Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4917
Revised: May 13, 2013
Accepted: June 8, 2013
Published online: August 14, 2013
Processing time: 146 Days and 11.8 Hours
AIM: To explore the role of S-phase kinase-associated protein-2 (Skp2) in gallbladder carcinoma and to identify whether depletion of Skp2 by Skp2-RNAi could attenuate proliferation and migration of gallbladder carcinoma.
METHODS: Skp2-RNAi was transduced into cells of the gallbladder carcinoma cell line GBC-SD, using a lentiviral vector. The effect of Skp2-RNAi on the proliferation, migration, invasion and cell cycle of GBC-SD cells was studied using in vitro assays for cell proliferation, colony formation, wound healing and cell cycle. The expression of Skp2 and p27 was detected by real-time polymerase chain reaction and Western immunoblotting. The effect of Skp2-RNAi on the proliferation of GBC-SD cells in vivo was investigated by tumorigenicity experiments in nude mice.
RESULTS: Lentivirus-mediated RNAi reduced the expression of Skp2 in cultured cells. The expression of the p27 protein increased along with the down-regulation of Skp2, although no significant difference was found in p27 mRNA expression. Flow cytometry revealed that Skp2-RNAi transfection significantly increased the proportion of cells in the S phase and significantly decreased the proportion of cells in the G2/M phase. No significant difference in the frequency of cells in the G0/G1 phase was observed. The results from the cell proliferation, colony formation and wound healing assays revealed that Skp2-RNAi transfection markedly inhibited the proliferation and migration of GBC-SD cells in vitro. Additionally, tumorigenicity experiments showed that suppression of Skp2 significantly decreased the weights of the tumors (0.56 ± 0.11 and 0.55 ± 0.07 g in the control and Scr-RNAi groups vs 0.37 ± 0.09 and 0.35 ± 0.08 g in the Skp2-RNAi-L and Skp2-RNAi-H groups).
CONCLUSION: The expression of Skp2 in GBC-SD cells was inhibited following Skp2-RNAi transfection. Silencing of the Skp2 gene inhibited proliferation, migration and invasiveness of GBC-SD cells by mechanisms dependent on enhanced expression of the p27 protein.
Core tip: The association between S-phase kinase-associated protein-2 (Skp2)/p27 and gallbladder carcinoma has rarely been reported. This study investigated the effects of Skp2-RNAi on in vitro and in vivo growth and the invasive potencies of gallbladder carcinoma cells. The authors proposed that the effects were due to the accumulation of the p27 protein following Skp2-depletion.
- Citation: Zhang B, Ji LH, Liu W, Zhao G, Wu ZY. Skp2-RNAi suppresses proliferation and migration of gallbladder carcinoma cells by enhancing p27 expression. World J Gastroenterol 2013; 19(30): 4917-4924
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4917
Primary gallbladder carcinoma is a common biliary malignancy. Its incidence is estimated to be approximately 1.2-10.6/100000, and this cancer accounts for almost 3% of all tumors[1]. Unfortunately, the majority of patients with primary gallbladder carcinoma have intermediate-advanced disease at presentation due, in part, to diagnostic difficulties and a high degree of malignancy. Thus, for these patients, the prognosis is extremely poor.
The cancer suppressor gene p27 (wherein p27 represents the gene and p27(Kip1) represents the protein) is a cyclin-dependent kinase inhibitor (CKI), which plays an important role in tumorigenesis and tumor development[2]. Altered expression of p27(Kip1) is closely associated with the prognosis in several types of human cancers[3,4]. It has been shown that the stability of p27(Kip1) can be enhanced by a specific proteasome inhibitor, which can further inhibit the growth of the tumor[5]. Over-expression of p27(Kip1) with an adenoviral vector (adenovirus-p27) can inhibit tumor growth and induce apoptosis[6,7]. In addition, the expression of p27 mRNA was determined to be constant during a normal cell cycle. The highest expression of p27(Kip1) was found during the G0/G1 phase of the cell cycle, and the lowest expression was throughout the S and M phases[8-10]. The expression of p27(Kip1) was found to be predominantly regulated by S-phase kinase-associated protein-2 (Skp2)[8,9].
Skp2 (wherein SKP2 represents the gene and Skp2 represents the protein) is an S-phase dependent protein kinase that was originally found by Rodriguez et al[11], constituting the F-box unit of the SCF-E3 ligase that specifically targets CKIs, such as p21(Cip1), p27(Kip1), p57(Kip2) and p130, for degradation[12]. Functional deletion of Skp2 leads to stabilization of CKIs, which can subsequently induce cell-cycle delay or arrest; conversely, the over-expression of Skp2 is frequently associated with a variety of human cancers[11,13]. Nelsen et al[14] reported that cotransfection of cyclin E and Skp2 synergistically promoted cell cycle progression in cultured primary hepatocytes in the absence of mitogen or in the presence of growth inhibitors. Furthermore, transfection of hepatocytes with cyclin E and Skp2 in vivo promoted abundant hepatocyte replication and hyperplasia of the liver. Hence, Skp2 is thought to be closely associated with cell cycle regulation, tumor emergence, tumor development and disease prognosis.
p27(Kip1) and Skp2 have been studied in many types of tumors[15-19]. The determination of an association between Skp2/p27(Kip1) and gallbladder carcinoma has been rarely reported[20,21]. In the current study, we constructed a lentiviral vector of Skp2-RNAi, and explored the role of Skp2/p27(Kip1) in the proliferation and metastasis of gallbladder carcinoma cells.
The gallbladder carcinoma cell line (GBC-SD) cells (Shanghai Cell Library, China) were divided into four groups: (1) control group: without any treatment; (2) Scr-RNAi group (Scr-RNAi group): GBC-SD cells were transfected with a negative control RNA interference sequence (TTCTCCGAACGTGTCACGT) using lentivirus vectors (SunBio, United States); (3) for the Skp2-RNAi-Low group (Skp2-RNAi-L group); and (4) the Skp2-RNAi-High group (Skp2-RNAi-H group), the cells were transfected with an RNA interference sequence of Skp2 (AGGTC-TCTGGTGTTTGTAA) at a dose of 10 and 20 MOI, respectively.
The GBC-SD cells were plated and cultured in 24-well plates until cell fusion reached 40%-60%. Next, the appropriate amounts of lentivirus were added to the cells according to the different MOI values (2.5 × 104 TU/well in Skp2-RNAi-L group and 5 × 104 TU/well in Skp2-RNAi-H group). The transduction efficiency was assessed by fluorescence microscopy (Nikon, Japan) after 96 h. The cells were harvested 10 d following transduction.
The effect of the RNAi-Lentivirus on the expression of Skp2 gene was assessed by determination of the mRNA and protein levels of Skp2 in the GBC-SD cells after infection with lentivirus for 5-7 d; real-time polymerase chain reaction (PCR) and Western immunoblotting were used for these assessments.
Cell proliferation ability was assessed with a methylthiazol tetrazolium (MTT) assay kit (SunBio, United States). The cells were inoculated into 96-well plates (1 × 104 cells per well). After incubation for 1, 2, 3, 4 and 5 d, 100 μL of sterile MTT (5 mg/mL, Sigma-Aldrich Corp, United States) was added to each well. The cells were further incubated at 37 °C for 4 h, and the reaction was stopped by adding 200 μL of dimethyl sulfoxide. After mixing for 10 min at room temperature, formazan production was determined by measurement of the optical density (OD) at 570 nm using an enzyme immunoassay analyzer (1420 multi-label counter).
Two hundred cells were prepared and plated into 35-mm culture plates for a period of 10 d. The resulting cellular clones were counted using an inverted microscope (BX45-72P15, Olympus, Japan). A cell clone was scored as positive following confirmation that the number of cells within the clone exceeded 50. The experiment was repeated 8 times.
Cells were inoculated into 6-well plates, and a 100-μL pipette tip was used to scribe a line across the cell monolayer. The cells that moved into the interspace of the wound line were counted 24 h later using a phase contrast microscope (BX45-72P15, Olympus, Japan). This assay was repeated 8 times.
Cells were seeded into a 6-well plate and harvested after infection for 10 d. After two washes in pre-cooled PBS, the cells were fixed in 70% alcohol. The percentage of cells in each stage of the cell cycle was determined by staining with propidium iodide (PI, Santa Cruz, United States). The cell cycle distribution was analyzed with a FAC-Scan Flow Cytometer (BD, United States), in accordance with the manufacturer’s guidelines.
Total RNA (2 μg) was isolated and reverse-transcribed into cDNA. The cDNA samples (2 μL) were employed for real-time PCR in a total volume of 20 μL on a GeneAmp Thermal Cycler 9700 (ABI, United States). The reactions were incubated in 96-well optical plate at 94 °C for 4 min, followed by 35 cycles of 94 °C for 10 s, 57 °C for 15 s and 72 °C for 20 s, and a final extension reaction at 86.5 °C for 5 s. Melting-curve analysis was performed from 72 °C to 99 °C at a rate of 1 °C every 5 s. The average of the triplicate data obtained for each sample was employed to calculate the relative change in gene expression after normalization to β-actin mRNA. The primer sequences were as follows: Skp2: 5’-CCTAAGCAGCTGTCCCAGAC-3’ (sense) and 5’-GTGTCAGTCGGCATTTGATG-3’ (antisense); p27: 5’-ACCCAACAATACCACCGACC-3’ (sense) and 5’-CCCGCCTAATCTGCACTGTG-3’ (antisense); β-actin: 5’-CCAAGGCCAACCGCGAGAAGATGAC-3’ (sense) and 5’-AGGGTACATGGTGGTGCCGCCAGAC-3’ (antisense).
After lysis with pre-cooled lysis buffer, 40 μg of protein extracted from the cells was loaded onto 10% SDS-PAGE gels, and the resolved proteins were transferred to a PVDF membrane over a 2-h period (Bio-Rad, United States). Next, the membrane was blocked in 5% non-fat milk for 1 h at room temperature and then probed overnight at 4 °C with antibodies against Skp2 (CST, United States, 1:1000 dilution) and p27 (CST, United States, 1:1000 dilution). After three washes with TBST (tris buffered saline with 0.5% Tween-20), the membrane was incubated with the appropriate secondary antibody (anti-mouse IgG, Santa Cruz, United States, 1:1000 dilution) for 2 h at room temperature. Following a further washing with TBST, the membrane’s image was developed using enhanced chemiluminescence (ECL + plus™, Amersham, United Kingdom). β-actin was employed as an internal standard, and expressions of Skp2 and p27 were determined and normalized against the level of β-actin.
Forty male nude mice weighing 18 to 21 g, provided by Shanghai Laboratory Animal Center (Chinese Academy of Science, China), were bred under aseptic conditions; the animals were housed in an area with a constant humidity of 60%-70% and a room temperature of 18 °C-20 °C. Animal maintenance, husbandry and experimental procedures were performed in accordance with the United States National Institute of Health Guidelines for the Use of Experimental Animals and approved by the Medical Animal Care and Use Committee of Renji Hospital (Shanghai, China). All of the mice were separated into four groups as described above: control, Scr-RNAi, Skp2-RNAi-L, and Skp2-RNAi-H groups. Lentivirus transfected cells from each group were administrated by subcutaneous injection (0.1 mL of a solution containing 1 × 104 cells/mL). The mice were examined every 4 d and were sacrificed 28 d after the initial subcutaneous injection. The tumors were resected and weighed.
All measurement data were expressed as the mean ± SD. The association analysis among the groups was performed using one-way analysis of variance with the SPSS17.0 statistical software package. Statistical significance was defined as having a P value less than 0.05.
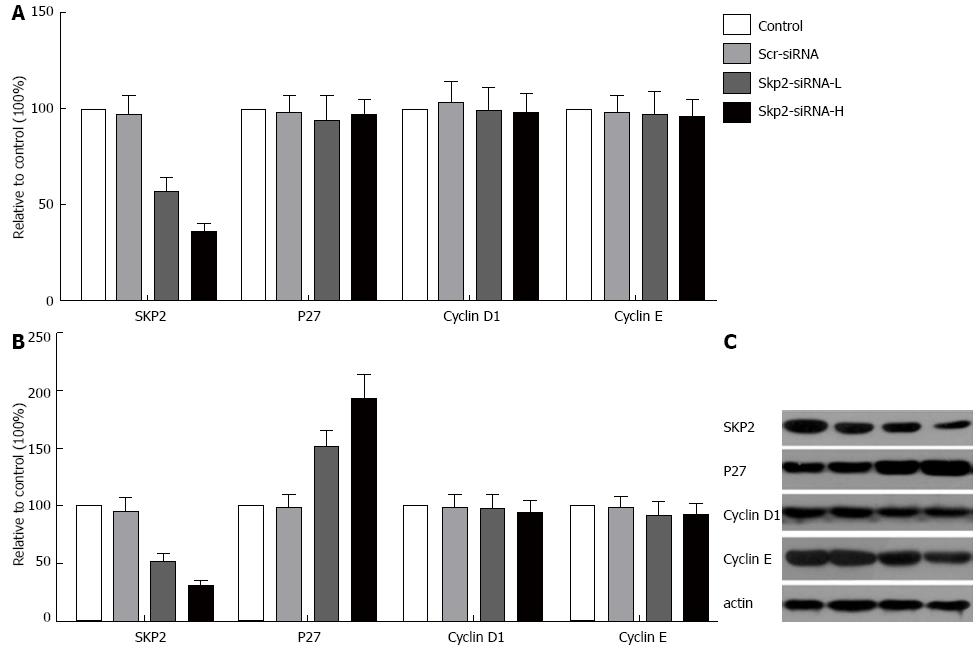
The studies showed that Skp2-RNAi transfection could significantly reduce the level of Skp2 mRNA (P < 0.05, Figure 1A) and protein (P < 0.05, Figure 1B and C) in the Skp2-RNAi-L and Skp2-RNAi-H groups, compared with the control and Scr-RNAi groups, and that these alterations were closely related to the dosage of Skp2-RNAi. In addition, the expression of p27 was also detected in GBC-SD cells after infection with RNAi-Lentivirus. Expression of p27 mRNA did not change following the down-regulation of Skp2. Densitometric analysis of the immunoblot images showed that the ratios between the p27 protein in the Scr-RNAi, Skp2-RNAi-L and Skp2-RNAi-H groups and the p27 protein in the control group were 0.99, 1.52 and 1.93, respectively (P < 0.05, Figure 1). This result suggests that p27 was increased at the protein level but not at the mRNA level following Skp2-RNAi transfection. Expression of cyclin D1 and E mRNA and protein was unaltered (data not shown).
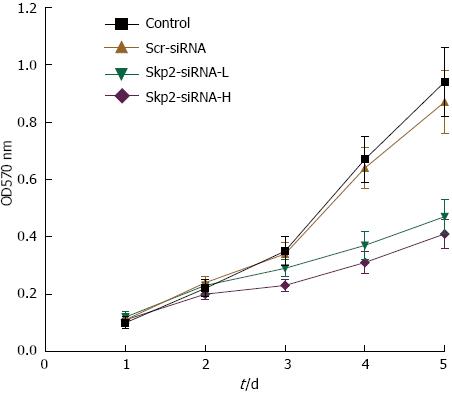
The effect of Skp2-RNAi on cell growth was evaluated using an MTT assay kit. As shown in Figure 2, the A values in the Skp2-RNAi-L and Skp2-RNAi-H groups were significantly higher than the values in the control and Scr-RNAi groups (0.94 ± 0.12 and 0.87 ± 0.11 vs 0.48 ± 0.06 and 0.41 ± 0.05, respectively, P < 0.01). This result suggests that cell growth was significantly inhibited along with the down-regulation of Skp2 by Skp2-RNAi.
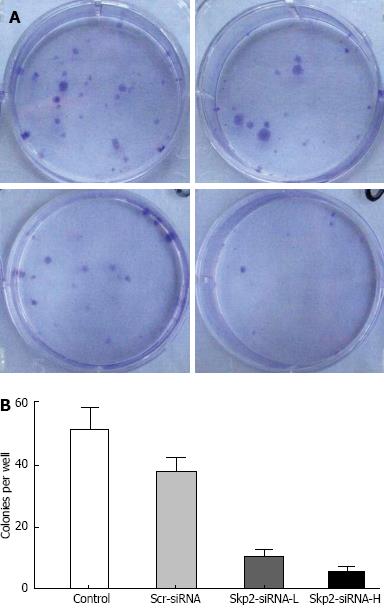
Cell colony formation was significantly decreased in the Skp2-RNAi-L and Skp2-RNAi-H groups, compared to that in the control and Scr-RNAi groups, (13.50 ± 5.90 and 7.25 ± 5.12 vs 51.25 ± 7.54 and 48.88 ± 11.93 cells per well, respectively, P < 0.01, Figure 3). Colony formation of GBC-SD cells was significantly inhibited after transfection with Skp2-RNAi, and those colonies formed were closely related to the dosage of Skp2-RNAi.
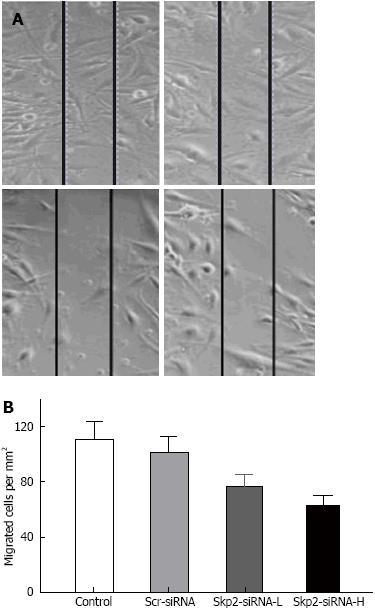
The migrated cells in the control, Scr-RNAi, Skp2-RNAi-L and Skp2-RNAi-H groups were found to be 111.75 ± 19.96, 101.38 ± 14.32, 76.50 ± 13.15 and 63.16 ± 11.00 cells per mm2, respectively. The migrated cells in the Skp2-RNAi-L and Skp2-RNAi-H groups were markedly decreased compared with the control and Scr-RNAi groups (P < 0.01, Figure 4).
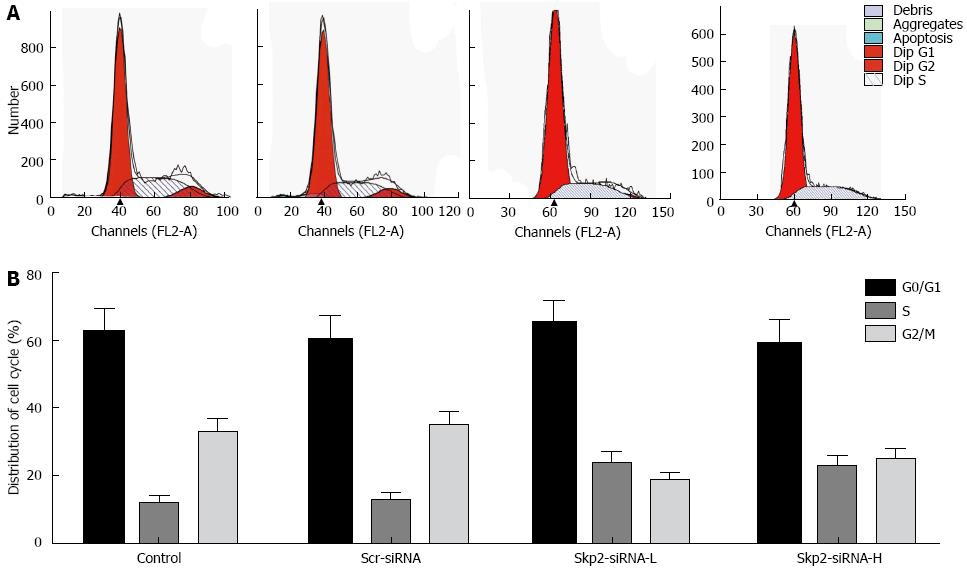
No significant difference was observed in the proportion of cells in the G0/G1 phase following inhibition of Skp2. However, in the Skp2-RNAi-L and Skp2-RNAi-H groups, the proportion of cells in S phase increased. Nevertheless, inhibition of Skp2 decreased the proportion of cells in the G2/M phase as compared with the control and Scr-RNAi groups (P < 0.05, Figure 5).
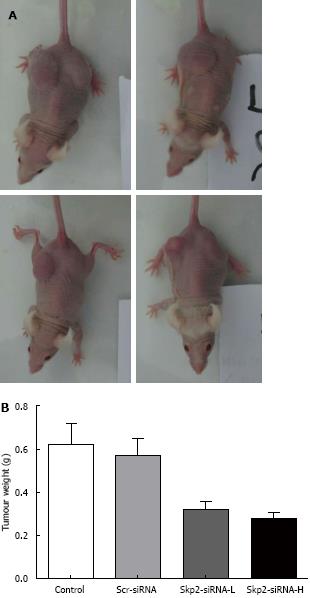
Twenty-eight days after the mice were injected with carcinoma cells, the weights of the tumors in the control, Scr-RNAi, Skp2-RNAi-L and Skp2-RNAi-H groups were 0.56 ± 0.11, 0.55 ± 0.07, 0.37 ± 0.09 and 0.35 ± 0.08 g, respectively. Thus, treatment with Skp2-RNAi inhibited the growth of tumors as compared with both the control and Scr-RNAi groups (P < 0.01, Figure 6).
Gallbladder carcinoma was first described by Clemente et al[1]. Despite advances in hepatobiliary imaging techniques, the preoperative diagnosis of this condition remains a daunting task. Furthermore, the long-term survival remains dismal, not only because of the non-specific presentation of the disease and its similarity to benign biliary tract disorders, but also because of the malignant entity. Currently, the mean survival time of advanced stage gallbladder carcinoma is approximately 6 mo, and the 5-year survival rate is less than 5%[22]. Hence, the prognosis of gallbladder carcinoma remains poor despite improvements in surgical techniques. Moreover, the molecular mechanisms underlying the development of gallbladder carcinoma remain largely unknown.
Skp2 is an F-box substrate-recognition subunit of the SCF ubiquitin-protein ligase complex, which regulates progression of the cell cycle by targeting regulators such as p27(Kip1) for ubiquitin-mediated degradation. Decreased levels of p27(Kip1) are thought to be associated with highly aggressive tumors and related to a poor prognosis in a variety of cancers[21,23-25].
In the current study, Skp2 expression was inhibited in GBC-SD cells by transfection of a Skp2 specific vector, namely, Skp2-RNAi. Consequently, cells in the S-phase of the cell cycle were increased, whereas cells in the G2/M phase were decreased. No significant difference was observed in the proportion of cells present in the G0/G1 phase; thereby, the cell cycle was blocked in the S phase. Cell growth was significantly decreased in several in vitro experiments, suggesting that silencing Skp2 could markedly reduce cell proliferation and the group-dependent capability to form colonies.
In most tumors, deletion or mutation of p27 rarely occurs, and its transcription is negligibly changed. Our research found that suppression of Skp2 had no effect on the mRNA expression of p27, but it was found to upregulate p27’s protein expression. This observation suggested that regulation of gallbladder carcinoma proliferation by Skp2-siRNA is dependent on p27 protein expression, but not expression at the gene level. Nuclear polyubiquitination of p27(Kip1) is dependent on Skp2 and phosphorylation of p27(Kip1) at threonine 187. However, Hara et al[26] found that polyubiquitination activity was also detected in the cytoplasm of Skp2(-/-) cells, even with a threonine 187 to alanine 187 mutant of p27(Kip1) as the substrate. This outcome suggested that the polyubiquitination activity in the cytoplasm might contribute to an early phase of p27(Kip1) degradation in a Skp2-independent manner.
In addition to inducing the degradation of p27(Kip1) and promoting cellular proliferation, Skp2 also plays an important role in tumor invasiveness and metastasis. The numbers of migrated cells in the two Skp2-RNAi treated groups were found to be significantly fewer than those in the control groups. This observation suggested that Skp2-RNAi could inhibit the proliferation, migration and invasiveness of GBC-SD cells. We also studied the antitumor effect of Skp2-RNAi on nude mice in tumorigenicity experiments; the weights of the resulting tumors were decreased. This outcome suggested that treatment with Skp2-RNAi repressed the growth of metastatic tumors in vivo. Furthermore, the inhibition was shown to be positively associated with the dose of the lentivirus used. Hung et al[27] established Skp2-overexpressing stable transfectants in A549 human lung cancer cells and found that these stable transfectants exhibited increased migratory and invasive capabilities. Additionally, the expression of matrix metalloproteinase-2 (MMP-2) and MMP-9 were up-regulated and neutralization of these two MMPs using antibody-mediated approaches reduced cellular invasion. These data suggest that Skp2 promoted both tumor growth and metastasis and that enhanced expression of both MMP-2 and MMP-9 may have provided a contributory mechanism.
Moreover, Skp2 and p27(Kip1) have been shown to be useful indicators of prognosis[28-31]. Sanada et al[21] reported that Skp2 and p27(Kip1) were independent predictors of poor prognosis in patients with biliary tract cancers (BTCs). Discrepancies between SKP2 DNA copy number and the level of Skp2 protein were observed, although a correlation was found between copy number and protein expression in some primary BTCs. Therefore, it is formally possible that Skp2 protein expression could be considered a more accurate prognostic marker for BTCs than SKP2 gene copy number. Hashimoto et al[32] revealed that low levels of protein expression of p27(Kip1) and high Skp2 were associated with aggressive tumor behavior and that both p27(Kip1) and Skp2 could be considered useful markers in predicting the outcome of patients with intrahepatic cholangiocarcinomas. In an immunohistochemical study of 62 cases using tissue microarray, Li et al[20] confirmed that Skp2 over-expression represented the most significant independent adverse prognostic indicator in gallbladder carcinoma. Beyond the prognostic importance of Skp2/p27(Kip1), the development of drugs targeting Skp2 may provide novel molecular therapeutic approaches.
In summary, the results from our studies support the idea that Skp2 inhibitors and/or Skp2 regulatory sequences could provide a useful therapeutic protocol for the treatment of gallbladder carcinoma. In the future, the role of Skp2/p27(Kip1) in gallbladder carcinoma can be expected to be gradually unveiled.
We would like to thank Jing Zheng for technical and methodological support.
Primary gallbladder carcinoma is a common biliary malignancy, with a poor prognosis. p27 and S-phase kinase-associated protein-2 (Skp2) may play an important role in tumorigenesis and tumor development, and are closely associated with prognosis.
Inhibition of Skp2 or over-expression of p27(Kip1) could inhibit tumor growth and induce apoptosis.
The authors explored the effect of Skp2-RNAi on GBC-SD cells, and found that suppression of the Skp2 gene inhibited proliferation, migration and invasiveness of GBC-SD cells by mechanisms dependent on enhanced expression of p27 protein.
The results indicated that Skp2 inhibitors and/or Skp2 regulatory sequences such as Skp2-RNAi could provide a useful therapeutic protocol for the treatment of gallbladder carcinoma.
The authors used several assays to explore the role of Skp2 in gallbladder carcinoma. The results indicated that Skp2-RNAi might provide a useful therapeutic protocol for the treatment of gallbladder carcinoma.
P- Reviewers Guo JM, Lichtor T S- Editor Gou SX L- Editor Logan S E- Editor Ma S
| 1. | Clemente G, Nuzzo G, De Rose AM, Giovannini I, La Torre G, Ardito F, Giuliante F. Unexpected gallbladder cancer after laparoscopic cholecystectomy for acute cholecystitis: a worrisome picture. J Gastrointest Surg. 2012;16:1462-1468. [PubMed] |
| 2. | Lee J, Kim SS. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009;41:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Guan X, Wang Y, Xie R, Chen L, Bai J, Lu J, Kuo MT. p27(Kip1) as a prognostic factor in breast cancer: a systematic review and meta-analysis. J Cell Mol Med. 2010;14:944-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | He W, Wang X, Chen L, Guan X. A crosstalk imbalance between p27(Kip1) and its interacting molecules enhances breast carcinogenesis. Cancer Biother Radiopharm. 2012;27:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Groth A, Willumsen BM. High-density growth arrest in Ras-transformed cells: low Cdk kinase activities in spite of absence of p27(Kip) Cdk-complexes. Cell Signal. 2005;17:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Koh TY, Park SW, Park KH, Lee SG, Seol JG, Lee DW, Lee CT, Heo DS, Kim KH, Sung MW. Inhibitory effect of p27KIP1 gene transfer on head and neck squamous cell carcinoma cell lines. Head Neck. 2003;25:44-49. [PubMed] |
| 7. | Luo J, Chen YJ, Wang WY, Zou SQ. Effect of mutant p27(kipl) gene on human cholangiocarcinoma cell line, QBC(939). World J Gastroenterol. 2008;14:5344-5348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Jonason JH, Gavrilova N, Wu M, Zhang H, Sun H. Regulation of SCF(SKP2) ubiquitin E3 ligase assembly and p27(KIP1) proteolysis by the PTEN pathway and cyclin D1. Cell Cycle. 2007;6:951-961. [PubMed] |
| 9. | Shiraso S, Katayose Y, Yamamoto K, Mizuma M, Yabuuchi S, Oda A, Rikiyama T, Onogawa T, Yoshida H, Hayashi H. Overexpression of adenovirus-mediated p27kip1 lacking the Jab1-binding region enhances cytotoxicity and inhibits xenografted human cholangiocarcinoma growth. Anticancer Res. 2009;29:2015-2024. [PubMed] |
| 10. | Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell. 2007;26:131-143. [PubMed] |
| 11. | Rodriguez S, Wang L, Mumaw C, Srour EF, Lo Celso C, Nakayama K, Carlesso N. The SKP2 E3 ligase regulates basal homeostasis and stress-induced regeneration of HSCs. Blood. 2011;117:6509-6519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Gao D, Fukushima H, Inuzuka H, Liu P, Wan L, Sarkar FH, Wei W. Skp2: a novel potential therapeutic target for prostate cancer. Biochim Biophys Acta. 2012;1825:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Chan CH, Lee SW, Wang J, Lin HK. Regulation of Skp2 expression and activity and its role in cancer progression. ScientificWorldJournal. 2010;10:1001-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Nelsen CJ, Hansen LK, Rickheim DG, Chen C, Stanley MW, Krek W, Albrecht JH. Induction of hepatocyte proliferation and liver hyperplasia by the targeted expression of cyclin E and skp2. Oncogene. 2001;20:1825-1831. [PubMed] |
| 15. | Ravaioli A, Monti F, Regan MM, Maffini F, Mastropasqua MG, Spataro V, Castiglione-Gertsch M, Panzini I, Gianni L, Goldhirsch A. p27 and Skp2 immunoreactivity and its clinical significance with endocrine and chemo-endocrine treatments in node-negative early breast cancer. Ann Oncol. 2008;19:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Liu S, Yamauchi H. p27-Associated G1 arrest induced by hinokitiol in human malignant melanoma cells is mediated via down-regulation of pRb, Skp2 ubiquitin ligase, and impairment of Cdk2 function. Cancer Lett. 2009;286:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Xiang-Lan M, Zu-Lan S, Dan H, Bi-Hong S, Ya-Qin P, Han-Liang L. Skp2/p27 expression profile is correlated with Epstein-Barr virus status in extranodal nasal-type natural killer cell lymphoma. Transl Res. 2008;151:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Miyamoto T, Horiuchi A, Kashima H, Suzuki A, Yamada T, Kurai M, Konishi I, Shiozawa T. Inverse correlation between Skp2 and p27(Kip1) in normal endometrium and endometrial carcinoma. Gynecol Endocrinol. 2010;26:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Akrish S, Ben-Izhak O, Peled M. P27/SKP-2 histochemical profile is relevant to malignant salivary gland tumors (MST) histogenesis and tumor grade. Head Neck Pathol. 2012;6:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Li SH, Li CF, Sung MT, Eng HL, Hsiung CY, Huang WW, Lin CN, Yu SC, Huang HY. Skp2 is an independent prognosticator of gallbladder carcinoma among p27(Kip1)-interacting cell cycle regulators: an immunohistochemical study of 62 cases by tissue microarray. Mod Pathol. 2007;20:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Sanada T, Yokoi S, Arii S, Yasui K, Imoto I, Inazawa J. Skp2 overexpression is a p27Kip1-independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci. 2004;95:969-976. [PubMed] |
| 22. | Cubertafond P, Mathonnet M, Gainant A, Launois B. Radical surgery for gallbladder cancer. Results of the French Surgical Association Survey. Hepatogastroenterology. 1999;46:1567-1571. [PubMed] |
| 23. | Wolters T, Vissers KJ, Bangma CH, Schröder FH, van Leenders GJ. The value of EZH2, p27(kip1), BMI-1 and MIB-1 on biopsy specimens with low-risk prostate cancer in selecting men with significant prostate cancer at prostatectomy. BJU Int. 2010;106:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Chen J, Li D, Killary AM, Sen S, Amos CI, Evans DB, Abbruzzese JL, Frazier ML. Polymorphisms of p16, p27, p73, and MDM2 modulate response and survival of pancreatic cancer patients treated with preoperative chemoradiation. Ann Surg Oncol. 2009;16:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Andre F, Conforti R, Moeder CB, Mauguen A, Arnedos M, Berrada N, Delaloge S, Tomasic G, Spielmann M, Esteva FJ. Association between the nuclear to cytoplasmic ratio of p27 and the efficacy of adjuvant polychemotherapy in early breast cancer. Ann Oncol. 2012;23:2059-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S, Nakayama K. Degradation of p27(Kip1) at the G(0)-G(1) transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem. 2001;276:48937-48943. [PubMed] |
| 27. | Hung WC, Tseng WL, Shiea J, Chang HC. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer Lett. 2010;288:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Skirnisdottir I, Seidal T. Association of p21, p21 p27 and p21 p53 status to histological subtypes and prognosis in low-stage epithelial ovarian cancer. Cancer Genomics Proteomics. 2013;10:27-34. [PubMed] |
| 30. | Liu J, Wei XL, Huang WH, Chen CF, Bai JW, Zhang GJ. Cytoplasmic Skp2 expression is associated with p-Akt1 and predicts poor prognosis in human breast carcinomas. PLoS One. 2012;7:e52675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Zhuang Y, Yin HT, Yin XL, Wang J, Zhang DP. High p27 expression is associated with a better prognosis in East Asian non-small cell lung cancer patients. Clin Chim Acta. 2011;412:2228-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Hashimoto N, Yachida S, Okano K, Wakabayashi H, Imaida K, Kurokohchi K, Masaki T, Kinoshita H, Tominaga M, Ajiki T. Immunohistochemically detected expression of p27(Kip1) and Skp2 predicts survival in patients with intrahepatic cholangiocarcinomas. Ann Surg Oncol. 2009;16:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |