Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3665
Revised: March 30, 2013
Accepted: May 16, 2013
Published online: June 21, 2013
Processing time: 135 Days and 17.9 Hours
AIM: To analyze the clinical characteristics of small bowel tumors detected by double-balloon enteroscopy (DBE) and to evaluate the diagnostic value of DBE in tumors.
METHODS: Four hundred and forty consecutive DBE examinations were performed in 400 patients (250 males and 150 females, mean age 46.9 ± 16.3 years, range 14-86 years) between January 2007 and April 2012. Of these, 252 patients underwent the antegrade approach, and 188 patients underwent the retrograde approach. All the patients enrolled in our study were suspected of having small bowel diseases with a negative etiological diagnosis following other routine examinations, such as upper and lower gastrointestinal endoscopy and radiography tests. Data on tumors, such as clinical information, endoscopic findings and operation results, were retrospectively collected.
RESULTS: Small bowel tumors were diagnosed in 78 patients, of whom 67 were diagnosed using DBE, resulting in a diagnostic yield of 16.8% (67/400); the other 11 patients had negative DBE findings and were diagnosed through surgery or capsule endoscopy. Adenocarcinoma (29.5%, 23/78), gastrointestinal stromal tumor (24.4%, 19/78) and lymphoma (15.4%, 12/78) were the most common tumors. Among the 78 tumors, 60.3% (47/78) were located in the jejunum, and the overall number of malignant tumors was 74.4% (58/78). DBE examinations were frequently performed in patients with obscure gastrointestinal bleeding (47.4%) and abdominal pain (24.4%). The positive detection rate for DBE in the 78 patients with small bowel tumors was 85.9% (67/78), which was higher than that of a computed tomography scan (72.9%, 51/70). Based on the operation results, the accuracy rates of DBE for locating small bowel neoplasms, such as adenocarcinoma, gastrointestinal stromal tumor and lymphoma, were 94.4%, 100% and 100%, respectively. The positive biopsy rates for adenocarcinoma and lymphoma were 71.4% and 60%, respectively.
CONCLUSION: DBE is a useful diagnostic tool with high clinical practice value and should be considered the gold standard for the investigation of small bowel tumors.
Core tip: This was a single-center study with a large sample size of patients who underwent 440 consecutive double-balloon enteroscopy (DBE) examinations. The detection rates of various tumors, location of the lesions, histological analyses and reasons for DBE were evaluated. Differences in the rates of detecting small bowel tumors between abdominal computed tomography, capsule endoscopy and DBE were compared. Based on the operation results, we analyzed the accuracy of DBE for locating neoplasms in addition to its positive biopsy rate. DBE’s high clinical practice value indicated that it should be considered as the gold standard for small bowel tumors.
- Citation: Chen WG, Shan GD, Zhang H, Li L, Yue M, Xiang Z, Cheng Y, Wu CJ, Fang Y, Chen LH. Double-balloon enteroscopy in small bowel tumors: A Chinese single-center study. World J Gastroenterol 2013; 19(23): 3665-3671
- URL: https://www.wjgnet.com/1007-9327/full/v19/i23/3665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i23.3665
Long considered to be a “black box” in the GI tract, the small bowel has been inaccessible to the endoscopist because of its anatomy, location and tortuosity. Small bowel tumors are relatively rare disorders and account for 3%-6% of all digestive neoplasms, most of which are malignant, and represent only 1.1%-2.4% of gastrointestinal malignancies[1]. This low incidence may be ascribed to its unique physiological features, which include an alkaline environment, fluidity, low bacterial count and a high level of IgA in the small intestine[2]. The diagnosis and management of small bowel tumors are formidable tasks for physicians.
The advent of capsule endoscopy (CE) and double-balloon enteroscopy (DBE) has completely changed our approach and launched a new era for small bowel diseases. DBE is a relatively noninvasive method, with a diagnostic yield of approximately 43%-80%[3,4]. Total enteroscopy can be achieved through antegrade and retrograde procedures. Compared with the use of CE in small bowel tumors, DBE has the particular advantage of biopsy and therapeutic potential, such as endoscopic stenting, balloon dilatation and localization before operation[5]. Additionally, DBE is not contraindicated in patients with stenosis of the intestine or an obstruction caused by neoplasms.
From January 2007 to April 2012, 440 examinations were performed in 400 patients, of whom 78 were diagnosed with small bowel tumors. However, little data involving large patient samples are available regarding the diagnostic value of DBE for small bowel tumors in China. In this context, our study was conducted to determine the characteristics of small bowel tumors in patients undergoing DBE and to evaluate the clinical value of DBE.
A retrospective, descriptive study involving all patients who were admitted to our hospital for DBE from January 2007 to April 2012 was conducted. Four hundred patients were enrolled in the present series (250 males and 150 females with a mean age of 46.9 ± 16.3 years, range 14-86 years). The indications included the following: obscure gastrointestinal bleeding (OGIB) in 149 cases, abdominal pain in 123 cases, chronic diarrhea in 40 cases, The other 88 cases involved weight loss, abnormalities on computed tomography (CT) scan or CE, and anemia. The main characteristics of the patients are shown in Table 1. All the patients were suspected of having small bowel diseases, and other routine examinations, such as gastroscopy, colonoscopy, abdomen CT, and radiography, did not reveal an etiological diagnosis. The data collected included age, sex, the indication for DBE, the insertion length, the diagnosis and the results of the operation.
| Characteristic | n = 400 |
| Age, yr, median (range) | 46.9 ± 16.3 (14-86) |
| Sex (male/female) | 250/150 |
| Reasons for DBE | |
| OGIB | 149 (37.3) |
| Abdominal pain | 123 (30.7) |
| Chronic diarrhea | 40 (10.0) |
| Others | 88 (22.0) |
| Tumors detected by DBE | n = 78 |
| OGIB | 37 (47.4) |
| Abdominal pain | 19 (24.4) |
| Intestinal obstruction | 8 (10.3) |
| Others | 14 (17.9) |
All DBE examinations were performed with a Fujinon enteroscope (EN450-P5/20, Fujinon, Inc, Saitama, Japan). The operating system consisted of a mainframe, an enteroscope, an overtube and an air pump. Two soft latex balloons, which could be inflated and deflated, were attached to the tip of the enteroscope and overtube. The balloons were connected to a pump that modulated the air automatically through an air channel in the endoscope, according to the different balloon pressures required. To reduce friction between the enteroscope and the overtube, olive oil and water were added as lubricants to the lacuna between them during the operation. When the procedure was performed as described by Yamamoto[6], the endoscope achieved deep advancement into the small bowel using the overtube in coordination with the serial inflation and deflation of the balloons.
DBE was performed via the oral, anal or both approaches at the discretion of the endoscopist and according to the presumed location of the suspected lesions. When the location was uncertain, the oral approach was preferred.
For both the antegrade and retrograde approaches, preparation included overnight fasting and the consumption of three boxes of polyethylene glycol electrolyte (69.56 g × 3) diluted in 3000 mL of water 5-6 h before the examination.
DBE was carried out under conscious or deep sedation when required. Sedation was achieved with the help of an anesthesiologist. Conscious sedation required the intravenous injection of midazolam and meperidine. General anesthesia was indicated for select patients who were administered a combination of propofol and fentanyl. Patients who underwent DBE via the oral approach with deep sedation requested a tracheal cannula. The cardiovascular risk status of the patients was evaluated before the examination. During DBE, oxygen was administered along with electrocardiographic monitoring when necessary.
The SPSS 16.0 software package was used for statistical analysis. Count data were expressed as a percentage, and measurement data were expressed as the mean ± SD. Differences were evaluated with the χ2 test. We used Fisher’s exact probability when the theoretical frequency was less than 5. P < 0.05 (two sided) was considered to be statistically significant.
In this study, 440 DBE procedures were performed in 400 patients (252 antegrade, 188 retrograde); 40 patients underwent both antegrade and retrograde procedures. Two patients completed the entire small intestine examination all at once via the oral approach. Seventy-eight cases of small bowel tumors were detected, giving a positive rate of 19.5% (78/400). Clinically positive DBE findings were observed in 67 patients.
All procedures were successfully performed, except for three patients who had a perforation after the examination. No hemorrhage, acute pancreatitis or other serious complications occurred. Uncomfortable feelings, such as nausea, abdominal distension and abdominal pain, occurred in some cases during the examination. However, these symptoms were transient and tolerable. The complication rate was 0.68% (3/440) in our study group.
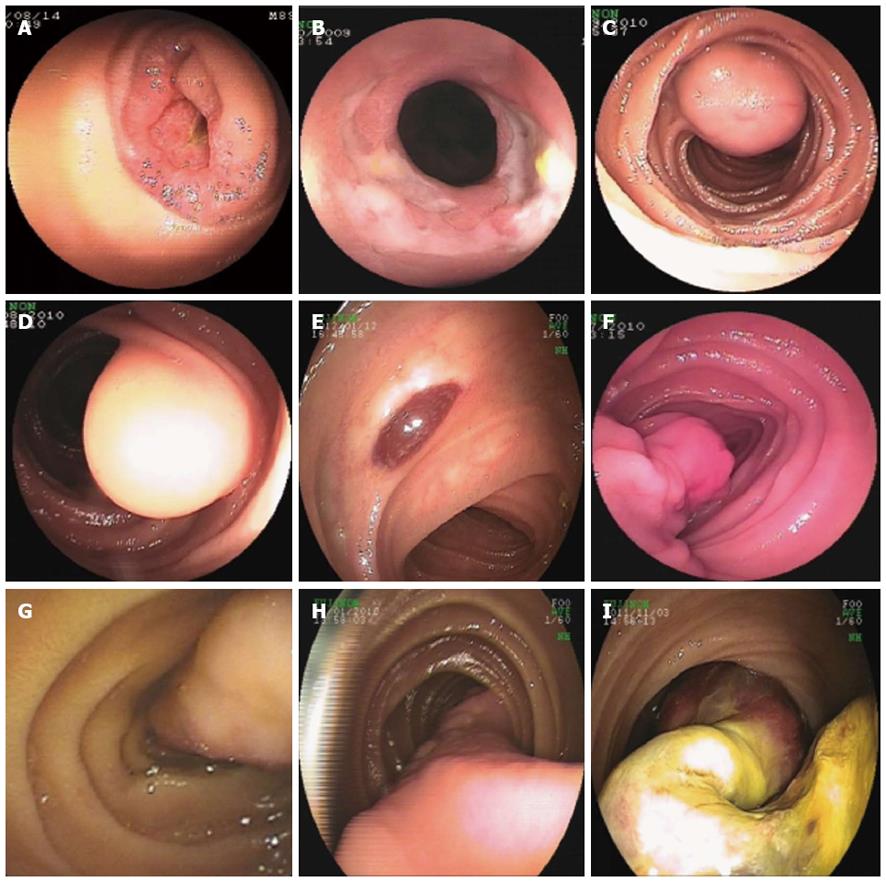
More than 10 types of tumors (Table 2) were found in our study. The majority of these tumors were adenocarcinoma, followed by gastrointestinal stromal tumor (GIST) and lymphoma. In contrast, some tumors had a low detection rate, such as lipoma, metastatic carcinoma and hamartoma. Some extremely rare cases, such as duodenal gangliocytic paraganglioma, jejunal mesangial fibrosarcoma, inflammatory myofibroblastic tumor and serosa fibromatosis, were also found in our group. The typical endoscopic images of the main tumors are shown in Figure 1.
| Tumor | DBE (positive/negative) | Detection rate | Benign/malignant | Duodenum /jejunum/ileum |
| Adenocarcinoma | 23 (22/1) | 29.50% | 0/23 | 6/16/1 |
| GIST | 19 (16/3) | 24.40% | 6/13 | 3/12/4 |
| Lymphoma | 12 (9/3) | 15.40% | 0/12 | 1/6/5 |
| Lipoma | 8 (7/1) | 10.30% | 8/0 | 0/8/0 |
| Metastatic carcinoma | 8 (8/0) | 10.30% | 0/8 | 3/2/3 |
| Hamartoma | 2 (2/0) | 2.60% | 2/0 | 1/0/1 |
| Others1 | 6 (3/3) | 7.70% | 4/2 | 2/3/1 |
| Total | 78 (67/11) | 100% | 20/58 | 16/47/15 |
In the 11 patients with negative DBE results, tumors were detected through surgery or capsule endoscopy and included three lymphomas, three GIST, one adenocarcinoma and one lipoma (Table 2). The reasons for the missed diagnoses were as follows: the depth of insertion was inadequate (five cases), the choice of insertion approach was not optimal and the tumors were located at the opposite end of the intestine (four cases), and the tumors were exophytic growths with normal intestinal mucosa (two cases).
Location of lesions: In general, we determined the approximate location through the inserted depth of the endoscope, the size of the enteric cavity, and the shape of the mucosal fold and villi. Among the 78 tumors identified in the patients, those located in the jejunum had the highest detection rate (60.3%, 47/78). The detection rate of tumors in the duodenum was 20.5% (16/78), which was similar to that of tumors located in the ileum (19.2%). Most of the tumors, such as adenocarcinoma, lymphoma, GIST and lipoma, had a high incidence rate in the jejunum.
Histological analysis: Malignant tumors were found in 58 patients, with a detection rate of 74.4% (58/78). The distribution of tumors was as follows: 23 adenocarcinoma, 13 malignant GIST, 12 lymphoma, eight metastatic carcinoma and two others. In our study, compared with malignant tumors, benign tumors had lower detection rates (25.6%, 20/78); the primary benign tumors were eight lipomas and six GIST.
Reasons for DBE: Of the 78 patients with small bowel tumors, OGIB was the most common reason for DBE, followed by abdominal pain, intestinal obstruction and others, which included abdominal distention, vomiting, diarrhea and weight loss (Table 1).
The positive detection rate for DBE in the 78 patients with small bowel tumors was 85.9% (67/78). Seventy of those 78 cases underwent an abdominal CT. If the CT showed a small mural mass, wall thickening with enhancement or luminal narrowing, then the results were considered positive. The positive rate for CT was 72.9% (51/70). CE was used to examine 27 cases, with a positive rate of 77.8% (21/27). Twenty-two patients underwent CE examinations before DBE; only five cases had the DBE examination first. We performed a statistical analysis of the detection rates for DBE, CE and abdominal CT. The results indicated that DBE had a higher detection rate compared with CT (P < 0.05). There were no significant differences between DBE and CE or CE and CT (P > 0.05).
In general, we determined the types of tumors through the endoscopic features, imaging results and other auxiliary examinations performed before the operation. An endoscopic biopsy was carried out for some tumors. Fifty-three cases (68.0%) underwent an operation in this series of patients.
As a result of the operation, 18 cases were confirmed to have an adenocarcinoma, which was the most common tumor type in this group, followed by GIST (14 cases) and lymphoma (8 cases); the other 13 cases included metastatic carcinoma and hamartoma. Of the 53 patients who underwent an operation in our study, the positive rate of DBE in the 18 cases of adenocarcinoma was 100% (18/18), whereas the positive rate was 78.6% (11/14) for GIST and 62.5% (5/8) for lymphoma. The accuracy rates of DBE in locating small bowel neoplasms, such as adenocarcinoma, GIST and lymphoma, were 94.4%, 100% and 100%, respectively. The positive biopsy rates for adenocarcinoma and lymphoma were 71.4% and 60%, respectively.
Although the small intestine comprises nearly 75% of the GI tract extension and nearly 90% of its mucosal surface, small bowel neoplasms are rare[7]. Recent epidemiological studies have indicated that the incidence of small bowel neoplasms has increased, particularly for malignant tumors, because of changes in diet and lifestyle[8]. Currently, the development of CE and DBE has made endoscopic examination of the entire small bowel practical. Although CE examination has revolutionized the standard small bowel evaluation[9], some technical limitations hamper its potential usefulness and effectiveness, including its inability to sample tissue and perform therapeutic procedures. These drawbacks have been overcome with the introduction of DBE, which allows dynamic observation with the controlled movement of the endoscope, the collection of biopsies and many types of interventional procedures[10]. Under some circumstances, it can be assumed that most investigational laparotomies will be replaced by DBE.
Among the 400 patients who were submitted to examination with DBE, 67 of the 78 patients (78/400, 16.8%) who were eventually found to have small bowel tumors were detected with DBE. The results revealed that adenocarcinoma, GIST and lymphoma were the three most common tumors. In our study, adenocarcinoma had the highest incidence among the malignant neoplasms, while lipoma was the most common benign tumor. Some extremely rare tumors reported only in single cases[11,12], including duodenal gangliocytic paraganglioma, jejunal mesangial fibrosarcoma, jejunal inflammatory myofibroblastic tumor and jejunal serosa fibromatosis, were also detected in our group. There are differences in reports regarding the incidence of small bowel tumors. In the United States, the most common small bowel tumors registered with the National Cancer Data Base are neuroendocrine (carcinoid) (44%), adenocarcinoma (33%) and lymphoma (17%)[13]. In contrast, in a Japanese multicenter study, small bowel tumors were identified in 144 of 1035 subjects (13.9%) who underwent DBE, of which lymphoma and GIST were the most frequent[14]. The hypothesis that adenocarcinoma is the most common tumor was corroborated by Safatle-Ribeiro et al[15]. These differences may be ascribed to racial differences and geographical distribution.
To date, approximately 40 different histological types of small bowel tumors, of which approximately two-thirds are malignant, have been identified[16]. Of the 78 small bowel tumors of our study, the detection rate for malignancy was 74.4%. The majority of the lesions were located in the jejunum, followed by the duodenum and ileum, which is similar to the distribution reported in the literature[17]. Most of the small bowel tumors that have been reported occurred primarily in the proximal small bowel (duodenum and jejunum), except for lymphomas, sarcomas and carcinoids[18,19]. In our study, 95.7% of the adenocarcinoma (22/23) cases were found in the proximal small bowel, and only one case was located in the ileum. Among the GIST cases, 78.9% (15/19) were detected in the proximal small bowel. In the 12 patients with lymphomas, the incidence in the jejunum was similar to that of the ileum. All of the lipomas were in the jejunum. Therefore, for patients with no clinical evidence indicating the tumor location, DBE via the oral approach is recommended in patients suspected of having tumors, especially adenocarcinoma, GIST and lipoma.
In large-sample studies, OGIB is the leading indication for DBE, and the diagnostic yield for OGIB is 43%-75%[20]. In our 400 patients, OGIB (37.3%, 149/400) was the main reason for DBE, which agreed with previously reported results, followed by abdominal pain (30.8%, 123/400) and chronic diarrhea (10%, 40/400). For small bowel tumors, early symptoms are often absent or nonspecific. The study by Talamonti MS indicated that obstruction, anemia and obscure bleeding were the most common symptoms of primary lesions[21]. In our group of 78 patients with small bowel tumors, the symptoms were not obviously different from those of the other patients; the top three causes for DBE were OGIB, abdominal pain and intestinal obstruction. Therefore, our research indicates that OGIB and abdominal pain were the most common reasons for DBE in both patients with small bowel tumors and patients with other diagnoses.
DBE and CE have diagnostic superiority over other routine procedures, such as push enteroscopy, abdominal CT and small bowel angiography, in detecting small bowel lesions[22]. In small bowel tumor patients, our study demonstrated that DBE had a higher detection rate than CT (85.90% vs 72.90%), whereas there was no difference between DBE and CE. Abdominal CT plays a pivotal role in the diagnosis, localization and staging of neoplasms and monitoring the treatment response[23]. At the same time, this examination is convenient and can determine the route of insertion for complementary DBE; therefore, it has become the initial screening method for tumors. However, it is not sufficient for the diagnosis of mucosal or small lesions of the small bowel. In the study of Cheung et al[24], tumors measuring less than 10 mm were missed with radiological techniques. CE examination has rapidly gained acceptance as the standard for small bowel evaluation. However, false-positive or false-negative results caused by the unique anatomical features of the small bowel are limitations of capsule endoscopy[25]. Imaoka et al[26] reported that two-thirds of patients in whom small bowel tumors were identified had stenosis or ulceration; CE is an inappropriate modality for those who have stenosis. The capsule retention incidence ranges from 9.7%-25% in patients with small bowel tumors, which is higher than the retention incidence in all patients receiving CE and even higher than in patients with small bowel Crohn’s disease[27]. DBE examination has no risk of obstruction and allows for the biopsy of tumors, which has high diagnostic value, especially for adenocarcinoma and lymphoma. In our group of 78 tumor patients, 53 cases who underwent an operation were compared regarding the DBE results, and the results indicated that the accuracy rate of DBE in locating small bowel tumors, such as adenocarcinoma, GIST and lymphoma, was very high. The positive biopsy rates for adenocarcinomas and lymphomas were 71.4% and 60%, respectively. All of the above results indicate that DBE possesses a high value in the qualitative and localization diagnosis of small bowel tumors and provides marked reference values for surgery.
In summary, our study results indicate that DBE examination has high clinical practice value in the diagnosis of tumors and confirms it as a useful diagnostic and therapeutic tool for the investigation of small bowel diseases. DBE can obtain direct visualization and histological characterization of small bowel tumors. DBE should be considered the gold standard for the diagnosis of small bowel tumors because of its unique advantages compared with other procedures[28].
Small bowel tumors are relatively rare, and the diagnosis of such tumors before surgery was difficult until the advent of double-balloon enteroscopy (DBE) and capsule endoscopy (CE).Compared with CE and other routine examinations used to identify small bowel tumors, DBE has particular advantages because of its diagnostic and therapeutic capabilities.
Studies are being performed to evaluate the diagnostic value of DBE in small bowel tumors.
This study was a single-center experience in China with a large sample size involving 440 consecutive DBE examinations. The difference between abdominal computed tomography, CE and DBE in the positive rates of detecting small bowel tumors was evaluated. At the same time, the detection rates of various tumors, the location of the lesions, the histological analysis, the reasons for DBE, the accuracy rates for localization and the positive biopsy rates for DBE were also analyzed in detail.
This study may encourage the use of DBE in the investigation of small bowel tumors. DBE has high diagnostic and therapeutic capabilities in clinical practice; therefore, should be considered as the gold standard for small bowel tumors. In the future, more therapies for small bowel tumors will be finished through DBE.
Double-balloon enteroscopy (DBE): a method of enteroscopy that can lead to the observation of the small intestine via the mouth or anus with the help of two balloons. One balloon is attached to the tip of the endoscope and the other balloon is attached to the distal end of a soft overtube.
This is an interesting paper with important results, which demonstrates the importance of DBE in the diagnosis of small bowel tumors and analyzes the clinical characteristics of 78 tumor patients who underwent DBE.
P- Reviewers Figueiredo P, Hondo FY, Yamamoto S S- Editor Gou SX L- Editor Stewart GJ E- Editor Zhang DN
| 1. | Gay G, Delvaux M. Small-bowel endoscopy. Endoscopy. 2008;40:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Anzidei M, Napoli A, Zini C, Kirchin MA, Catalano C, Passariello R. Malignant tumours of the small intestine: a review of histopathology, multidetector CT and MRI aspects. Br J Radiol. 2011;84:677-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Kuga R, Safatle-Ribeiro AV, Ishida RK, Retes F, Uemura RS, Sakai P. Small bowel endoscopy using the double-balloon technique: four-year results in a tertiary referral hospital in Brazil. Dig Dis. 2008;26:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Kita H, Yamamoto H, Yano T, Miyata T, Iwamoto M, Sunada K, Arashiro M, Hayashi Y, Ido K, Sugano K. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small intestinal disorders. Inflammopharmacology. 2007;15:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 7. | Moglia A, Menciassi A, Dario P, Cuschieri A. Clinical update: endoscopy for small-bowel tumours. Lancet. 2007;370:114-116. [PubMed] |
| 8. | Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, Longo WE. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg. 2007;142:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Maieron A, Hubner D, Blaha B, Deutsch C, Schickmair T, Ziachehabi A, Kerstan E, Knoflach P, Schoefl R. Multicenter retrospective evaluation of capsule endoscopy in clinical routine. Endoscopy. 2004;36:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Ross A, Mehdizadeh S, Tokar J, Leighton JA, Kamal A, Chen A, Schembre D, Chen G, Binmoeller K, Kozarek R. Double balloon enteroscopy detects small bowel mass lesions missed by capsule endoscopy. Dig Dis Sci. 2008;53:2140-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | De Man M, De Gendt S, De Raeve H, Vandervoort J. Gangliocytic paraganglioma of the duodenum: a rare entity. Acta Gastroenterol Belg. 2012;75:462-463. [PubMed] |
| 12. | Ntloko S, Gounden A, Naidoo M, Madiba TE, Singh Y, Ramdial PK, Hadley GP. Intestinal inflammatory myofibroblastic tumour. S Afr J Surg. 2011;49:190-193. [PubMed] |
| 13. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [PubMed] |
| 14. | Mitsui K, Tanaka S, Yamamoto H, Kobayashi T, Ehara A, Yano T, Goto H, Nakase H, Tanaka S, Matsui T. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc. 2009;70:498-504. [PubMed] |
| 15. | Zouhairi ME, Venner A, Charabaty A, Pishvaian MJ. Small bowel adenocarcinoma. Curr Treat Options Oncol. 2008;9:388-399. [PubMed] |
| 16. | O’Riordan BG, Vilor M, Herrera L. Small bowel tumors: an overview. Dig Dis. 1996;14:245-257. [PubMed] |
| 17. | Achour J, Serraj I, Amrani L, Amrani N. Small bowel tumors: what is the contribution of video capsule endoscopy? Clin Res Hepatol Gastroenterol. 2012;36:222-226. [PubMed] |
| 18. | Giri K, Sudar C, Arya M, Haber G, Chandra P. Diagnosis of marginal cell lymphoma of small intestine by double balloon enteroscopy. South Med J. 2008;101:561-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC 2nd. A proposed staging system for small bowel carcinoid tumors based on an analysis of 6380 patients. Am J Surg. 2008;196:896-903 [PMID 19095106]. |
| 20. | Westerhof J, Weersma RK, Koornstra JJ. Investigating obscure gastrointestinal bleeding: capsule endoscopy or double balloon enteroscopy? Neth J Med. 2009;67:260-265. [PubMed] |
| 21. | Talamonti MS, Goetz LH, Rao S, Joehl RJ. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137:564-570; discussion 570-571. [PubMed] |
| 22. | Trifan A, Singeap AM, Cojocariu C, Sfarti C, Stanciu C. Small bowel tumors in patients undergoing capsule endoscopy: a single center experience. J Gastrointestin Liver Dis. 2010;19:21-25. [PubMed] |
| 23. | Kamaoui I, De-Luca V, Ficarelli S, Mennesson N, Lombard-Bohas C, Pilleul F. Value of CT enteroclysis in suspected small-bowel carcinoid tumors. AJR Am J Roentgenol. 2010;194:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Cheung DY, Lee IS, Chang DK, Kim JO, Cheon JH, Jang BI, Kim YS, Park CH, Lee KJ, Shim KN. Capsule endoscopy in small bowel tumors: a multicenter Korean study. J Gastroenterol Hepatol. 2010;25:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Lee BI, Choi H, Choi KY, Byeon JS, Jang HJ, Eun CS, Cheon JH, Shin SJ, Kim JO, Lee MS. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56:2920-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Imaoka H, Higaki N, Kumagi T, Miyaike J, Ohmoto M, Yamauchi K, Murakami T, Murakami H, Ikeda Y, Yokota T. Characteristics of small bowel tumors detected by double balloon endoscopy. Dig Dis Sci. 2011;56:2366-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Bailey AA, Debinski HS, Appleyard MN, Remedios ML, Hooper JE, Walsh AJ, Selby WS. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol. 2006;101:2237-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Almeida N, Figueiredo P, Lopes S, Gouveia H, Leitão MC. Double-balloon enteroscopy and small bowel tumors: a South-European single-center experience. Dig Dis Sci. 2009;54:1520-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |