Published online Jun 28, 2013. doi: 10.3748/wjg.v19.i24.3841
Revised: May 24, 2013
Accepted: June 1, 2013
Published online: June 28, 2013
Processing time: 45 Days and 12.1 Hours
AIM: To investigate the safety and efficacy of anus-preserving rectectomy via telescopic colorectal mucosal anastomosis (TCMA) for low rectal cancer.
METHODS: From August 1993 to October 2012, 420 patients including 253 males and 167 females with low rectal cancer underwent transabdominal and transanal anterior resection, followed by TCMA. The distance between the anus and inferior margin of the tumor ranged from 5 to 7 cm, and was 5 cm in 6 patients, 6 cm in 127, and 7 cm in 287 patients. Tumor-node-metastasis staging showed that 136 patients had stage I, 252 had stage II and 32 had stage III. Fifty-six patients with T3 or over received preoperative neoadjuvant chemoradiotherapy.
RESULTS: The postoperative follow-up rate was 91.9% (386/420) with a median time of 6.4 years. All 420 patients underwent radical resection. No postoperative death occurred. Postoperative complications included anastomotic leakage in 13 (3.1%) patients and anastomotic stenosis in 7 (1.6%). The local recurrence rate after surgery was 6.2%, the hepatic metastasis rate was 13.2% and the pulmonary metastasis rate was 2.3%. The 5-year survival rate was 74.0% and the disease-free survival rate was 71.0%. Kirwan classification showed that continence was good in 94.4% of patients with stage I when scored 12 mo after resection.
CONCLUSION: TCMA for patients with low rectal cancer leads to better quality of life and satisfactory defecation function, and lowers anastomotic leakage occurrence, and might be one of the safe operative procedures in anus-preserving rectectomy.
Core tip: Li et al developed the telescopic colorectal mucosal anastomosis technique based on the experiences and lessons from several sphincter-preserving operations under preconditions of low rectal resection, which improved the anastomotic stoma and alleviated tension. With this modified technique used over the past 20 years, the incidence of anastomotic leakage was significantly decreased and the long-term outcome was satisfactory with good anal function and a lower rate of incontinence.
-
Citation: Li SY, Chen G, Bai X, Zuo FY, Chen G, Du JF, Wei XJ, Cui W. Anus-preserving rectectomy
via telescopic colorectal mucosal anastomosis for low rectal cancer: Experience from a Chinese cohort. World J Gastroenterol 2013; 19(24): 3841-3846 - URL: https://www.wjgnet.com/1007-9327/full/v19/i24/3841.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i24.3841
Abdominoperineal resection (APR) is thought to be the gold standard in the treatment of low rectal cancer less than 5 cm from the anal verge[1,2]. However, over the last 50 years, surgeons realized that permanent colostomy led to inconvenience in terms of the social life of patients and mental health issues[3,4]. Dixon developed the operative procedure of anterior resection, in which end-end anastomosis of the sigmoid colon and rectum was performed after radical resection of low rectal cancer. However, it was difficult to perform this operation in overweight patients with a narrow pelvis[5]. Parks et al[6] proposed a colon-anal anastomosis, which was modified by Bacon’s operation with the preservation of both internal and external anal sphincters. Due to satisfactory clinical results, this type of operation was popular in European countries, however, a temporary diverting stoma was routinely required to ensure healing of the anastomotic stoma[7]. Heald et al[8] reported total mesorectal excision (TME) for the first time, involving resection of the mesorectum more than 5 cm from the distal margins of the tumor, which reduced the local recurrence rate, and improved the survival rate of patients with rectal cancer[9,10]. However, potential ischemia of the distal bowel during such surgery could lead to an increased rate of anastomotic leakage.
Li et al[11] developed the telescopic colorectal mucosal anastomosis (TCMA) based on the experiences and lessons from several sphincter-preserving operations under preconditions of low rectal resection, which improved the anastomotic stoma and alleviated tension. With this modified technique, the incidence of anastomotic leakage was significantly decreased and the long-term outcome was satisfactory with good anal function and a lower rate of incontinence[11-13]. In this study, we summarized the influential factors for high-incidence anastomotic leakage after sphincter-preserving surgery in radical rectal resection (8.1%-18.0%), including anastomotic skills, blood supply and tension of the anastomotic stoma[6,8,14-16].
From August 1993 to August 2012, we treated 1510 patients with rectal cancer surgically at the Department of General Surgery, General Hospital of Beijing Military Command, China. Of these patients, 576 (38.1%) underwent Miles’ procedure and 420 (27.8%) underwent TCMA for rectal carcinoma less than 7 cm from the anal verge. There were 253 male and 167 female patients, with an average age of 55.7 years (range: 21-91 years). The distance between the lower margin of the tumor and the anal verge varied from 5 to 7 cm, and was 7 cm in 287 patients, 6 cm in 127, and 5 cm in 6 patients. The distance was measured with a rigid sigmoidoscope. All 420 patients were examined by rectal touch, colonoscopy, barium enema, magnetic resonance imaging (MRI) and endorectal ultrasonography. Primary malignant rectal neoplasms were confirmed by biopsy, and preoperative tumor-node-metastasis (TNM) staging of the patients was also carried out.
Preoperative TNM staging in the 420 patients was as follows: stage I, n = 136; stage II, n = 252 and stage III, n = 32. Of these patients, 56 above T3 received neoadjuvant chemoradiotherapy. They were given capecitabine 1500 mg twice a day orally for 1 mo. Radiotherapy was added during the chemotherapy cycle (45-50 Gy in 25-28 fractions to the pelvis). The tumor staging (assessed by MRI) after 6-8 wk of neoadjuvant therapy was as follows: T0, n = 4; T1, n = 24; T2, n = 26; T3, n = 2 (Table 1).
| Preoperative stage | Cases | Postoperative stage (n) |
| T3 | 50 | T0 (4), T1 (24), T2 (22) |
| T4 | 6 | T0 (0), T1 (0), T2 (4), T3 (2) |
| Total | 56 | T0 (4), T1 (24), T2 (26), T3 (2) |
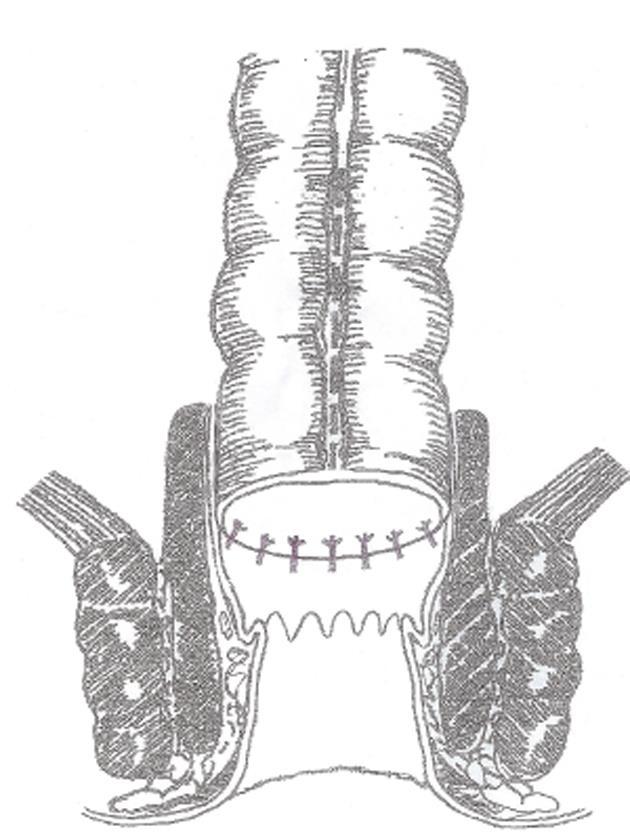
Surgical procedures were performed according to the TME principles and the methods previously described by Li et al[11]. The schematic layout of the anus-preserving procedure via TCMA is shown in Figure 1. Under general anesthesia and continuous epidural anesthesia, the patient was placed in the lithotomy position. The procedures routinely involved high ligation of the inferior mesenteric artery and dissection to the levator ani under direct vision. The rectum was mobilized to the pelvic floor as low as possible to facilitate the perianal approach. If the lower edge of the tumor was reached, a clamp was applied below the tumor to close the rectum when possible.
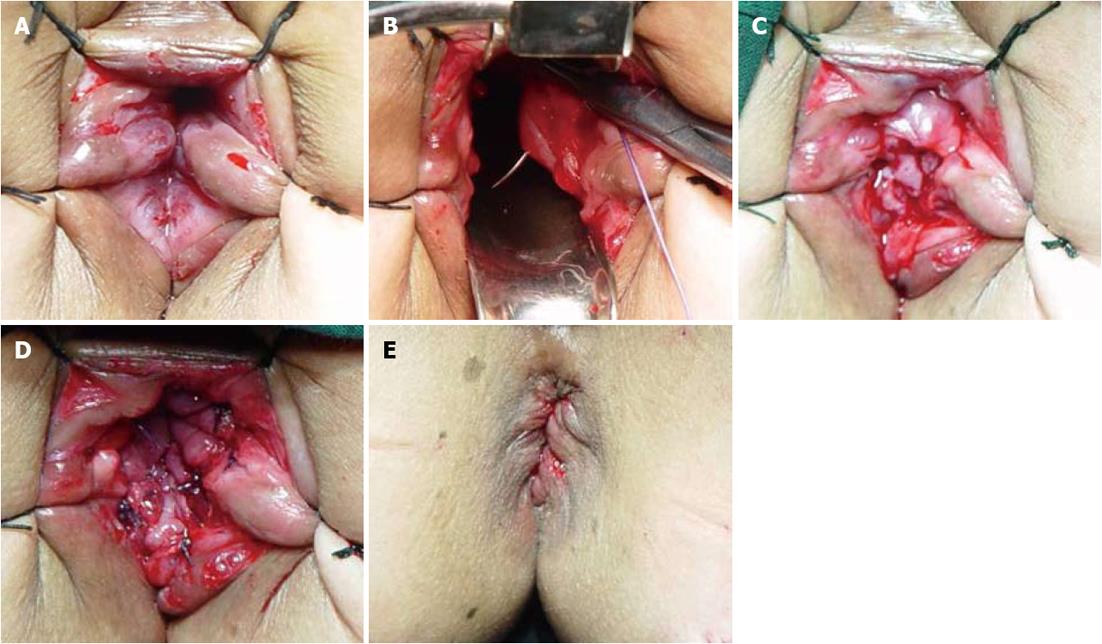
Following the abdominal approach, sufficient relaxation of the anal sphincter was achieved by finger expansion under continuous epidural anesthesia. Wide exposure of the operative field above the dentate line was achieved using the “5-stitches-suspension” method (Figure 2A). To prevent bleeding, 2-3 mL of saline adrenaline solution (1:10000) was injected into the anal canal 1.0 cm above the dentate line, which resulted in swelling of the mucosa. A circumferential incision of the mucosa was made at 1.5-2.0 cm above the dentate line. Dissection was performed by mobilizing the rectum through the mucosal plane to approximately 2-4 cm (Figure 2B), and then the distal margin of the rectum was clamped and cut, with preservation of the entire muscular sheath of the rectum. Later, the distal end of the colon was pulled through the anus, and TCMA of the sero-muscular layer and muscular sheath was performed at 2.0 cm above the dentate line (Figure 2C). Four interrupted absorbable sutures were placed at the 12, 3, 6 and 9 o’clock positions in the lithotomy position, respectively, for fixation and relaxation. Similarly, 4 interrupted absorbable sutures in the distal end of the colon and the residual rectal mucosa were also placed at the 12, 3, 6 and 9 o’clock positions, followed by 4-8 additional sutures (Figure 2D). To remove the dermal sutures and reposition the anastomotic stoma back in the anal canal (Figure 2E), a pelvic drainage tube was placed before closure of the abdominal wall, and was removed 4-5 d after surgery.
Patients with greater than T2 stage received 7-12 cycles of postoperative systemic chemotherapy with the mFOLFOX4 protocol (oxaliplatin, 5-fluorouracil and calcium folinate). Eighty-eight patients with T4 stage and 23 patients with positive circumferential margins after resection were given postoperative pelvis radiotherapy at a total dose of 10-20 Gy before adjuvant chemotherapy.
Patients were seen within one month following resection for monitoring postoperative complications, such as bleeding, pelvic abscess and anastomotic leakage. Follow-up was performed every 3 mo for 2 years, every 6 mo for 3 years and then every 1 year thereafter. All patients underwent digital examination, laboratory studies (stool analysis including occult blood, serum carcinoembryonic antigen levels) and imaging examination (abdominal ultrasound, chest X-ray, and pelvic computed tomography/MRI). Colonoscopy was performed every 6 mo for 5 years after surgery. Anastomotic stenosis was confirmed by colonoscopy under direct vision within 1 year after operation. Local recurrence was defined as the first clinical, radiologic and/or pathologic evidence of tumor of the same histologic type within the pelvis 2-3 years after surgery. Distant recurrence was defined as clinical, radiologic, and/or pathologic evidence of systemic disease outside the pelvis, at sites including liver and lungs 5 years after surgery. Death of patients was recognized as the end of follow-up. All the clinical data were collected from the follow-up records at different time-points.
A questionnaire on anal function was completed after surgery according to Kirwan staging criteria[17].
Overall survival and disease-free survival were calculated by the Kaplan-Meier method. Statistical analysis was performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, United States).
The postoperative follow-up rate in this series was 91.9% (386/420), with a median time of 6.4 years. All 420 patients underwent radical resection. The distance between the distal margins of the tumor ranged from 2 to 5 cm (mean 3.2 cm). Negative distal margins were confirmed pathologically in all 420 cases, while positive circumferential margins were observed in 23 cases (5.4%). A pathological diagnosis was made in 148 patients with well differentiated adenocarcinoma, in 249 patients with moderately differentiated adenocarcinoma, in 16 patients with poorly differentiated adenocarcinoma, and in 7 patients with adenomatous canceration.
According to the TNM staging principles of the 2010 National Comprehensive Cancer Network guidelines, postoperative pathological staging showed: 142 patients with stage I, 250 patients with stage II (IIa: 177 patients, IIb: 61 patients, and IIc: 12 patients) and 28 patients with stage III (IIIa: 13 patients, IIIb: 9 patients, and IIIc: 6 patients) (Table 2).
| Characteristics | Data |
| Patients (n) | 420 |
| Age (yr) | 55.7 (range: 21.0-91.0) |
| Gender | |
| Male | 253 |
| Female | 167 |
| Distance of tumor from anal verge (cm) | |
| 7 | 287 |
| 6 | 127 |
| 5 | 6 |
| Preoperative tumor stage | |
| I | 136 |
| II | 252 |
| III | 32 |
| Postoperative tumor stage | |
| I | 142 |
| II | 250 (IIa: 177, IIb: 61, IIc: 12) |
| III | 28 (IIIa: 13, IIIb: 9, IIIc: 6) |
| Differentiation of tumors | |
| Well differentiated | 148 |
| Moderately differentiated | 249 |
| Poorly differentiated | 16 |
| Adenomatous canceration | 7 |
| Temporary diverting stoma | |
| Yes | 0 |
| No | 420 |
| Surgical time (min) | 130 (range: 110-190) |
| Intraoperative blood loss (mL) | 360 (range: 150-1200) |
| Hospital stay (d) | 13 (range: 7-31) |
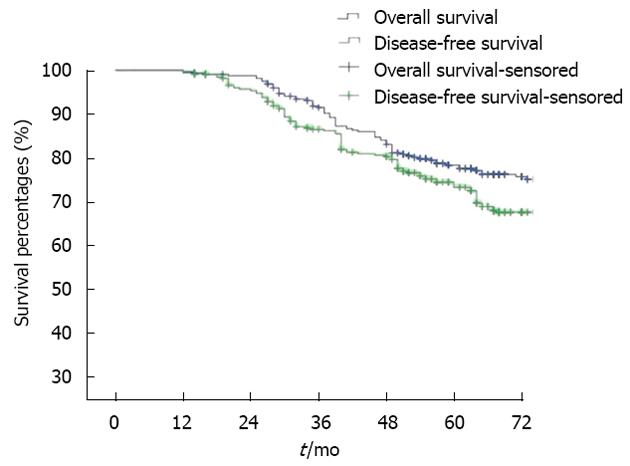
No postoperative death occurred in this series. Anastomotic leakage occurred in 13 patients (3.1%), of whom 7 received conservative therapy (total parenteral nutrition and continuous drainage), and 5 underwent transverse colostomy (stoma apothesis after 3 mo). Anastomotic stenosis occurred in 7 patients (1.6%), these patients recovered with continuous expansion of the anus for 1-3 mo. The postoperative local recurrence rate in the series was 6.2% (26 cases), recurrence was seen 2-3 years after surgery. The rate of metastasis to the liver and the lung was 13.2% (51 cases) and 2.3% (9 cases), respectively. The postoperative 5-year survival rate was 74% and the 5-year disease-free survival rate was 71% (Figure 3).
Enteral nutrition was administered to patients during the early postoperative period. These patients had poor continence, with approximately 6-9 bowel movements per day, which could be controlled to 3-6 times per day following oral intake of compound diphenoxylate 2 pills three times per day. Two to 4 mo after surgery, patients had better continence and recovered anal function 12 mo after surgery. Kirwan staging[17] was stage I in 369 patients (94.4%), stage II in 20 patients (5.1%), and stage III in 2 patients (0.5%).
Despite the improved clinical results of anterior resection via all types of anus-preserving procedures for treating low rectal cancer, several issues remain controversial such as the incidence of anastomotic leakage, the local recurrence rate and anal function outcome[11,18,19]. In 1993, Li et al developed the telescopic anastomosis technique for treating low rectal cancer, focusing on relaxation sutures, while strengthening the anastomotic stoma. The use of this method with wide exposure and a refined surgical technique effectively controlled anastomotic leakage. In our series, the rate of anastomotic leakage was 3.1%, which was significantly lower than the rate of 8.0%-18.0% reported elsewhere. Clinical data indicated that TCMA was a reliable, safe and superior surgical procedure[6,8,14].
The mechanisms of defecation involve both sphincter contraction reflection[20] and complete physiological reflection of the rectal mucosa[21]. In anus-preserving procedures, low anastomosis may impair the regions of these reflections. Thus, the intraoperative prevention of impairment in such regions could contribute to better anal function after surgery. In TCMA, colorectal anastomosis should be performed on the rectum plane 1.5-2.0 cm above the dentate line, with the preservation of anal sphincter function and enough residual rectum to protect complete physiological nervous reflection of defecation, which is completely different from that of anus-preserving procedures with resection of the internal sphincter[22-24]. Obviously, without impairment of the internal sphincter and normal anal construction, patients could have a recovery of 97.6%-99.5% Kirwan stages I and II defecation function 6-12 mo after operation, with improved quality of life.
Long-term clinical outcome depends on radical resection of the tumor and sufficient dissection of lymph nodes according to the TME principles[25], which help to obtain both negative distal and circumferential margins for lowering local recurrence after surgery[26,27]. In our series, patients with T3-4 staging received preoperative neoadjuvant radiochemotherapy to downgrade tumor staging and to facilitate radical resection and anus-preserving procedures. Patients with stage II or above underwent postoperative systemic chemotherapy, and those with T4 staging or positive circumferential margins confirmed by pathological examination received postoperative pelvic radiotherapy. The data showed that the patients who received comprehensive treatment exhibited a local recurrence rate of 6.2% and a 5-year survival rate of 74%, which were not significantly different from those of Miles. With 20 years of use in clinical practice, TCMA has been proved to be a safe and feasible treatment for low rectal cancer and one of the effective standard anus-preserving procedures.
Wide exposure of the operative field by the “5-stitches-suspension” method (Figure 3) and adequate muscle relaxation during anesthesia play a dominant role in TCMA[11]. Perianal anastomosis could be facilitated after satisfactory relaxation of the anal sphincter, and the injury of perianal architectures could be avoided and anal function restored after surgery[11,13].
Perianal dissection through the rectal mucosa plane requires prevention of bleeding by the circumferential injection of saline adrenaline solution (1:10000) 1.0 cm above the dentate line and adequate exposure of the internal sphincteric plane to achieve complete excision of the distal mucosa, while avoiding injury of the internal sphincter[11,13]. In brief, a circumferential incision of the mucosa and internal anal sphincter is made at 1.5-2.0 cm above the dentate line. The dissection continues upward between the mucosa and the superficial layer of the internal sphincter for the resection of 2-4 cm of distal rectal mucosa (Figure 2B).
Relaxation and strengthening prevent anastomotic leakage, using a 4-stitches relaxation suture of the colonal sero-muscular layer and residual rectal muscular sheath[11,13] (Figure 2C). More importantly, penetrating the whole layer of the bowel wall and/or the posterior wall of the vagina is avoided to prevent the occurrence of intestinal and vaginal fistula.
TCMA involves telescopic anastomosis between the whole layer of the colon and residual mucous and the submucous layer of the rectum. The distal margin of the colon should be modified by the resection of adipose tissue with well preserved blood supply to facilitate healing of the anastomotic stoma. Absorbable interrupted sutures are placed at the 6 and 12 o’clock positions, then at the 3 and 9 o’clock positions, followed by the addition of 4-8 sutures to avoid postoperative stenosis (Figure 2D).
After TCMA, the 5-suspension-stitches are removed, and the anastomotic stoma is repositioned. Vaseline gauze can be used for support and is removed 48-72 h after surgery (Figure 2E).
Anus-preserving operations should be performed under the conditions mentioned above in addition to sufficient mobilization of the rectum.
In conclusion, anus-preserving procedures via trans-abdominal radical anterior resection and trans-anal TCMA to treat patients with low rectal cancer can achieve satisfactory recovery of anal function with a decreased incidence of anastomotic leakage and a moderate local recurrence rate. In comparison with APR, this modified treatment can improve patient quality of life. TCMA might be one of the standard surgical options in treating low rectal cancer.
For many years, abdominoperineal resection (APR) was the treatment of choice for most patients with rectal cancer. Recent advances in surgical technique and other treatment modalities have led to a marked increase in the rate of sphincter-sparing operations, with a concomitant decrease in APR as permanent colostomy leads to inconvenience in terms of the social life of patients and mental health issues. In 1993, Li et al developed the telescopic colorectal mucosal anastomosis (TCMA) for treating low rectal cancer, focusing on relaxation sutures, while strengthening the anastomotic stoma. This modified technique effectively controlled anastomotic leakage.
With the modified surgical techniques used over the past 20 years in this study, the incidence of anastomotic leakage was decreased significantly and the long-term outcome was satisfactory with good anal function and a lower rate of incontinence. Anus-preserving procedures via trans-abdominal radical anterior resection and trans-anal TCMA in treating patients with low rectal cancer could achieve a satisfactory recovery of anal function with a decreased incidence of anastomotic leakage and a moderate local recurrence rate. In comparison with APR, TCMA can greatly improve the quality of life of the patients, and could be one of the standard surgical options in treating low rectal cancer.
Despite the improved clinical results of anterior resection in treating low rectal cancer by all kinds of anus-preserving procedures, several issues remain controversial such as the incidence of anastomotic leakage, the local recurrence rate and anal functional outcome. The telescopic anastomosis technique was developed to treat low rectal cancer while strengthening the anastomotic stoma. Using this method with wide exposure and refined surgical technique, the anastomotic leakage could be controlled effectively. In this series, the rate of anastomotic leakage was significantly lower than the reported rate elsewhere. Clinical data indicated that TCMA is a reliable, safe and superior surgical procedure for low rectal cancer.
TCMA could be one of the standard surgical options in treating low rectal cancer. The transabdominal and transanal anterior resection for low rectal cancer via colorectal mucosal anastomosis leads to a better life quality with satisfactory defecation function, while lowering the occurrence of anastomotic leakage. Telescopic anastomosis is one of the safe operative procedures in anus-preserving rectectomy for patients with low rectal cancer.
APR is considered to be a gold standard for the treatment of low rectal cancer less than 5 cm from the anal verge. It completely removes the distal colon, rectum, and anal sphincter complex using both anterior abdominal and perineal incisions, resulting in a permanent colostomy. The technique of TCMA was developed by Li et al for anus-preserving rectectomy in patients with low rectal cancer, which improved the anastomotic stoma and alleviated tension. With this modified technique, the incidence of anastomotic leakage was significantly decreased and the long-term outcome was satisfactory with good anal function and a lower rate of incontinence.
This study is innovative and is of interest for surgical community. Methods used are innovative and advanced. Detailed description is provided to allow other investigators to reproduce or validate authors’ findings. Results provide sufficient evidence to draw firm scientific conclusions. Sample size and statistical data, especially graphic data, are adequate for a clinical study. However, some revisions should be made on the presentation and evaluation of the results.
P- Reviewers Barauskas G, Kim YJ, Kopljar M S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Practice parameters for the treatment of rectal carcinoma. The American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 1993;36:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Nicholls RJ, Hall C. Treatment of non-disseminated cancer of the lower rectum. Br J Surg. 1996;83:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Li SY. [Some issues deserve consideration in sphincter-preserving operations of low rectal cancer]. Zhonghua Waike Zazhi. 2007;45:1153-1155. [PubMed] |
| 4. | Bai X, Li S, Yu B, Su H, Jin W, Chen G, DU J, Zuo F. Sphincter-preserving surgery after preoperative radiochemotherapy for T3 low rectal cancers. Oncol Lett. 2012;3:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Aytac E, Lavery IC, Kalady MF, Kiran RP. Impact of Obesity on Operation Performed, Complications, and Long-term Outcomes in Terms of Restoration of Intestinal Continuity for Patients With Mid and Low Rectal Cancer. Dis Colon Rectum. 2013;56:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Parks AG, Percy JP. Resection and sutured colo-anal anastomosis for rectal carcinoma. Br J Surg. 1982;69:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 184] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Neuman HB, Park J, Fuzesi S, Temple LK. Rectal cancer patients’ quality of life with a temporary stoma: shifting perspectives. Dis Colon Rectum. 2012;55:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1937] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 9. | Arenas RB, Fichera A, Mhoon D, Michelassi F. Total mesenteric excision in the surgical treatment of rectal cancer: a prospective study. Arch Surg. 1998;133:608-611; discussion 611-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1056] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 11. | Li SY, Yu B, Gao QX, Liang ZJ, Yuan SJ, Wu E, Chen G, Chen G, BAI X. Sphincter-preserving transabdominal-anal radical resection of middle-lower rectal cancer by mucosa-mucosa coloanal anastomosis. Zhongguo Puwai Zazhi. 2005;20:622-624. |
| 12. | Li SY, Liang ZJ, Yuan SJ, Yu B, Chen G, Chen G, Bai X, Zuo FY, Wei XJ, Wu E. [Clinical study of 231 cases of radical excision with sphincter preservation by casing anastomosis in low rectal cancer]. Zhonghua Waike Zazhi. 2007;45:1170-1172. [PubMed] |
| 13. | Li SY, Yu BM. Further improve the colorectal cancer treatment level. Zhongguo Puwai Zazhi. 2001;39:422-424. |
| 14. | Shao YF, Wu TC, Shan Y, Wu JX, Zhou ZX, Xu LB, Wang B. Clinical experience in the use of stapler anastomosis in anterior resection of rectal carcinoma. Zhongguo Puwai Zazhi. 2005;20:30-32. |
| 15. | Richardson DP, Porter GA, Johnson PM. Population-based use of sphincter-preserving surgery in patients with rectal cancer: is there room for improvement? Dis Colon Rectum. 2013;56:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum. 2013;56:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Kirwan WO, Turnbull RB, Fazio VW, Weakley FL. Pullthrough operation with delayed anastomosis for rectal cancer. Br J Surg. 1978;65:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Gong J, Shi DB, Li XX, Cai SJ, Guan ZQ, Xu Y. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol. 2012;18:7308-7313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, Rahbari NN, Büchler MW, Weitz J. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery. 2013;153:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Williams NS. The rationale for preservation of the anal sphincter in patients with low rectal cancer. Br J Surg. 1984;71:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Braun J, Treutner KH, Winkeltau G, Heidenreich U, Lerch MM, Schumpelick V. Results of intersphincteric resection of the rectum with direct coloanal anastomosis for rectal carcinoma. Am J Surg. 1992;163:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumours. Br J Surg. 1994;81:1376-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 320] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Saito N, Ono M, Sugito M, Ito M, Morihiro M, Kosugi C, Sato K, Kotaka M, Nomura S, Arai M. Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoid a permanent colostomy. Dis Colon Rectum. 2004;47:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1474] [Cited by in RCA: 1365] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 26. | den Dulk M, Marijnen CA, Putter H, Rutten HJ, Beets GL, Wiggers T, Nagtegaal ID, van de Velde CJ. Risk factors for adverse outcome in patients with rectal cancer treated with an abdominoperineal resection in the total mesorectal excision trial. Ann Surg. 2007;246:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 245] [Article Influence: 6.6] [Reference Citation Analysis (0)] |