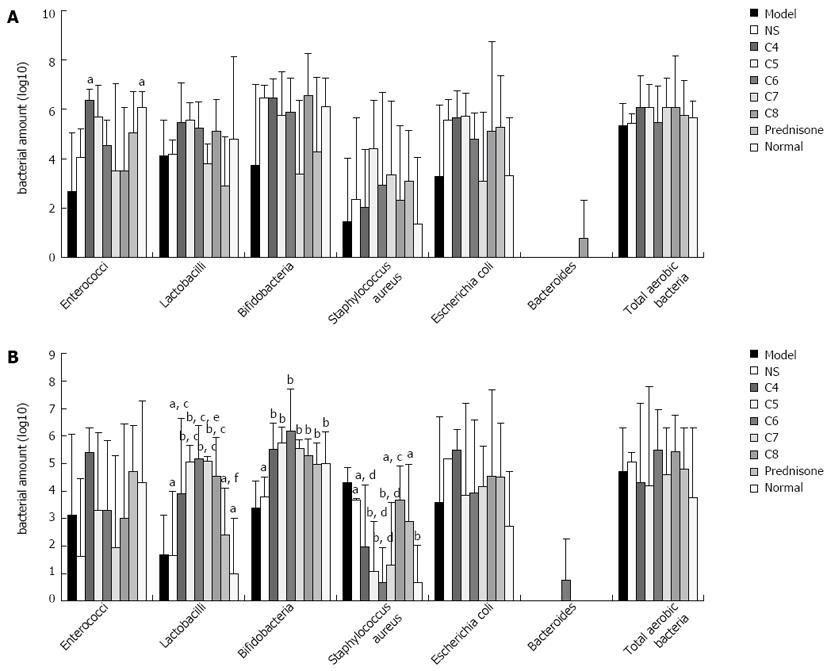
RESULTS: All treatments (L. acidophilus and prednisone acetate) protected against colitis-induced weight loss (P < 0.05 vs model and normal control groups). The extent of colitis-induced colonic shortening was significantly reduced by all treatments (prednisone acetate > C4 > C5 > C7 > C8 > C6; P < 0.05 vs untreated model group), and the C6 group showed colonic length similar to that of the normal control group (P > 0.05). The C6 group also had the lowest disease activity index scores among the model groups. The bacterial profiles in the proximal colon were similar between all of the experimental-treatment model groups (all P > 0.05). In contrast, the bacterial profile in the distal colon of the C6 group showed the distinctive features (P < 0.05 vs all other experimental-treatment model groups) of Lactobacillus sp. and Bifidobacterium sp. being the most abundant bacteria and Staphylococcus aureus being the least abundant bacteria.
CONCLUSION: The most therapeutically efficacious concentration of L. acidophilus (106 CFU/10 g) may exert its effects by modulating the bacterial profile in the distal colon.
Core tip: Efficacies of the current treatments for ulcerative colitis (UC) are limited by procedure-related complications, poor patient compliance, and high relapse rates. Administration of supplemental probiotics represents a promising new therapy of UC. Since UC pathogenic sites mainly involve the rectum and colon and UC patients show differential composition profiles of intestinal bacteria, this study was designed to evaluate the therapeutic efficacies of various concentrations for the standard probiotic, Lactobacillus acidophilus, using a well-known murine model of experimental colitis to examine the changes in colitis symptoms and the corresponding effects on the bacterial flora in the distal and proximal colon.
-
Citation: Chen LL, Zou YY, Lu FG, Li FJ, Lian GH. Efficacy profiles for different concentrations of
Lactobacillus acidophilus in experimental colitis. World J Gastroenterol 2013; 19(32): 5347-5356 - URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5347.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5347
Ulcerative colitis (UC), a non-specific chronic inflammatory bowel disease, has emerged as a significant human health burden in Western countries and its prevalence is rising worldwide. As such, extensive research efforts have focused on determining its pathogenic mechanisms and developing efficacious therapies. While these studies have helped to define the clinical course of UC, identification of a safe and effective treatment has remained elusive. The primary drug therapies currently in use, including the anti-infective salazosulfamide, anti-inflammatory glucocorticoids and immunosuppressant probiotics, are limited by considerable side-effects, which lead to poor patient compliance and may contribute to the high relapse rates of UC[1].
Four fundamental components underlying UC pathogenesis have been identified, which represent likely sources for the yet undefined etiological factors: environment, microbiota, immune system, and genome[2,3]. A large number of experimental studies using animal models and clinical studies of human UC subjects have demonstrated that the intestinal microbiota, in particular, plays an important role in both UC onset and progression[4-7]. Moreover, sterile conditions (i.e., germfree environments) have been shown to induce UC in mice and differential distributions of specific bacteria (i.e., Campylobacter sp.) have been correlated with UC in adult humans[8,9]. Interactions between the microbiota and the immune system are well-described and recognized for their critical roles in normal physiological processes; accordingly, aberrant development and response of the immune system related to the microorganism environment in the gut, have been associated with UC[2,10].
The profile of normal human intestinal flora consists of about 30 genera of bacteria, representing hundreds of species and unknown thousands of strains. The most abundant species are anaerobic (including Bifidobacteria, Lactobacilli and Bacteroides), among which the Lactobacillus sp. appear to be the predominant flora, especially in the colon. Furthermore, quantitative analysis has indicated that many of these anaerobes are present at concentrations between 109-1012 CFU/mL. Many of these anaerobes, such as Lactobacillus sp. and Bifidobacterium sp., are characterized as probiotics, exerting beneficial effects on the human body. Indeed, detection of latent pathogenic species, such as Clostridium and Staphylococcus, is rare under normal physiological conditions[4].
Probiotics are considered a promising alternative therapy for UC. To date, Lactobacilli, Bifidobacteria, VSL#3 (a compound probiotic preparation composed of four Lactobacilli strains, three Bifidobacteria strains, and one Streptococcus salivarius strain), and Escherichia coli (E. coli) Nissle 1917 have been applied to UC subjects (both animal models and human cases) and shown to effectively resolve disease symptoms[11-14]. In addition, our laboratory showed that administration of Lactobacillus acidophilus (L. acidophilus) at the early stage is an effective therapy for UC but that administration of different probiotics does not provide the same efficacies[15,16]. These results suggest that the distinctive features of different bacteria, including their secretory functions and interactions with other bacteria and the host system, may have significant functional implications for their therapeutic efficacies in specific host tissues. Thus, while it is possible that introducing a large amount of a probiotic (or a mixture of probiotics) may beneficially impact the overall profile of intestinal bacteria, such an effort may also provide no benefit or be detrimental, possibly promoting latent pathogenicity, in specific intestinal regions.
Since UC pathogenic sites mainly involve the rectum and colon[3] and UC patients show differential composition profiles of intestinal bacteria[17,18], we hypothesize that development of probiotic therapy as an effective UC treatment modality will depend upon the particular probiotic’s effects at a specific tissue site, dosage concentration, and relationship to other flora. Thus, the current study was designed to evaluate the efficacy profiles of different concentrations (104-108/10 g body weight) of the probiotic L. acidophilus in the proximal and distal colon of a well-established murine model of experimental colitis.
L. acidophilus was isolated from a normal human intestinal tract SMC-S095 sample and sequence-identified by our laboratory. After culturing under anaerobic conditions with MRS medium for 24 h, the bacteria was collected, quantified by a spectrophotometer, and diluted with normal saline (NS) to 1010 CFU/mL.
Seventy-two female BALB/c mice (6-8-wk-old, 20.0 ± 2.0 g mean body weight) were purchased from Hunan Agricultural University and housed under standard conditions (50% ± 10% humidity, 12 h light/dark cycle, ad libitum access to standard mouse chow). Colitis was established in 64 of the mice by adding 5% dextran sodium sulfate (DSS, MW 50000; Sigma Corp, St. Louis, MO) to the drinking water and allowing ad libitum access for 7 d. The mice were then randomly divided into the following control and experimental model groups (n = 8 each; day 0): untreated model control; negative-treatment model control (administered gavage of 1 mL/10 g normal saline); experimental-treatment models C4-C8 (administered gavage of 104, 105, 106, 107, or 108 CFU/10 g L. acidophilus, respectively); positive-treatment model control (administration of the anti-inflammatory agent prednisone acetate at 45 μg/10 g). Eight mice given regular water (no DSS) and no subsequent treatments served as the normal control group.
Body weight, fecal traits, presence of fecal occult blood, and disease activity index (DAI) scores were assessed daily, as previously described[19]. On post-treatment day 7, all mice were sacrificed by ether anesthesia overdose. The resected colon was measured (length-wise). Colonic segments (0.5 cm) were obtained starting from 1 cm to the ileocecus to 1 cm to the anus and used for bacterial analysis (described below). The remaining colonic segments approximately 0.5 cm to the anus were fixed in neutral formalin, prepared as paraffin-embedded sections, stained with hematoxylin and eosin (HE), and subjected to histological analysis by light microscopy and the damage scoring procedure described by Dieleman et al[20].
Colonic segments were weighed, homogenized, serially diluted in NS, and used to inoculate nonselective and selective culture mediums. For each animal, 10 L of intestinal fluid (100-10-5L. acidophilus concentration) was collected and also inoculated in corresponding media. After culturing, three isolates each of anaerobic bacteria (Lactobacilli, Bifidobacteria, and Bacteroides) and aerobic bacteria [Staphylococcus aureus (S. aureus), E. coli, and Enterococci], as well as a sample of total aerobic bacteria, were selected for further analysis.
The anaerobic bacteria were incubated in the BAC.III-IE anaerobic workstation (Shel Lab, Cornelius, OR) at 37 °C for 48-72 h using the appropriate medium (Lactobacilli, LBS medium; Bifidobacteria, BS medium; Bacteroides, BDS medium; total aerobic bacteria, ordinary nutrition agar medium). The aerobic bacteria were incubated in the BSG-4 biochemical incubator (WanTong Precision Instruments Co., Ltd, Wuhan, China) at 37 °C for 24-48 h using the appropriate medium (S. aureus, high-salt mannitol medium; E. coli, eosin-methylene blue medium; Enterococci, TTC sodium azide medium). The resultant bacterial colonies were counted and converted to CFU/g by the following formula: number of bacterial colonies × [(diluted liquid volume + sample weight)/sample weight] × dilution multiple.
Preliminary identification was performed on colonies of different bacteria according to morphological and Gram staining characteristics detected by light microscopy.
Identification of anaerobic bacteria (Lactobacilli and Bifidobacteria): Using Bifidobacteria as an example, the bacterial colonies of various forms were obtained from the BS medium, cultured respectively in MRS liquid culture medium. Resultant colonies were analyzed by light microscopy to confirm normal homogeneous morphology of Bifidobacteria, and isolates were inoculated onto MRS solid culture medium and cultured in the anaerobic incubator at 37 °C for 24 h. The resultant colonies were rescreened. After several generations’ of serial cloning, the bacterial colonies with normal homogeneous appearance and morphology were selected for bacterial identification via the API20 A test (bioMérieux Vitek, Inc., Hazelwood, MO), according to the manufacturer’s instructions. Results were within the API20 Analytical Profile Index. The protocol for Lactobacillus identification was similar.
Identification of Enterococci: The serially purified bacteria isolated from TTC culture were verified as Enterococci according to results of catalase test and verified by Gram staining. A single colony was resuspended in 0.3 mL sterile water, inoculated on a sheep blood agar plate, and incubated in the biochemical incubator at 37 °C for 24 h. One of the resultant colonies was selected for testing with the API20 Strep test (bioMérieux Vitek, Inc.), according to the manufacturer’s instructions. Results (at 4 and 24 h of culture) were within the API20 Analytical Profile Index.
All statistical analyses were carried out with the SPSS statistical software suite (version 16.0; SPSS Inc, Chicago, IL). Results are expressed as mean ± SD. Quantitative data were converted to logarithm values and tested for homogeneity of variance. If the variance was homogeneous, single-factor analysis of variance (one-way ANOVA) was used to analyze the differences between groups. Mean values with significant difference (P < 0.05) were subjected to post-hoc pairwise comparison by the Student-Newman-Keuls test.
General condition: Mice in the normal control group were alert and had sleek, healthy coats. Mice in the untreated model control and the negative-treatment model control groups were apathetic, inert, had horripilated, dry coats with blood staining around the perianal area, and showed an obviously leaner body configuration than the normal controls. All experimental-treatment model groups and the positive-treatment model group showed similar disruptions in alertness, motor function, and coat condition as their model control counterparts, but to a much lesser extent.
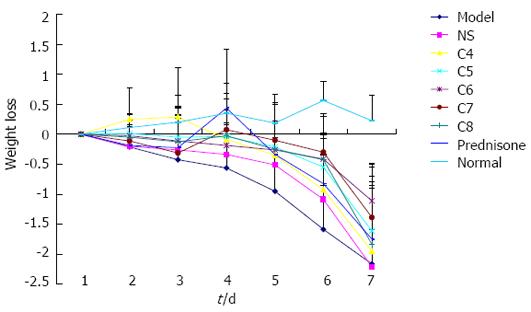
Body weight: Mice in the normal control group experienced an increase in body weight over the duration of the study course; all mice were within normal weight range at sacrifice. In contrast, all other mice experienced a decrease in body weight over the study course. The greatest weight loss occurred in the untreated model control group, and the negative-treatment model control group showed only slightly less (and statistically similar; P > 0.05) weight loss. The body weight loss experienced by all experimental-treatment model groups and the positive-treatment model group showed trends of a more gradual decline over time than that of the untreated model control group. On the day of sacrifice, the extent of body weight loss among the model groups (Figure 1) showed the following hierarchical pattern: untreated model control = negative-treatment (NS) model control > C4 > C8 > positive-treatment (prednisone acetate) model control > C5 > C7 > C6.
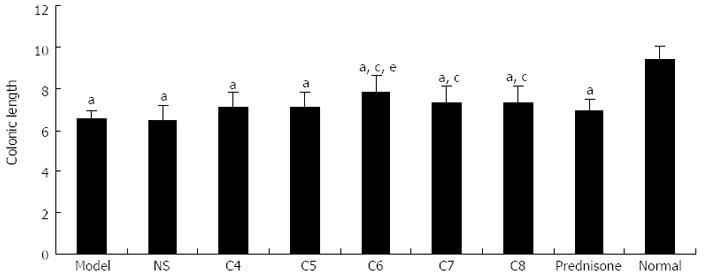
Colonic length: All model groups had significantly shorter colonic lengths than the normal control group (P < 0.05), with the greatest extent of shortening observed in the untreated model control and negative-treatment model control groups. As shown in Figure 2, the following hierarchical pattern was observed for shortening degree among the treatment model groups: positive-treatment (prednisone acetate) model control > C4 > C5 > C7 > C8 > C6. Compared to the untreated model control group, the mean colonic lengths of C7, C8, and C6 were significantly longer (P < 0.05). Among those groups, the C6 group experienced the smallest degree of colonic shortening, and its mean colonic length was similar to that of the normal control group (P > 0.05).
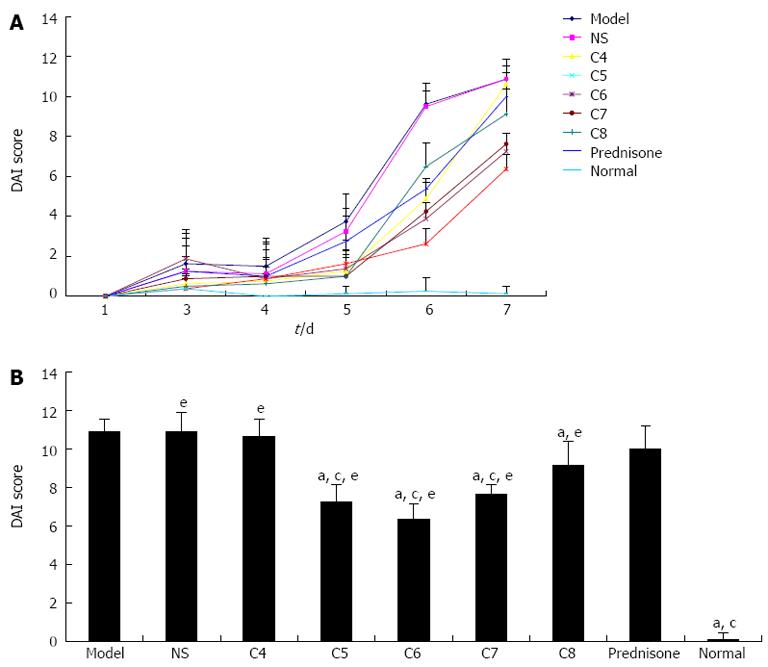
When the mean DAI score of the normal control group was set to zero, the mean DAI score of all model groups showed a trend of significantly increasing values (indicating increasing detrimental pathology) over the study course. On the day of sacrifice, the untreated model control and the negative-treatment model control groups showed the highest (and similar) DAI scores. During the first four days after treatment, no significant differences were observed between the model groups (controls and experimentals). However, starting at day 5 post-treatment, the scores of the untreated model control and the negative-treatment model control groups markedly increased, indicating that the UC in these mice was aggravated. In addition, at this time, therapeutic effects begin to appear among the -treatment model groups. As shown in Figure 3A, while all model groups showed a sharp increase in mean DAI scores at day 7, the scores for the C4 and prednisone acetate treatment groups became significantly higher than the other experimental-treatment groups and statistically similar to those of the model control groups (both P < 0.05). As shown in Figure 3B, the experimental-treatment groups showed the following hierarchical pattern of decreasing DAI scores on day 7: C8 > C7 > C5 > C6.
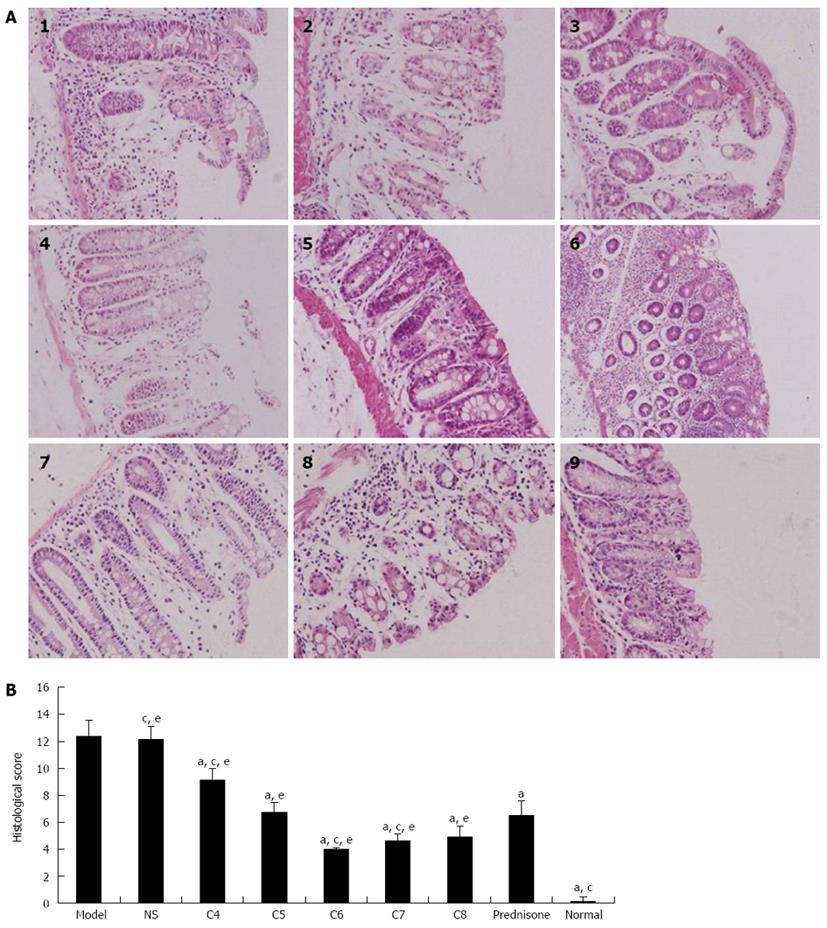
Mice in the normal control group showed normal orderly arrangement of the cellular structure of rectal and colonic tissue glands, with no obvious perturbations in goblet cell number or mucosal integrity. In contrast, mice in the untreated model control and the negative-treatment model control groups showed multifocal and deep ulcers distributed throughout the entire colon; moreover, the extent of ulceration increased along with disease severity, as evidenced by recess depth, histological perturbations, mucosal erosion, bleeding, necrosis, partially or completely damaged epithelial cell structure, and inflammation extending towards the submucosa and serosa. Mice in the experimental-treatment model groups and the positive-treatment model group also showed mucosal defects similar to but less extensive than those in the model control groups; moreover, the extent of relief of the mucosal defects varied among the different treatment models. The C6 group, in particular had the most apparent relief of colitis-induced mucosal defects, showing partially incomplete glands, rare occurrences of mucosal erosion, bleeding and necrosis, and only a small quantity of inflammatory cell infiltration (Figure 4A, panels 1-9).
The histological scores of colon damage observed in the various groups at day 7 are shown in Figure 4B. When the mean damage score of the normal control group was set to zero, the mean damage scores of all model groups showed a trend of significantly increasing values over the study course. The untreated model control and the negative-treatment model control groups had the highest mean damage scores. Compared to the untreated model control group, the mean damage scores of all experimental-treatment groups and the positive-treatment control model group were significantly lower (P < 0.05). Comparisons among the treatment model groups revealed that the mean damage scores of C6 and C7 groups were significantly lower than that of the prednisone acetate group (P < 0.05), with the C6 group having the lowest score.
The bacterial profiles detected in the proximal colons of the control, model, and treatment model groups were similar, and none of the between-group differences reached statistical significance (Figure 5A). In contrast, the bacterial profiles detected in the distal colons showed distinct features between the various groups (Figure 5B). While the most abundant bacteria in all of the profiles were Lactobacilli, Bifidobacteria and Staphylococcus, the levels of each were different. For example, the levels of Lactobacilli among the untreated, negative-treatment, and positive-treatment model control groups were significantly higher than those in the normal control group (P < 0.05) but similar to one another (P > 0.05), while the experimental-treatment groups showed the highest levels (P < 0.05 vs the untreated model control group and the positive-treatment model control group), The levels of Bifidobacteria showed the same trends as the Lactobacilli levels. The levels of S. aureus, however, were similar between the untreated model control group, the negative-treatment model control group, the positive-treatment model control group, the C4 group, and the C8 group (P > 0.05), but all five were significantly higher than that in the normal control group (P < 0.05). The C6 group showed the lowest level of S. aureus among all the model groups (P < 0.05), and this amount was not significantly different from that in the normal control group (P > 0.05). No other bacteria detected showed significantly different levels between any of the groups.
To date, investigations of the potential correlations between bacterial profiles and UC have been carried out from the perspective of disease cause (etiological factors) and resolution (therapeutic modalities). Despite the focused efforts of many experimental and clinical studies, no particular bacterial genus or species, or combined panel of such, has been identified as a causative agent of UC onset. Profiling of the organic acids metabolized by bacteria that are present in stool samples has indirectly provided some insights into this issue, suggesting that UC is likely to be related to a panel of multiple bacteria, rather than a single species or phenotype[21]. Profiling of human intestinal flora has indicated that individuals with UC have significantly higher numbers of intestinal Bacteroides sp., Streptococcus sp., and facultative anaerobes, but significantly lower numbers of Lactobacillius sp. and Bifidobacterium sp. than their healthy counterparts[21-23]. These results are considered to have clinical implications in that they suggest administration of corresponding probiotics may restore the profile of enteric microorganisms to match that of a non-UC status.
Indeed, several studies to date have evaluated the therapeutic efficacy of probiotic administration using Lactobacilli[24,25], Bifidobacteria[11,26,27], E. coli Nissle 1917[16,28], or VSL#3[15,29-31]. The degrees to which these individual supplements successfully resolved the UC varied, which led to the hypothesis that administration of a combination of probiotics may provide more benefit to the patients. In order to determine the most efficacious composition, the overall profile of bacteria present at the mucus barrier in UC needs to first be determined[32]. Another important issue that needs to be elucidated is the activities and effects of the various probiotics on the normal intestinal flora; otherwise, a suboptimal dose of any particular probiotic may negatively impact the overall efficacy of the treatment.
Gionchetti et al[30] demonstrated that high-dose VSL#3 helps to maintain the remission status achieved by surgery to treat mild pouchitis of UC; lower doses were not evaluated. An informal review of the research studies of probiotic treatment for UC published in the publicly accessible science and medical literature databases suggested that most common concentrations of probiotics used range between 106-109 CFU/mL; the lack of a standardized concentration precludes direct comparison of the results from these studies. In addition, we have found no published reports of comparative analyses to evaluate the differential effects of probiotics at varying concentrations.
L. acidophilus was chosen as the focus of the current study based upon previous results showing its therapeutic benefit for early-stage experimental colitis and its abundance in the normal human intestine[33]. The current results indicated that the therapeutic effect of L. acidophilus did not increase in a concentration-dependent manner, but revealed that a moderate-dose concentration (106 CFU/10 g) provided the most alleviation of UC symptoms, as evidenced by the significant reductions in DAI and tissue damage scores. Most importantly, this moderate-dose restored UC-related parameters to the levels in non-UC healthy control mice.
Our detailed analyses of the proximal and distal colonic intestinal flora provided further insights into the relationship between the therapeutic effects observed and the concentration of Lactobacillus in the lesion. The symptoms’ resolution mediated by the moderate-dose of L. acidophilus was accompanied by distinct and significant changes in the distal colon bacterial profile (i.e., increases in Lactobacilli and Bifidobacteria, and decreases in S. aureus). Since UC lesions frequently involve the proximal colon and rectum, and the L. acidophilus intervention used in this study mainly affected the distal colonic bacterial flora, it is possible that the different therapeutic efficacies observed for the various probiotics in previous studies may result from effects in specific tissues.
In the current study, the number of Lactobacilli detected in specific lesions did not increase in conjunction with increased concentrations of the administered L. acidophilus. Since the Lactobacilli population is composed of many species, such as L. acidophilus, L. casei, L. plantarum and L. bulgaricus, researchers have started to investigate the therapeutic effects on UC related to the individual species[12,34]. Similarly, efficacy studies on the Lactobacilli compound bacteria VSL#3 have been carried out, and their results confirmed that the combined panel of Lactobacillus sp. in this compound is beneficial for treating inflammatory bowel diseases[15,35]. We hypothesize that, in the intestine, interactions between L. acidophilus and other local Lactobacilli (at low concentrations) may serve to promote each other mutually. It is possible then that increasing the concentration of L. acidophilus (via administration of the probiotic supplement) may serve to intensify this mutual promotion. However, the dose of the L. acidophilus supplement is important; too high of a dose may instead create an imbalance between the different Lactobacillus sp. and disrupt the mutual promotion, thereby leading to a decrease in the total number of the beneficial Lactobacilli. Gaining a detailed understanding of the mechanisms of the interactions between Lactobacillus sp. will provide important insights into the related roles in UC pathogenesis.
The optimal dose of L. acidophilus will promote the growth of endogenous probiotics, such as Bifidobacteria, and inhibit the growth of pathogenic bacteria, such as S. aureus. The moderate-dose of L. acidophilus in the current study increased the amount of other probiotics and reduced the amounts of the pathogenic species. However, it appears that simply modifying the dose of L. acidophilus will not be sufficient for designing a supplemental regimen with optimal efficacy as we also found that the concentration of endogenous Lactobacilli in the lesion is relatively positively correlated with the efficacy.
In conclusion, administration of L. acidophilus supplement at a dose of 106 CFU/10 g body weight provides optimal therapeutic effect on experimental colitis in a mouse model. The treatment-induced relief of UC symptoms was correlated with changes in the concentrations of endogenous Lactobacilli and other probiotic and pathogenic bacteria in the distal colon. Future studies should aim to determine the mechanisms underlying the interactions between L. acidophilus and other endogenous bacteria, as well as molecular effects on the host immune system, both of which may identify novel manipulable targets to further increase the therapeutic efficacy of this approach.
Ulcerative colitis (UC) is a nonspecific chronic inflammatory bowel disease with a high rate of recurrence. The etiological factors of UC remain largely unknown, but a large number of studies have demonstrated that the intestinal microorganism environment plays an important role in the development of UC.
Despite the increased incidence of UC, there remains a distinct lack of efficacious and non-invasive methods of treatment. Besides surgical intervention, which is performed in the later stages of UC, the primary therapies are drug-based, with the most frequently administered agents being salazosulfamide, glucocorticoids and immunosuppressant probiotics. Yet all of these drugs produce significant side-effects that limit patient compliance, which may actually promote the high relapse rates. Because probiotics are beneficial (and predominant) components of the normal intestinal flora, they represent promising therapeutic agents for UC; yet, to date, no study has reported the comparative efficacies of different kinds of probiotics or different doses in UC.
Although probiotics are beneficial to the human body, the supplement preparation of these living bacteria is not static and their dynamic activities, such as proliferation and secreted signaling mechanisms, may influence the therapeutic effects at different sites within the host system. It is necessary to identify the most suitable probiotic type for use a supplemental therapy for UC, as well as the optimal dose that will replenish the endogenous probiotics and inhibit any latent pathogenic species.
By determining the most suitable concentration of Lactobacillus acidophilus (L. acidophilus) for use as a supplemental probiotic treatment of UC, and providing novel insights into the relationship between L. acidophilus and the other endogenous flora, this study not only promotes the clinical potential for probiotic treatment but also expands the base of knowledge about UC pathogenesis.
Inflammatory bowel diseases, such as UC and Crohn’s disease, are nonspecific chronic inflammatory conditions with highly complex etiologies. The dextran sodium sulfate-induced mouse model of experimental colitis is similar to human UC.
Grammatical errors should be corrected but this manuscript will provide a good addition to the medical literature.
P- Reviewer Leitman M S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Furuta R, Ando T, Watanabe O, Maeda O, Ishiguro K, Ina K, Kusugami K, Goto H. Rebamipide enema therapy as a treatment for patients with active distal ulcerative colitis. J Gastroenterol Hepatol. 2007;22:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1277] [Article Influence: 67.2] [Reference Citation Analysis (2)] |
| 3. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1538] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 4. | Knight DJ, Girling KJ. Gut flora in health and disease. Lancet. 2003;361:1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224-5231. [PubMed] |
| 6. | Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Jess T, Simonsen J, Nielsen NM, Jørgensen KT, Bager P, Ethelberg S, Frisch M. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359-2364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 818] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 9. | Mukhopadhya I, Thomson JM, Hansen R, Berry SH, El-Omar EM, Hold GL. Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS One. 2011;6:e21490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Fiocchi C. Future of IBD pathogenesis: how much work is left to do? Inflamm Bowel Dis. 2008;14 Suppl 2:S145-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Imaoka A, Shima T, Kato K, Mizuno S, Uehara T, Matsumoto S, Setoyama H, Hara T, Umesaki Y. Anti-inflammatory activity of probiotic Bifidobacterium: enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J Gastroenterol. 2008;14:2511-2516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Gardlik R, Palffy R, Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol (Praha). 2012;58:238-245. [PubMed] |
| 14. | Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 15. | Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 492] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Looijer-van Langen MA, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. 2009;15:454-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Meijer BJ, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011;45 Suppl:S139-S144. [PubMed] |
| 19. | Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin Exp Immunol. 1999;117:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 927] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 21. | Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001;29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailón E, Nieto A, Concha A, Olivares M, Zarzuelo A, Xaus J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Oishi K, Sato T, Yokoi W, Yoshida Y, Ito M, Sawada H. Effect of probiotics, Bifidobacterium breve and Lactobacillus casei, on bisphenol A exposure in rats. Biosci Biotechnol Biochem. 2008;72:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Setoyama H, Imaoka A, Ishikawa H, Umesaki Y. Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microbes Infect. 2003;5:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56-63. [PubMed] |
| 28. | Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 822] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 29. | Chapman TM, Plosker GL, Figgitt DP. Spotlight on VSL#3 probiotic mixture in chronic inflammatory bowel diseases. BioDrugs. 2007;21:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, Brigidi P, Vitali B, Straforini G, Campieri M. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50:2075-2082; discussion 2075-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Qin X. Etiology of inflammatory bowel disease: a unified hypothesis. World J Gastroenterol. 2012;18:1708-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 33. | Yang WF, Li L, Lu FG. The effect of probiotics CMS-H002 strain combined with CMS-H003 strain on mice with acute ulcerative colitis. J Prac Medi. 2007;11:1613-1615. |
| 34. | Kokesová A, Frolová L, Kverka M, Sokol D, Rossmann P, Bártová J, Tlaskalová-Hogenová H. Oral administration of probiotic bacteria (E. coli Nissle, E. coli O83, Lactobacillus casei) influences the severity of dextran sodium sulfate-induced colitis in BALB/c mice. Folia Microbiol (Praha). 2006;51:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 35. | Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66:1371-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |