Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5261
Revised: July 7, 2013
Accepted: July 17, 2013
Published online: August 28, 2013
Processing time: 100 Days and 17.1 Hours
AIM: To identify the role of human development in the incidence and mortality rates of gastrointestinal cancers worldwide.
METHODS: The age-standardized incidence and mortality rates for gastrointestinal cancers, including cancers of the esophagus, stomach, pancreas, liver, gallbladder, and colorectum, were obtained from the GLOBOCAN 2008 database and United States Cancer Statistics (USCS) report. The human development index (HDI) data were calculated according to the 2011 Human Development Report. We estimated the mortality-to-incidence ratios (MIRs) at the regional and national levels, and explored the association of the MIR with development levels as measured by the HDI using a modified “drug dose to inhibition response” model. Furthermore, countries were divided into four groups according to the HDI distribution, and the MIRs of the four HDI groups were compared by one-way ANOVA followed by the Tukey-Kramer post-hoc test. State-specific MIRs in the United States were predicted from the estimated HDI using the fitted non-linear model, and were compared with the actual MIRs calculated from data in the USCS report.
RESULTS: The worldwide incidence and mortality rates of gastrointestinal cancers were as high as 39.4 and 54.9 cases per 100000 individuals, respectively. Linear and non-linear regression analyses revealed an inverse correlation between the MIR of gastrointestinal cancers and the HDI at the regional and national levels (β < 0; P = 0.0028 for regional level and < 0.0001 for national level, ANOVA). The MIR differed significantly among the four HDI areas (very high HDI, 0.620 ± 0.033; high HDI, 0.807 ± 0.018; medium HDI, 0.857 ± 0.021; low HDI, 0.953 ± 0.011; P < 0.001, one-way ANOVA). Prediction of the MIRs for individual United States states using best-fitted non-linear models showed little deviation from the actual MIRs in the United States. Except for 28 data points (9.93% of 282), the actual MIRs of all gastrointestinal cancers were mostly located in the prediction intervals via the best-fit non-linear regression models.
CONCLUSION: The inverse correlation between HDI and MIR demonstrates that more developed areas have a relatively efficacious healthcare system, resulting in low MIRs, and HDI can be used to estimate the MIR.
Core tip: This study is the first to explore the exact relationship between the epidemiology of gastrointestinal cancers and area-specific development disparities. We showed the association between the mortality-to-incidence ratios (MIRs) and the human development index at the regional and national levels using a modified “drug dose to inhibition response” model. Further prediction of the MIRs for individual United States states on the basis of best-fitted non-linear models showed little deviation from the actual MIRs in the United States.
- Citation: Hu QD, Zhang Q, Chen W, Bai XL, Liang TB. Human development index is associated with mortality-to-incidence ratios of gastrointestinal cancers. World J Gastroenterol 2013; 19(32): 5261-5270
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5261
The digestive system includes multiple organs within or alongside the alimentary tract and is of vital importance in the proper functioning of the body. Currently, gastrointestinal cancer is a leading cause of cancer-related deaths in many developed countries, and it has been predicted to have the highest incidence and mortality rates worldwide, irrespective of the level of a country’s resources[1-3]. Gastrointestinal cancers are known to notably affect the pathophysiological condition and functioning of the digestive system. Both cancer incidence and mortality in highly developed countries such as the United States peaked in the early 1990s and have since declined because of enhanced awareness, preventive measures, earlier detection and the availability of new and more effective treatment regimens, although very little progress has been made in the treatment of some cancers such as pancreatic cancer[4]. In contrast, limited or inaccessible healthcare resources in developing areas remain barriers to the effective control of future changes in incidence and mortality rates[5,6]. The expected cancer burden will continue to be a serious public health problem in the coming decade, particularly in developing countries[3,7,8].
Disparities in healthcare have received considerable attention from international organizations and national governments[9,10]. The socioeconomic determinants of the inequality reflect regional imbalances in human development. A previous study found that 35% of the cancer deaths may be attributable to nine modifiable risk factors: alcohol, smoking, low fruit and vegetable intake, overweight and obesity, physical inactivity, urban air pollution, unsafe sex, contaminated injections in health care settings, and indoor smoke from household activities such as cooking or indoor heating[11]. Most of these risk factors vary widely among populations in areas with different levels of development[12,13]. However, there is little knowledge about the healthcare disparities in the individuals suffering from gastrointestinal cancers. This study is the first to explore the exact relationship between the epidemiology of gastrointestinal cancers and area-specific development disparities. We aimed to identify the role of human development in the incidence and mortality rates of gastrointestinal cancers worldwide.
The global incidence and mortality estimates for gastrointestinal cancers in 184 countries were obtained from the GLOBOCAN 2008 database (http://globocan.iarc.fr/) maintained by the WHO International Agency for Research on Cancer[14,15]. GLOBOCAN also provided regional estimates for each continent.
United States Cancer Statistics (USCS) reported the incidence and mortality rates associated with cancers in United States states[16]. State-specific incidence data were collected from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Mortality information was collected by the National Vital Statistics System, National Center for Health Statistics and United States Centers for Disease Control and Prevention (United States-CDC).
We obtained the data of the incidence and mortality rates of gastrointestinal cancers in six major sites, namely, the esophagus, stomach, pancreas, liver, gallbladder, and colorectum. The USCS did not provide gallbladder cancer data. The overall rates of gastrointestinal cancers were estimated by addition of the rates of the six cancers. The rates were age-standardized using the world standard population and a previously proposed method[17], and presented as age-standardized rates (ASR). ASR is a summary measure of the rate that a population distribution would have if it had a standard age structure. Since age has a powerful influence on the risk of cancer, standardization is necessary when comparing several populations that differ with respect to age[14].
The mortality-to-incidence ratios (MIRs) were calculated from the obtained incidence and mortality rates provided by the GLOBOCAN database[14] and USCS report[16]. Extreme MIRs (0, 1, or > 1) were considered abnormal because of (1) illogical data (zero mortality or mortality more than incidence); and (2) zero incidence, and these results were excluded from the regression fit and further analysis. Respectively, 25, 13, 62, 82, 46, and 0 extreme MIR results were excluded in the analysis for cancers of the esophagus, stomach, pancreas, liver, gallbladder, and colorectum.
The human development index (HDI) data of Union Nation members in 1980-2011 were available in the United Nations Development Programme (UNDP) database (http://hdr.undp.org/en/statistics). The HDI was calculated according to the 2011 Human Development Report (HDR 2011)[18]. The HDI of Taiwan was obtained from the National Statistics (Taiwan) website (http://www.stat.gov.tw), and subsequently verified.
We further estimated the state-specific HDI in the United States on the basis of data provided by various data agencies. Information on life expectancy at birth provided by the CDC was adapted by the American HDI Project[19]. The gross domestic product (GDP) per capita was acquired from the Bureau of Economic Analysis at the United States Department of Commerce, and compiled by the Bureau of Business and Economic Research, University of New Mexico[20]. The GDP values were converted to international dollars using purchasing power parity rates. The mean duration of education was estimated from the 2009 American Community Survey data provided by the United States Census Bureau[21], according to the method of Barro and Lee[22]. The expected duration of education in the United States was defined as 12 years; this value was adapted from the United Nations Educational, Scientific and Cultural Organization Institute for Statistics[23].
Only the countries with both epidemiologic data from the GLOBOCAN database and HDI from the UNDP program were included in the analysis. Taiwan was not excluded because its HDI value was available at the National Statistics (Taiwan) website. The number of countries included in our research was 165. Patterns in the MIR of gastrointestinal cancers with respect to the levels of socioeconomic development were investigated by correlating the MIRs to the corresponding HDIs via linear or non-linear regression. Linear regression fit was conducted to determine the existence of correlations. Derivation of the slope parameter β from 0 was defined by ANOVA. Correlation existence referred to the significantly non-zero β value. Non-linear regression fit was based on a modified “drug dose to inhibition response” model using the formula:
where “HDI50” was the HDI value at half maximal MIR and “slope” was a parameter that indicated the steepness of the slope. The MIRs of the four HDI groups were compared by one-way ANOVA followed by the Tukey-Kramer post-hoc test. A P value of less than 0.05 was considered statistically significant. Statistical analysis and plotting were performed using Prism 5 (GraphPad, San Diego, CA, United States). The geographical map showing MIR was created using the open source software TileMill (a GitHub project maintained by MapBox, Washington, WI, United States), with map data sources from the Natural Earth database rendered by the Mapnik Library.
In 2008, gastrointestinal cancers were estimated to have affected a total of 3878986 individuals and caused 2824985 deaths worldwide. The global mortality and incidence rates were as high as 39.4 and 54.9 cases per 100000 individuals, respectively. Colorectal cancer was the third most common cancer with 1235108 incidences among the 27 cancers included in the GLOBOCAN database, and it was the most common cancer among the six gastrointestinal cancers included in the current study. Other prevalent cancers according to the incidences reported in the database included stomach cancer (ranked 4th, with 988602 incidences), liver cancer (6th, 749744 incidences), esophageal cancer (8th, 481645 incidences), pancreatic cancer (13th, 278684 incidences), and gallbladder cancer (21st, 145203 incidences). However, stomach cancer had the highest mortality rate (26.1%, 737419 deaths) among all gastrointestinal cancers. In terms of the mortality rate, liver cancer (ranked 3rd with 695726 deaths), colorectal cancer (4th, 609051 deaths), esophageal cancer (6th, 406533 deaths), pancreatic cancer (8th, 266669 deaths) and gallbladder cancer (17th, 109587 deaths) contributed to 24.6%, 21.6%, 14.4%, 9.4% and 3.9% of all deaths caused by gastrointestinal cancers, respectively.
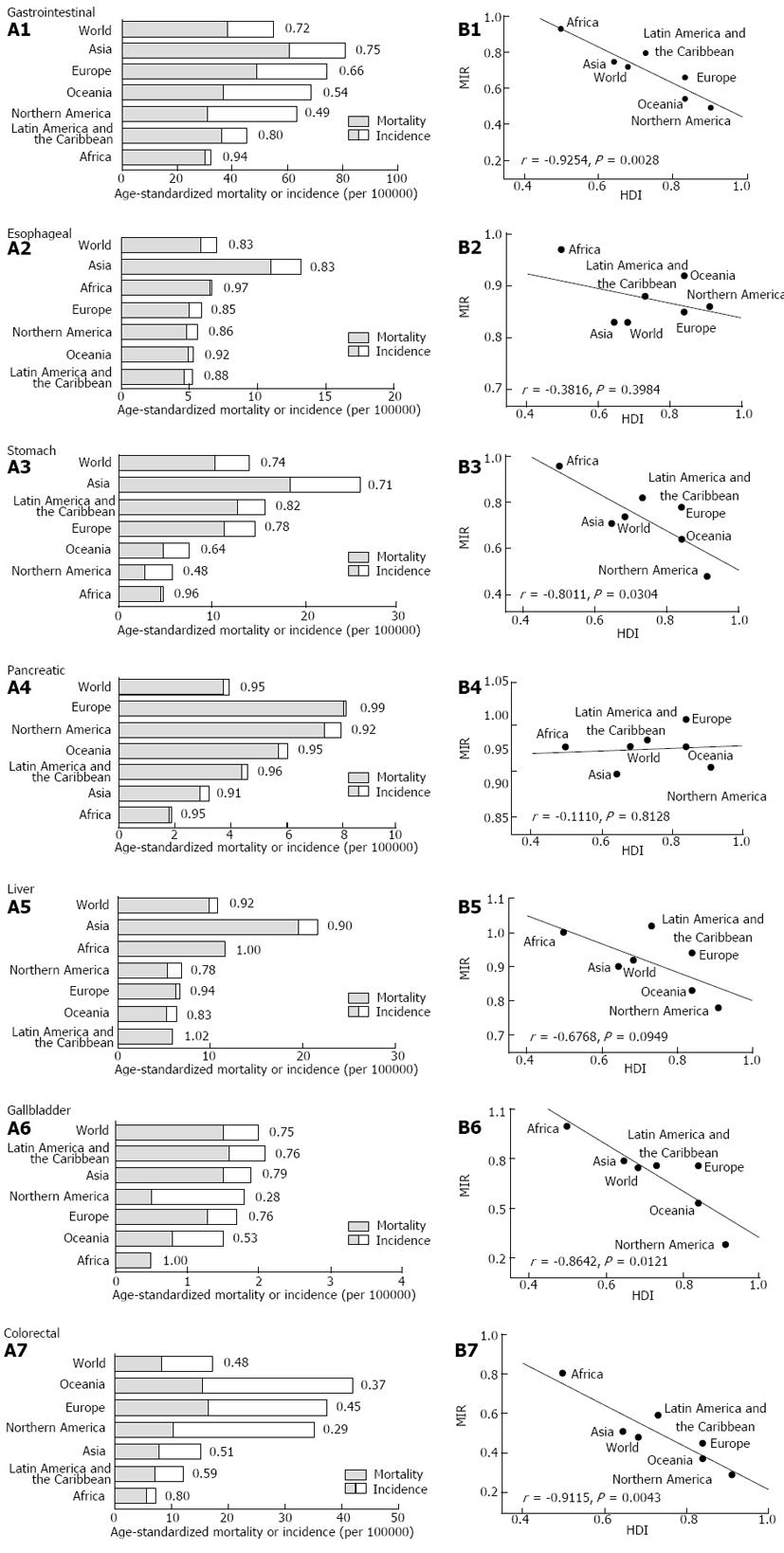
The regional incidence and mortality rates varied among different continents and regions (Figure 1A). Asia had the highest incidence rates of esophageal, stomach and liver cancers, as well as gastrointestinal cancers overall. Interestingly, the MIRs for gastrointestinal cancers, except for pancreatic cancer, were higher in Africa compared with other continents. Linear regression analysis revealed a significant inverse correlation between the regional HDI and MIR of stomach, gallbladder and colorectal cancers and gastrointestinal cancer overall (P < 0.05, ANOVA) (Figure 1B).
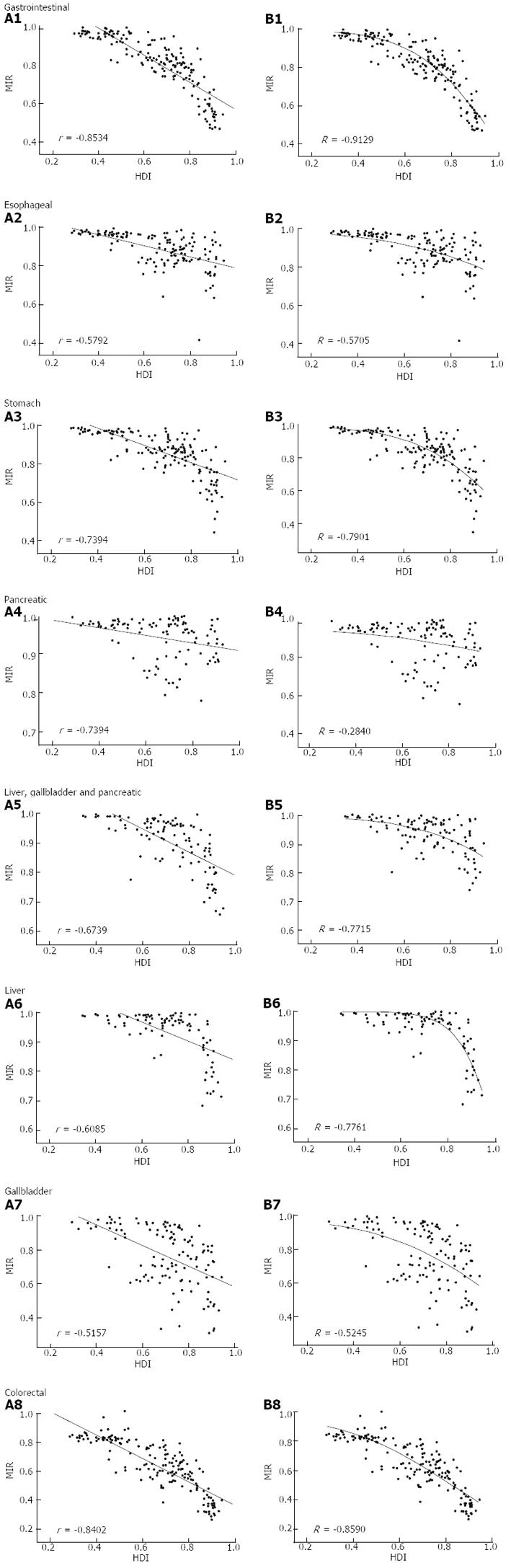
The national MIR varied across different countries with different levels of development, as measured by HDI (Figure 2). Countries with high HDI tended to have relatively low MIR. Cross-national analysis demonstrated that the MIRs of gastrointestinal cancers consistently showed an inverse correlation with the national HDI values via linear regression (β < 0; P < 0.05, ANOVA; Table 1, Figure 3A). Furthermore, non-linear regression based on the “drug dose to inhibition response” model was used to analyze this correlation, and a more satisfactory fitting result with larger R square values was achieved for all gastrointestinal cancers (Table 1, Figure 3B). The HDI values at half-maximal MIR (HDI50) in gastrointestinal cancers overall and colorectal cancer were 0.946 and 0.825, respectively. Five other cancers had an HDI50 of more than 1.
| Cancer | Linear regression | Non-linear regression1 | ||||
| β | P | r | HDI50 | Slope | R | |
| All gastrointestinal | -0.703 | < 0.001 | -0.853 | 0.946 | 2.746 | -0.9129 |
| Esophageal | -0.295 | < 0.001 | -0.579 | 1.362 | 1.344 | -0.5705 |
| Stomach | -0.536 | < 0.001 | -0.739 | 1.023 | 2.372 | -0.7901 |
| Pancreatic | -0.097 | 0.0019 | -0.301 | 2.391 | 0.706 | -0.2840 |
| Liver | -0.322 | < 0.001 | -0.609 | 1.026 | 5.247 | -0.7761 |
| Gallbladder | -0.611 | < 0.001 | -0.516 | 1.027 | 1.697 | -0.5245 |
| Liver, gallbladder and pancreas | -0.216 | < 0.001 | -0.543 | 1.386 | 1.726 | -0.5704 |
| Colorectal | -0.808 | < 0.001 | -0.840 | 0.825 | 1.785 | -0.8590 |
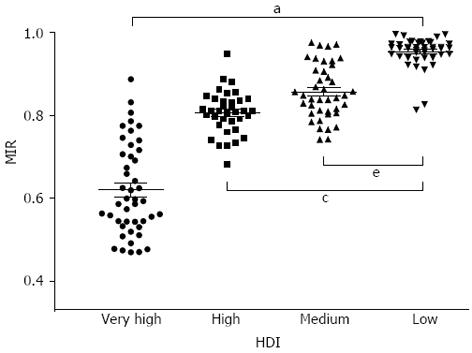
Countries were divided into four groups according to the HDI distribution reported in HDR 2011[18]. The MIR of gastrointestinal cancers differed significantly among these four groups (P < 0.001, one-way ANOVA). The mean MIR of countries with very high HDI was 0.620 ± 0.033 (95%CI), which was significantly lower than the corresponding values of countries with high, medium, and low HDIs (0.807 ± 0.018, 0.857 ± 0.021, and 0.953 ± 0.011, respectively; P < 0.05, Tukey-Kramer post-hoc test; Figure 4). Furthermore, there was a significant difference among the four groups in each specific cancer (P < 0.001, one-way ANOVA).
The individual HDIs of 51 United States states were calculated as previously described in HDR 2011. The HDI values in each state ranged from 0.847 to 0.962. To verify the effectiveness of the fitted models, the MIRs of gastrointestinal cancers in each of the United States states were predicted using respective best-fit equations. Except for 28 data points (9.93% of 282), the actual MIRs of all gastrointestinal cancers were mostly located in the prediction intervals via the best-fit non-linear regression models. In California, for example, the estimated HDI was 0.907 and the predicted MIR of gastrointestinal cancers was 0.560 ± 0.118 (95% prediction interval, 95%PI). The actual MIR calculated from the reported incidence and mortality was 0.533, and the difference between the actual and predicted MIRs (ΔMIR) was -0.027 (23.1% of 95%PI). The actual MIRs of the six cancers were also in the 95%PI predicted by the respective regression fitting equations (Table 2).
| Cancer | Incidence(ASR, per100000) | Mortality(ASR, per100000) | Actual MIR | Predicted MIR(95%PI)1 | ΔMIR |
| All gastrointestinal | 74.3 | 39.6 | 0.533 | 0.560 ± 0.118 | -0.027 |
| Esophageal | 3.8 | 3.4 | 0.895 | 0.803 ± 0.148 | 0.091 |
| Stomach | 7.4 | 4.3 | 0.581 | 0.653 ± 0.158 | -0.072 |
| Pancreatic | 11.3 | 10.3 | 0.912 | 0.918 ± 0.104 | -0.006 |
| Liver | 8.4 | 6.8 | 0.810 | 0.807 ± 0.101 | 0.002 |
| Colorectal | 43.4 | 14.8 | 0.341 | 0.416 ± 0.173 | -0.075 |
Gastrointestinal cancers have high incidence and mortality rates worldwide[1,24]. We found that both the incidence and mortality rates differed greatly from region to region. Interestingly, the ratio of the mortality rate to the incidence rate, i.e., the MIR, appeared to be higher in less developed regions such as Africa. The development level was quantified by HDI, which is a composite measure of human development. Estimation of national HDI is based on the following parameters: a long and healthy life, access to knowledge, and a decent standard of living[18]. As an indicator of the socioeconomic factor of health, the HDI may serve as the gold standard for international comparisons of development.
MIR is derived as a surrogate indicator of the effectiveness of the health system. It has been proposed as an indirect measure of true biological differences in disease phenotypes or health system-related attributes such as screening, diagnostic modalities, treatment and follow-up[25,26]. An MIR-associated derivative form was identified as a good approximation of the 5-year relative survival for most, but not all, cancers[27]. The MIR is computed from age-standardized rates, and it also reflects a population-based approximation of survival[25]. Accordingly, it could be used to assess the diagnosis proficiency and treatment effectiveness in gastrointestinal cancers.
Africa, which had a relatively low HDI, showed a high MIR for most gastrointestinal cancers, whereas Northern America, which had a higher HDI, showed a low MIR. Furthermore, we found a significant inverse correlation between the regional MIR and corresponding HDIs in some, but not all, gastrointestinal cancers. However, only seven data points were included in the region-specific linear regression analysis. Insufficient sample size for regression analysis might cause fitting inaccuracy[28]. To avoid such inaccuracies, country-specific regression was performed. Linear regression analysis in this study revealed a correlation between the national HDI and MIR in all gastrointestinal cancers. The impact of human development on the effectiveness of healthcare for gastrointestinal cancers, as reflected by the relationship between HDI and MIR, was assumed to bear a similarity to the dose-dependent inhibitory response by anticancer drugs. HDI-to-MIR and dose-to-response patterns both have several characteristics in common, such as (1) MIR or response approaches 1 as HDI or dose approaches 0; (2) MIR or response decreases as HDI or dose increases; and (3) MIR or response approaches 0 as HDI or dose approaches infinity. Non-linear regression using the modified “drug dose to inhibition response” model confirmed the assumption and provided the HDI50 value, which was found to be a potential estimate of healthcare effectiveness on gastrointestinal cancers. The progress in screening, diagnostic and therapeutic techniques for colorectal cancer in recent decades[1,29] has resulted in an HDI50 of 0.825, which is the lowest value among all the gastrointestinal cancers investigated in this study.
Inequality in healthcare has been regarded as a major cause of variation in the effectiveness of cancer care[30], reflected by the inverse correlation between MIR and HDI. Although eliminating such disparities in healthcare has become the focus of an initiative of healthcare reform in many countries, quality improvement in medical care is not yet obvious[9]. Region- or country-specific disparities in cancer care still exist, even in highly developed countries[7]. Apart from healthcare inequality, the inverse correlation between HDI and MIR is also influenced by the factors such as socioeconomic conditions, lifestyle (particularly diet and tobacco use), and genetic variances among individuals or races[7,9]. Infection with Helicobacter pylori, hepatitis virus or other cancer-inducing micro-organisms is another risk factor for gastrointestinal cancers[31-33]. A very recent study analyzed world cancer burden by HDI groups and suggested disparities in cancer distributions[3]. We further demonstrated that HDI influenced cancer MIRs on a country level, which resembled the effect of drug dose on inhibitory response. Therefore, relatively high MIRs indicate the premature mortality from cancer in lower HDI areas. Healthcare disparities emphasize the need for efforts in cancer control in low human development settings.
Cancer health disparities occur not only between countries, but also within a single country[7,34,35]. The health outcomes in the United States were related to socioeconomic factors and racial diversity[9,36]. Health inequality between different socioeconomic levels also contributed to the health disparities observed in the United States. Therefore, we supposed that the observed association between HDI and MIR could be applied to United States states. Prediction based on the modified “drug dose to inhibition response” model turned out to be relatively satisfactory.
The methods used to estimate cancer-specific incidence and mortality rates at the national level in the GLOBOCAN database depend on the availability and accuracy of local data sources[3]. Despite the various provisos concerning data quality and methods of estimation, the estimates in GLOBOCAN are the most accurate that can be made at present, and may be used in the setting of priorities for cancer control actions in different regions and countries of the world[14]. Countries without high quality data are usually those countries with lower development levels. Limiting analysis to high quality data could eliminate biases due to data inaccuracy, but would lead to excessive absence of epidemiological data in the less developed countries. Since our study aimed to show the disparities of cancer MIRs between low and high HDI countries, the data with relatively low quality were essential to this study and therefore remained in our analysis.
In conclusion, the results of this study obtained by collating excellent data resources revealed an inverse correlation between HDI and MIR at the regional and national levels. This association illustrated that more developed areas tend to have relatively more effective healthcare systems, resulting in low MIRs. Further prediction of the state-specific MIR of gastrointestinal cancers obtained using a fitted non-linear regression model revealed the potential application of HDI for estimation of the MIR.
Gastrointestinal cancer is a common, highly fatal disease. The expected cancer burden will be a serious public health problem in the coming decade, particularly in developing countries. However, little is known about healthcare disparities in individuals suffering from gastrointestinal cancers.
There is little knowledge about the healthcare disparities in individuals suffering from gastrointestinal cancers. Inequality in healthcare has been regarded as a major cause of variation in the effectiveness of cancer care. Region- or country-specific disparities in cancer care still exist, even in highly developed countries.
According to the authors of this study, this study is the first to explore the exact relationship between the epidemiology of gastrointestinal cancers and area-specific development disparities. The authors showed the association between the mortality-to-incidence ratios (MIRs) and the human development index (HDI) at the regional and national levels using a modified “drug dose to inhibition response” model. Further prediction of the MIRs for individual United States states on the basis of best-fitted non-linear models showed little deviation from the actual MIRs in the United States.
Based on the modified “drug dose to inhibition response” model, more developed areas have relatively more effective healthcare systems, resulting in low MIRs. Prediction of the state-specific MIR of gastrointestinal cancers obtained using a fitted non-linear regression model revealed the potential application of HDI for estimation of the MIR.
MIR is derived as a surrogate indicator of the effectiveness of the health system, and is proposed as an indirect measure of true biological differences in disease phenotypes or health system-related attributes such as screening, diagnostic modalities, treatment and follow-up. HDI is a composite measure of human development based on the following parameters: a long and healthy life, access to knowledge, and a decent standard of living.
The authors, using the GLOBOCAN 2008 database, obtained age-standardized incidence and mortality rates for gastrointestinal cancers. They estimated the MIRs at the regional and national levels, and explored the association between the MIR and development levels as measured by the HDI. Furthermore, they have predicted state-specific MIRs in the United States from the estimated HDI using the fitted non-linear model. Finally, the authors have managed to show an inverse correlation between HDI and MIR at the regional and national levels and that more developed areas tend to have relatively more effective healthcare systems, resulting in low MIRs. Overall, the manuscript is very well written and well organized. The language is satisfactory and the tables along with the figures are well structured.
P- Reviewers Akbulut S, Koukourakis GV, Motoo Y S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 2. | Keighley MR. Gastrointestinal cancers in Europe. Aliment Pharmacol Ther. 2003;18 Suppl 3:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1245] [Cited by in RCA: 1401] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 4. | Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 680] [Cited by in RCA: 697] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 5. | Farmer P, Frenk J, Knaul FM, Shulman LN, Alleyne G, Armstrong L, Atun R, Blayney D, Chen L, Feachem R. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 516] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 6. | Kapiriri L. How effective has the essential health package been in improving priority setting in low income countries? Soc Sci Med. 2013;85:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Jones LA, Chilton JA, Hajek RA, Iammarino NK, Laufman L. Between and within: international perspectives on cancer and health disparities. J Clin Oncol. 2006;24:2204-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Kulendran M, Leff DR, Kerr K, Tekkis PP, Athanasiou T, Darzi A. Global cancer burden and sustainable health development. Lancet. 2013;381:427-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 797] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 10. | Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118:293-302. [PubMed] |
| 11. | Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 783] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 12. | Baker EA, Metzler MM, Galea S. Addressing social determinants of health inequities: learning from doing. Am J Public Health. 2005;95:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 1231] [Article Influence: 87.9] [Reference Citation Analysis (1)] |
| 14. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 15. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. 2010; Available from: http: //globocan.iarc.fr. |
| 16. | US Cancer Statistics Working Group. United States Cancer Statistics: 1999-2008 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2012. Available from: http: //www.cdc.gov/uscs. |
| 17. | Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer Incidence in Five Continents, Vol. IX. Lyon, IARC: IARC Scientific Publications 2007; 160. |
| 18. | United Nations Development Programme. Human Development Report 2011: Sustainability and Equity: A Better Future for All, 2012. Available from: http: //hdr.undp.org. |
| 19. | Lewis K, Burd-Sharps S. The Measure of America, 2010-2011: Mapping Risks and Resilience. New York: NYU Press 2010; . |
| 20. | US Department of Commerce, Bureau of Economic Analysis. Real Per Capita Gross Domestic Product by State, 2012. Available from: http: //bber.unm.edu/econ/st-gdp5.htm. |
| 21. | US Census Bureau. Educational Attainment by State. 2009 American Community Surveys. Available from: http: //www.census.gov/acs. |
| 22. | Barro RJ, Lee JW. A new data set of educational attainment in the world, 1950-2010. National Bureau of Economic Research Working Paper Series No. 15902, 2010. Available from: http: //www.nber.org/papers/w15902. |
| 23. | United Nations Educational, Scientific and Cultural Organization (UNESCO). UNESCO Institute for Statistics: Data Centre. Available from: http: //stats.uis.unesco.org. |
| 24. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1882] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 25. | Hébert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, Puett R, Burch JB, Steck SE, Bolick-Aldrich SW. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115:2539-2552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Patel AR, Prasad SM, Shih YC, Eggener SE. The association of the human development index with global kidney cancer incidence and mortality. J Urol. 2012;187:1978-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Asadzadeh Vostakolaei F, Karim-Kos HE, Janssen-Heijnen ML, Visser O, Verbeek AL, Kiemeney LA. The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur J Public Health. 2011;21:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Dupont WD, Plummer WD. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 743] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 29. | Lieberman D. Progress and challenges in colorectal cancer screening and surveillance. Gastroenterology. 2010;138:2115-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Murray SA, Grant E, Grant A, Kendall M. Dying from cancer in developed and developing countries: lessons from two qualitative interview studies of patients and their carers. BMJ. 2003;326:368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1844] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 32. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 33. | Yan TL, Hu QD, Zhang Q, Li YM, Liang TB. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther. 2013;37:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Gouws L, Eedes D, Marais E, Valodia P, De Villiers M. Revolutionising cancer care in South Africa. Lancet Oncol. 2012;13:447-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample. Med Care. 2002;40:52-59. [PubMed] |
| 36. | Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100 Suppl 1:S186-S196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 958] [Article Influence: 63.9] [Reference Citation Analysis (0)] |