Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4252
Revised: May 4, 2013
Accepted: May 16, 2013
Published online: July 14, 2013
Processing time: 100 Days and 8.4 Hours
Lymphocytic and collagenous colitis are forms of microscopic colitis which typically presents in elderly patients as chronic watery diarrhea. The association between microscopic colitis and inflammatory bowel disease is weak and unclear. Lymphocytic colitis progressing to ulcerative colitis has been previously reported; however there is limited data on ulcerative colitis evolving into microscopic (lymphocytic or collagenous) colitis. We report a series of six patients with documented ulcerative colitis who subsequently were diagnosed with collagenous colitis or lymphocytic colitis suggesting microscopic colitis could be a part of the spectrum of inflammatory bowel disease. The median duration of ulcerative colitis prior to being diagnosed with microscopic colitis was 15 years. We noted complete histological and/or symptomatic remission in three out of six cases while the other three patients reverted back into ulcerative colitis suggesting lymphocytic or collagenous colitis could present as a continuum of ulcerative colitis. The exact molecular mechanism of this histological transformation or the prognostic implications is still unclear. Till then it might be prudent to follow up these patients to assess for the relapse of inflammatory bowel disease as well as for dysplasia surveillance.
Core tip: Lymphocytic colitis (LC), together with collagenous colitis (CC) is a part of the spectrum of “microscopic colitis” (MC) characterized by profuse non-bloody watery diarrhea, without endoscopic or radiological lesions, but with histological abnormalities. The association between LC and inflammatory bowel disease (IBD) is weak and unclear. The case reports of CC progressing to ulcerative colitis (UC) and vice versa has been previously reported but however to our knowledge we report the first case series of six patients with chronic UC subsequently developing into CC or LC suggesting MC could be a part of the spectrum of IBD.
- Citation: Jegadeesan R, Liu X, Pagadala MR, Gutierrez N, Butt M, Navaneethan U. Microscopic colitis: Is it a spectrum of inflammatory bowel disease? World J Gastroenterol 2013; 19(26): 4252-4256
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4252
Lymphocytic colitis (LC), together with collagenous colitis (CC) is a part of the spectrum of “microscopic colitis” (MC) characterized by profuse non-bloody watery diarrhea, without endoscopic or radiological lesions, but with histological abnormalities. LC is characterized by increased lymphocytic infiltration of the colonic epithelium and lamina propria. CC in addition to the inflammatory infiltrate is characterized by a markedly thickened sub epithelial collagen band adjacent to the basal membrane. The association between LC and inflammatory bowel disease (IBD) is weak and unclear. The case reports of CC progressing to ulcerative colitis (UC) and vice versa has been previously reported but however to our knowledge we report the first case series of six patients with chronic UC subsequently developing into CC or LC suggesting MC could be a part of the spectrum of IBD[1-4].
We evaluated more than 1000 UC patients from a retrospectively collected UC colonoscopy database who had more than 3000 colonoscopies at our institution from 1998-2011. We identified a total of six patients with documented UC who subsequently were diagnosed with biopsy proven CC or LC from this database. All six patients were seen at our institution with the underlying UC for further management and the diagnosis of UC was reconfirmed by colonoscopic study in our institution. When these patients were followed up either for the change in symptoms or surveillance with the colonoscopic studies, colonic biopsies revealed CC or LC with no evidence of UC (Table 1).
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| Age at the time of last follow-up (yr) | 81 | 75 | 62 | 60 | 52 | 36 |
| Gender | Female | Male | Male | Female | Female | Female |
| Age at diagnosis of UC | 65 | 42 | 28 | 46 | 34 | 18 |
| Age at diagnosis of LC/CC | 79 | 72 | 48 | 54 | 45 | 33 |
| Duration of UC when LC/CC was diagnosed | 14 | 30 | 20 | 8 | 11 | 15 |
| Extent of UC when LC/CC was diagnosed | Extensive colitis | Extensive colitis | Left-sided | Left-sided | Left-sided | Extensive colitis |
| Extent of LC/CC at diagnosis | Extensive colitis | Extensive colitis | Right-sided | Extensive colitis | Extensive colitis | Extensive colitis |
| Endoscopic findings at the time of LC/CC diagnosis | Mild generalized redness | Mild erythema at left colon | Normal | Normal | Normal | Mild erythema at distal rectum |
| Medication at the time of LC/CC diagnosis | Mesalamine | None | Sulfasalazine, 6- mercaptopurine | Mesalamine | Mesalamine | Mesalamine, 6- mercaptopurine |
| Medications added when LC/CC was diagnosed | None | Sulfasalazine | None | Bismuth | None | None |
| Outcome on subsequent follow up | Asymptomatic | Asymptomatic | Ulcerative colitis | Asymptomatic | Ulcerative colitis | Ulcerative colitis |
| Family history of IBD/LC/CC | UC | None | None | None | None | UC |
| Smoking | Yes | Ex-smoker | No | Yes | Yes | Ex-smoker |
| History | ||||||
| Alcohol use history | Yes alcoholic | Yes | No | No | Yes | No |
| History of aspirin/NSAIDS intake | No | Aspirin | No | NSAIDS | No | No |
| Period of follow up after LC/CC was diagnosed | 3 mo | 3 yr | 10 yr | 3 yr | 8 yr | 3 yr |
Diagnostic criteria used by our pathologists to diagnose LC is increased intraepithelial lymphocytes (IELs > 20/100 colonic surface epithelial cells) in an architecturally normal colonic mucosa, accompanied by surface epithelial damage and a mixed mononuclear inflammatory infiltrate in the lamina propria[5]. These patients were treated for LC and on subsequent follow up three out of six patients reverted back to UC. All the slides were re-examined by a single pathologist and the diagnosis was confirmed (Liu X).
A 79-year-old female with a history of UC for 14 years presented with complaints of left lower quadrant abdominal pain for 1 month duration. She also had intermittent watery diarrhea and fecal urgency over the past 2 years. She denied bloody diarrhea or recent weight loss. She was on maintenance mesalamine, and UC was kept under complete remission. She was evaluated with colonoscopy for the present complaints which revealed very mild generalized redness throughout colon. Random colonic biopsies suggested marked surface epithelial lymphocytosis with collagen deposition in all areas of the colon consistent with the diagnosis of CC without any evidence of UC. She was treated with mesalamine 2400 mg/d and complete resolution of symptoms occurred within a month. She was asked to continue mesalamine and follow up if symptoms recur again. She has not had any recurrence of any symptoms.
A 75-year-old male with a history of UC for 25 years came for the surveillance colonoscopy to our institution. The surveillance colonoscopy with random colon biopsies revealed chronic quiescent UC involving the entire colon with no dysplasia. Subsequently, the patient developed intermittent watery diarrhea with fecal urgency. He denied nocturnal symptoms, bloody diarrhea and recent weight loss. He had a history of aspirin intake but there was no temporal relationship between aspirin intake and onset of diarrhea. Further work up with colonoscopy revealed mild erythema in left colon. The colon biopsies were consistent with LC throughout the colon with a significant increase in the number of intraepithelial lymphocytes without any evidence of UC. He was started on sulfasalazine and symptoms resolved within a month. Surveillance colonoscopy done 2 years later was macroscopically normal. Histopathological studies were negative both for UC and LC. He was asymptomatic to follow up till date.
A 61-year-old male with a long standing history of left-sided UC maintained on remission with sulfasalazine and 6-mercaptopurine had a surveillance colonoscopy performed for UC at our institution 20 years later and was macroscopically normal. Colonic biopsies revealed evidence of LC in the right colon. He was not treated for LC since he was asymptomatic on maintenance treatment. Four years later another surveillance colonoscopy revealed chronic quiescent UC. He was completely asymptomatic through these years.
A 59-year-old female was diagnosed with left-sided UC at the age of 46 and was maintained on mesalamine with complete symptomatic remission. She subsequently developed symptoms of intermittent watery diarrhea accompanied by abdominal cramps. She was treated with prednisone and mesalamine with no symptomatic improvement. She had a history of ibuprofen intake for back pain for a long time and there was no association between ibuprofen and the development of new symptoms. She was further evaluated with colonoscopy which revealed increased intraepithelial lymphocytes with collagen deposition throughout the colon consistent with CC. She was treated with bismuth and her symptoms resolved within 2 mo. The follow up colonoscopy a year later was negative for CC.
A 53-year-old female with a history of left-sided UC for 16 years had surveillance colonoscopy performed which revealed inactive UC in rectum and a tubular adenoma in the descending colon. She was referred to our institution for an opinion regarding UC and tubular adenoma. She was treated with mesalamine for UC in the past and she was kept under complete remission. She was evaluated with a colonoscopy in our institution which suggested macroscopically normal colon with a histop anthology consistent with LC without any evidence of dysplasia. There was no change in treatment. Subsequently, surveillance colonoscopy done a year later revealed mild inactive UC in rectum and sigmoid colon without any evidence of LC and dysplasia. Two subsequent surveillance colonoscopies were positive for chronic UC in sigmoid colon and rectum without any dysplasia.
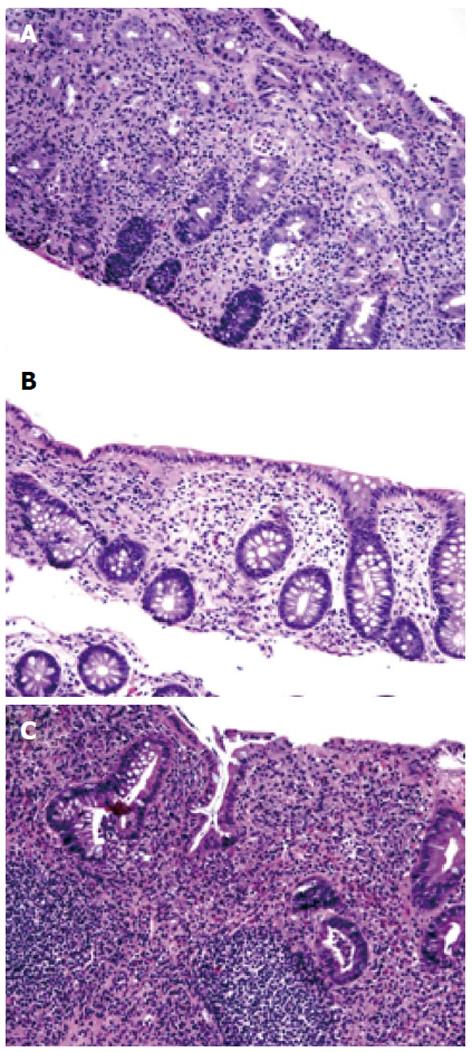
A 36-year-old female had a long standing history of extensive colitis for 18 years. She developed steroid dependent UC and required 6-mercaptopurine and mesalamine for symptom control (Figure 1A). She was maintained on complete remission on these medications. She had a surveillance colonoscopy performed which revealed mild erythema in distal rectum. The colonic biopsy studies were consistent with LC (Figure 1B) in all other areas of the colon. She had a follow up colonoscopy three years later which showed left-sided chronic active UC without any evidence of LC (Figure 1C). She has been asymptomatic at the time of last follow-up.
LC is characterized by chronic watery diarrhea and specific histopathological changes in a macroscopically normal colonic mucosa. The incidence has been reported to be 5.5 per 100000 and prevalence is 63.7 per 100000[6], but the incidence and prevalence appears to be increasing over time. The median age at diagnosis is 59 years with female: male ratio of 2.4:1. The most frequent symptoms at presentation are diarrhea, abdominal pain, weight loss and fecal urgency. Most patients are in remission with a limited disease duration of 6 mo; it can be chronic intermittent or chronic continuous in minority of patients. 7 However, relatively limited data has been published on the relationship between inflammatory bowel disease (IBD) and LC[7-11].
The etiology of LC is largely unknown and probably multifactorial. At present, it is thought to be caused by immunological reaction to different mucosal insults in predisposed individuals. The frequent association of LC with other autoimmune disorders (thyroid disease, diabetes mellitus, celiac disease, psoriasis, and rheumatoid arthritis), inflammation in the lamina propria with increased intraepithelial lymphocytes and the fair response to steroids support this theory. Infectious agents, drugs, or food antigen such as gluten may be precipitating factors. The importance of genetic factors in LC is still unclear but Olesen et al[7] suggested a family history of IBD in patients with LC. We describe six cases of UC that subsequently evolved into CC or LC. The median age at the time of UC diagnosis was 38 years in our series. The median duration of UC prior to being diagnosed with CC or LC was 15 years. The median age at the time of LC diagnosis was 51 years. We noted complete histological and/or symptomatic remission in three out of six cases while the other three patients reverted back into UC suggesting that LC could present as a continuum of UC. The triggering factor for this transformation is still unknown. The association between MC and IBD is found predominantly in patients with extensive colitis[12]. However, in our case series only 50% of patients had extensive colitis when LC or CC was diagnosed.
The relationship between LC and IBD is unclear. Surveillance colonoscopic biopsies from IBD patients with inactive disease may show a collagenous colitis pattern in UC and a focal LC-like pattern with Crohn’s disease[1,12,13]. In our patients, the histopathological diagnosis of LC when UC was in complete remission further raises the question whether the observed LC pattern is an expression of healing and inactive UC disease in reality. However, the presence of symptoms in some patients and lack of IBD flare immediately prior to LC diagnosis makes this “healing” theory unlikely. A previous study had demonstrated that the transcriptional factor nuclear factor κB activation occurs in both UC and CC patients. However in CC patients, nuclear factor κB activation occurs only in epithelial cells whereas it occurs both in epithelial cells and lamina propria macrophages in UC patients[14]. Hence it is possible that the site of nuclear factor κB activation determines the pathological manifestation of the disease. Either UC or MC may precede the onset of the other. Based on these data, it seems reasonable to assume that UC and MC could represent both ends of the spectrum of the same disorder.
With the aggregate of cases we have reported along with other few case reports it seems highly reasonable to assume that LC or CC could be a part of the spectrum of IBD. Whether it is a random coincidence of MC and IBD in the same patient remains to be answered. Since there was no specific cause of LC in our patients such as an infection or drugs, along with the absence of autoimmune conditions usually associated with LC further supports our current view. The prognostic implication of this histological transformation to LC in inactive UC patients is still unknown and has to be further evaluated with prospective studies. Until then it might be prudent to consider MC as a part of the natural history of IBD, at least in some cases, and follow up these patients to assess for the relapse of IBD as well as for dysplasia surveillance.
P- Reviewer Keszthelyi D S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Freeman HJ, Berean KW, Nimmo M. Evolution of collagenous colitis into severe and extensive ulcerative colitis. Can J Gastroenterol. 2007;21:315-318. [PubMed] |
| 2. | Pokorny CS, Kneale KL, Henderson CJ. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol. 2001;32:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Aqel B, Bishop M, Krishna M, Cangemi J. Collagenous colitis evolving into ulcerative colitis: a case report and review of the literature. Dig Dis Sci. 2003;48:2323-2327. [PubMed] |
| 4. | Haque M, Florin T. Progression of ulcerative colitis to collagenous colitis: chance, evolution or association. Inflamm Bowel Dis. 2007;13:1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Mahajan D, Goldblum JR, Xiao SY, Shen B, Liu X. Lymphocytic colitis and collagenous colitis: a review of clinicopathologic features and immunologic abnormalities. Adv Anat Pathol. 2012;19:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Pardi DS, Loftus EV, Smyrk TC, Kammer PP, Tremaine WJ, Schleck CD, Harmsen WS, Zinsmeister AR, Melton LJ, Sandborn WJ. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Baert F, Wouters K, D’Haens G, Hoang P, Naegels S, D’Heygere F, Holvoet J, Louis E, Devos M, Geboes K. Lymphocytic colitis: a distinct clinical entity A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Mullhaupt B, Güller U, Anabitarte M, Güller R, Fried M. Lymphocytic colitis: clinical presentation and long term course. Gut. 1998;43:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Giardiello FM, Lazenby AJ, Bayless TM, Levine EJ, Bias WB, Ladenson PW, Hutcheon DF, Derevjanik NL, Yardley JH. Lymphocytic (microscopic) colitis. Clinicopathologic study of 18 patients and comparison to collagenous colitis. Dig Dis Sci. 1989;34:1730-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Pardi DS, Ramnath VR, Loftus EV, Tremaine WJ, Sandborn WJ. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol. 2002;97:2829-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Goldblum JR, Wang N. Lymphocytic and collagenous colitis as possible patterns of Crohn’s colitis. Am J Surg Pathol. 2000;24:755-756; author reply 755-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Goldstein NS, Gyorfi T. Focal lymphocytic colitis and collagenous colitis: patterns of Crohn’s colitis. Am J Surg Pathol. 1999;23:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Andresen L, Jørgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |