Published online Jun 14, 2013. doi: 10.3748/wjg.v19.i22.3487
Revised: May 15, 2013
Accepted: May 22, 2013
Published online: June 14, 2013
Processing time: 152 Days and 1.2 Hours
AIM: To explore the role of nesfatin-1 on irritable bowel syndrome (IBS)-like visceral hypersensitivity.
METHODS: The animal model of IBS-like visceral hypersensitivity was induced by intracolonic infusion of 0.5% acetic acid (AA) in saline once daily from postnatal days 8-21. Experiments were performed when rats became adults. The visceral sensitivity of rats was evaluated by abdominal withdrawal reflex (AWR) and electromyographic (EMG) activity of the external oblique muscle to graded colorectal distension. The content of nesfatin-1 in serum was determined using enzyme-linked immunosorbent assay. After implantation of an intracerebroventricular (ICV) cannula and two electrodes into the external oblique muscle, model rats were randomly divided into four groups. Animals then received ICV injection of 8 μg of anti-nesfatin-1/nucleobindin-2 (NUCB2), 50 μg of α-helical corticotropin releasing factor (CRF) 9-41 (non-selective CRF receptor antagonist), 50 μg of NBI-27914 (selective CRF1 receptor antagonist) or 5 μL of vehicle. After 1 h of ICV administration, visceral sensitivity of each group was measured again, and comparisons between groups were made.
RESULTS: Rats treated with AA showed higher mean AWR scores and EMG activity at all distension pressures compared with controls (P < 0.05). On histopathologic examination, no evidence of inflammation or abnormalities in structure were noted in the colon of either control or AA-treated groups. Myeloperoxidase values were not significantly different between the two groups. The level of nesfatin-1 in serum was significantly higher in the AA-treated group than in the control group (5.34 ± 0.37 ng/mL vs 4.81 ± 0.42 ng/mL, P < 0.01). Compared with rats injected with vehicle, rats which received ICV anti-nesfatin-1/NUCB2, α-helical CRF9-41 or NBI-27914 showed decreased mean AWR scores and EMG activity at all distension pressures (P < 0.05).
CONCLUSION: Nesfatin-1 may be associated with IBS-like visceral hypersensitivity, which may be implicated in brain CRF/CRF1 signaling pathways.
Core tip: This is a well conducted experimental study on the possible effect of nesfatin-1 in visceral hypersensitivity. Currently no reports have been published concerning the role of nesfatin-1 in irritable bowel syndrome (IBS). In a well-established visceral hypersensitivity animal model, we found an elevated nesfatin-1 level in the serum, and there was a reduction in evoked abdominal electromyography and abdominal withdrawal reflex scores after treatment with nesfatin-1 antibody, a non-selective corticotropin releasing factor (CRF) receptor antagonist, or a selective CRF1 receptor antagonist. These results suggest that nesfatin-1 may be associated with visceral hypersensitivity in model rats and implicated in brain CRF/CRF1 signaling pathways, which contribute to the visceral hypersensitivity of IBS.
- Citation: Jia FY, Li XL, Li TN, Wu J, Xie BY, Lin L. Role of nesfatin-1 in a rat model of visceral hypersensitivity. World J Gastroenterol 2013; 19(22): 3487-3493
- URL: https://www.wjgnet.com/1007-9327/full/v19/i22/3487.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i22.3487
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by abdominal pain and alterations in bowel habits. The pathogenic mechanism of IBS remains incompletely understood. Visceral hypersensitivity is common in IBS patients and probably plays a major role in development of symptoms[1]. Nesfatin-1 is a newly discovered anorexigenic peptide derived from the precursor peptide nucleobindin-2 (NUCB2)[2]. Putative post-translational processing of NUCB2 by the enzyme pro-hormone convertase-1/3 results in nesfatin-1, nesfatin-2 and nesfatin-3[2]. Intracerebroventricular (ICV) injection of nesfatin-1 decreased dark phase food intake in freely fed rats, whereas injection of an antibody specific to nesfatin-1 potently stimulated appetite[2]. The hypothalamic selective corticotropin releasing factor-2 (CRF2) signaling system was also shown to be involved in the underlying mechanisms of nesfatin-1 induced reduction of dark phase food intake[3]. Nesfatin-1 is distributed in stress-related brain areas, such as the supraoptic nucleus, hypothalamic paraventricular nucleus (PVN), nucleus of the solitary tract, locus coeruleus and raphe pallidus nucleus[4,5]. Furthermore, nesfatin-1 is colocalized with CRF in the PVN[5]. The hormone CRF is the hallmark initiator of the stress response[6]. It exerts its biological actions by interacting with CRF1 and CRF2 receptors[7]. It has been well established that the brain CRF/CRF1 signaling system modulates pain responses although the exact sites mediating this modulation remain unidentified[8,9]. These observations suggest that nesfatin-1 may be involved in the autonomic regulation of visceral sensation. Given that visceral hypersensitivity is the major pathophysiology of IBS, nesfatin-1 may be an important contributor to the symptoms of IBS. Currently no reports have been published concerning the role of nesfatin-1 in IBS. The purpose of the present study, therefore, was to investigate the effect of nesfatin-1 on visceral sensitivity in IBS and the possible underlying mechanisms of this action. This was achieved by establishing a rat model of visceral hypersensitivity associated with IBS.
Experiments were performed on male Sprague-Dawley rats bought from the Experimental Animal Center of Nanjing Medical University (China). Rats were housed with ad libitum food and water in standard rodent cages at 22 ± 2 °C in a 12-h light-dark controlled room. All animal procedures strictly adhered to the guidelines of the Institution Council of Animal Care and were approved by the Ethics Committee of Nanjing Medical University.
Pups received an infusion of 0.3 mL of 0.5% acetic acid (AA) solution in saline into the colon 2 cm from the anus once daily on postnatal days 8-21[10]. Controls received an equal volume of saline. Experiments were conducted in these rats between 6 and 9 wk of age.
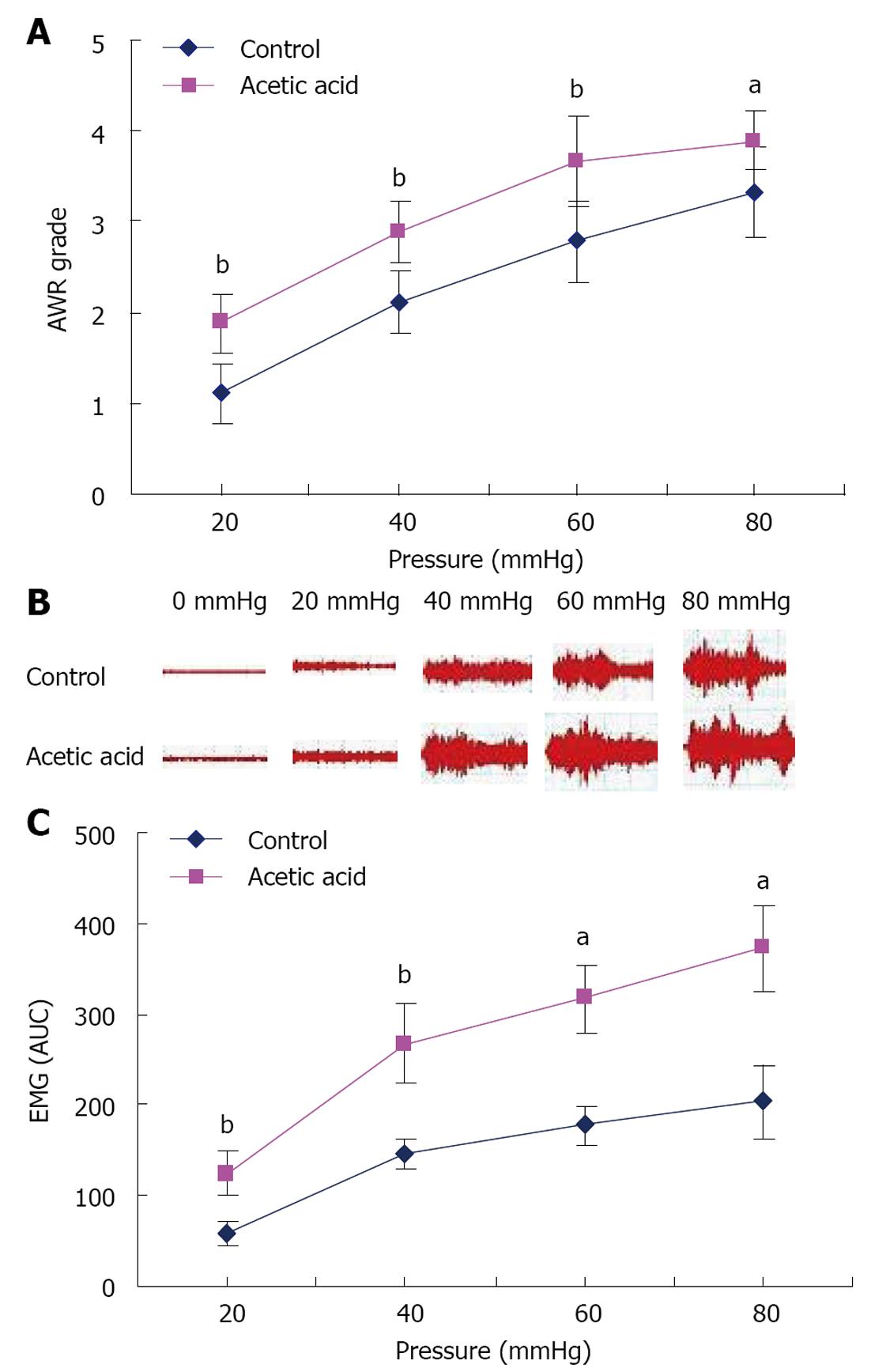
We used a grading system based on the abdominal withdrawal reflex (AWR), as well as a measure of the electromyographic (EMG) activity of the external oblique muscle to evaluate visceral hypersensitivity 6 wk after treatment, by grading the response of rats to colorectal distention (CRD). Briefly, under mild sedation with ether, a flexible balloon (5 cm) constructed from a surgical glove finger attached to tygon tubing was inserted (8 cm) into the descending colon and rectum via the anus and held in place by taping the tubing to the tail. Rats were placed in small lucite cubicles (20 cm × 6 cm × 8 cm) and allowed to adapt for 30 min. CRD was performed by rapidly inflating the balloon to a constant pressure using a sphygmomanometer connected to a pressure transducer. The balloon was inflated to various pressures (20, 40, 60 and 80 mmHg) for 20 s followed by a 2-min rest. Behavioral responses to CRD were measured by visual observation of the AWR by a blinded observer. The assignment of an AWR score was as follows: 1 = normal behavior without response; 2 = contraction of abdominal muscles; 3 = lifting of abdominal wall; and 4 = body arching and lifting of pelvic structures[11].
To obtain EMG measurements of visceromotor responses, two electrodes were implanted, under anesthesia with pentobarbital sodium (100 mg/kg, intraperitoneally), into the external oblique muscle and externalized behind the head. Rats were allowed 1 wk recovery from surgery. CRD was performed as described previously with 20 s of distention followed by 2-min rest between distentions of 20, 40, 60 and 80 mmHg. Wires were connected to a Bio Amp, which was connected to a power lab (AD Instruments, Australia) used as an EMG acquisition system with Chart 7 software. The area under the curve during the 20-s distention for the EMG signal was calculated by subtracting the area under the curve for the 20 s before distention[12].
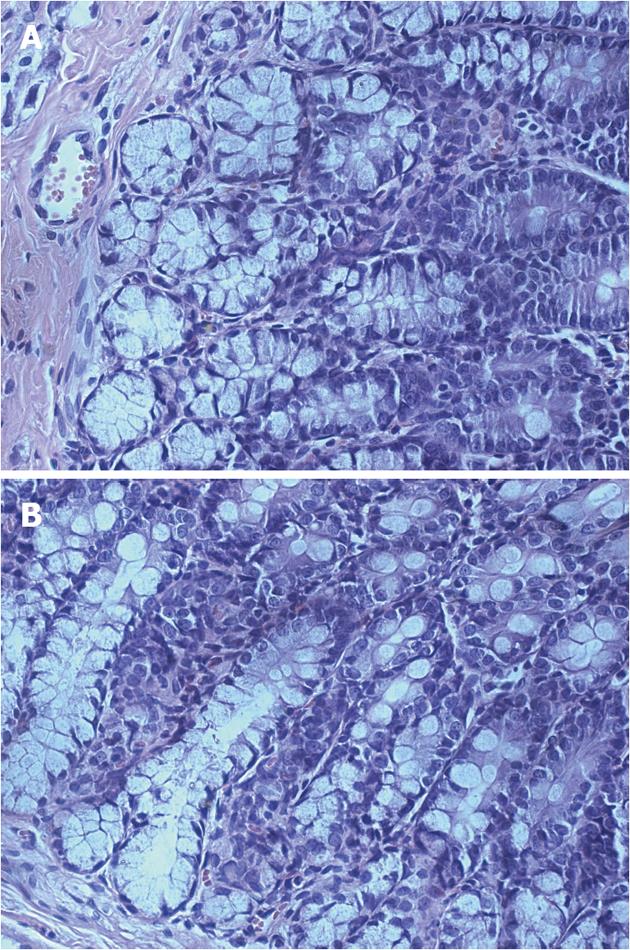
After behavioral testing, 4 cm of colonic tissue proximal to the anus was removed and rinsed briefly with saline. The proximal half of each colon was placed in 4% paraformaldehyde, embedded in paraffin, cut into 4 μm sections, and used for histologic examination. The distal half was snap-frozen and stored at -80 °C until use. Myeloperoxidase (MPO) activity was determined later by an enzyme-linked immunosorbent assay (ELISA) kit (RB, Minneapolis, MN, United States).
Under mild sedation with ether, approximately 1 mL blood was taken from the orbital canthus vein plexus. The blood was centrifuged, then the serum was separated and stored at -80 °C until assayed. Serum nesfatin-1 levels were measured using the ELISA kit (RD, CA, United States).
Model rats were selected and anaesthetized with pentobarbital sodium (100 mg/kg ip). A chronic guide cannula was implanted into the right lateral ventricle of the brain following coordinates from Bregma: anteroposterior, -0.8 mm; lateral, -1.5 mm; dorsoventral, -3.5 mm[13]. Two stainless steel screws were fixed to the skull, and then the cannula was secured with dental cement. Finally, two electrodes were implanted in the external oblique muscle as described above. After surgery, rats were housed individually and allowed to recuperate for 1 wk.
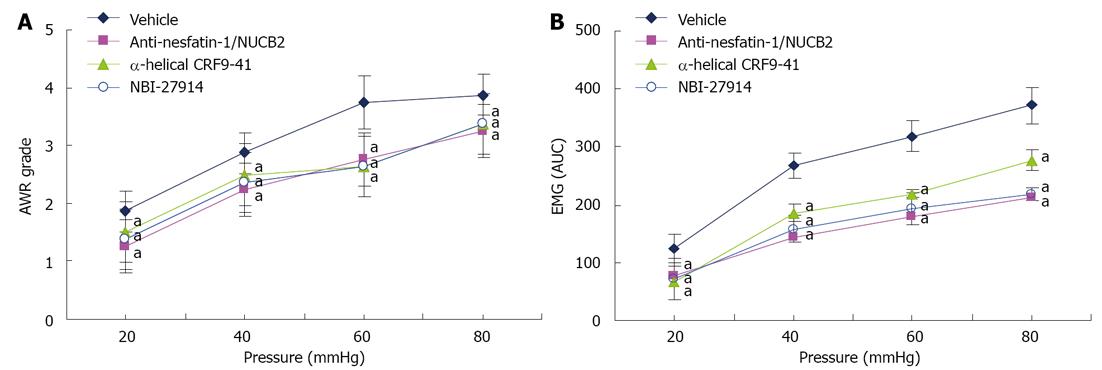
After ICV cannula and external oblique muscle electrode implantation, model rats were randomly divided into 4 groups and administered, by ICV injection, 8 μg of anti-nesfatin-1/NUCB2[2] (Bioss, Beijing, China), 50 μg of α-helical CRF9-41[14] (Tocris, Minneapolis, MN, United States ), 50 μg of NBI-27914[13] (Tocris, Minneapolis,MN, United States) or 5 μL of vehicle[2]. After 1 h, the visceral sensitivity of each group to CRD was measured and compared between groups.
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, United States). All data are expressed as mean ± SE. For AWR behavioral grades, a Friedman analysis of variance (ANOVA) was used. Median AWR scores at each distention pressure were compared between treatment groups using the Mann-Whitney U rank sum test. EMG data were analyzed by two-way repeated-measures ANOVA. Other data were analyzed by the Student t test or one-way ANOVA where appropriate. P values < 0.05 were considered statistically significant.
Visceral sensitivity to CRD was determined at 6 wk of age. Rats treated with AA exhibited higher mean AWR scores at all distension pressures tested than controls (Figure 1A, P < 0.05). In a separate experiment, EMG activity, measured in response to graded CRD, was significantly higher in neonatal AA-treated rats than in controls (Figure 1B and C, P < 0.05). Taken together, these data showed that rats treated with AA between postnatal days 8 and 21 were more sensitive to CRD than controls, suggesting that neonatal AA treatment produced persistent visceral hypersensitivity when animals became adults.
To determine whether persistent visceral hypersensitivity achieved in adults of neonatal AA-treated rats was due to the development of chronic colitis in adults, hematoxylin and eosin-stained sections and MPO activity of the colons of adult rats were examined for histopathologic signs of inflammation. On examination, no significant inflammation or abnormalities in structure were noted in either saline- or AA-treated groups and no inflammatory infiltrates were observed (Figure 2). Likewise, there was no significant difference in the level of MPO between control and neonatal AA-treated rats (Figure 3A, P > 0.05). Thus, these data showed that inflammation/abnormalities were absent in our model, ruling out their involvement in persistent hypersensitivity. Therefore, we have a rat model of persistent visceral hypersensitivity caused by neonatal exposure to a mild acid in the absence of ongoing inflammation.
The mean serum nesfatin-1 level in the neonatal AA-treated group (5.34 ± 0.37 ng/mL) was significantly higher than in the control rats (4.81 ± 0.42 ng/mL; Figure 3B, P = 0.003).
To determine the effect of nesfatin-1 on visceral sensitivity, adult model rats were given ICV injection of anti-nesfatin-1/NUCB2 or vehicle, and visceral sensitivity was measured 1 h later. The AWR scores of nesfatin-1 antibody-treated rats were significantly lower than those of vehicle-treated rats at 20, 40, 60 and 80 mmHg (Figure 4A). Similarly, in model rats, anti-nesfatin-1/NUCB2 treatment caused a significant decline in the EMG activity to graded CRD when compared with vehicle injection. To examine whether brain CRF/CRF1 signaling pathways were involved in visceral hypersensitivity in model rats, animals were injected intracerebroventricularly with non-selective CRF receptor antagonists (α-helical CRF9-41), selective CRF1 receptor antagonist (NBI-27914) or vehicle 1 h before graded CRD. In comparison with model rats receiving a vehicle injection, model rats that received α-helical CRF9-41 and NBI-27914 showed decreased mean AWR scores and EMG activity at all distension pressures (Figure 4B).
IBS is one of the most common functional gastrointestinal disorders worldwide. The mechanism of this disease is not clear, but an important role for visceral hypersensitivity in the development of symptoms compatible with IBS has become evident. It has been confirmed that a lower pain threshold to colonic distention was observed in patients with IBS compared with healthy subjects[1], and visceral hypersensitivity is a biological marker for IBS[15].
Visceral hypersensitivity may be associated with intestinal irritation (pain or inflammation) in the neonatal period. Nociceptive neuronal circuits are formed during the embryonic and postnatal period when painful stimuli are normally absent or limited. Pain and inflammation during this critical period, particularly before the maturation of the descending inhibitory systems, can lead to prolonged structural and functional alterations in pain pathways that can last into adult life. This visceral hypersensitivity is associated with central neural sensitization, as well as central sensitization[16].
In this study, a rat model of visceral hypersensitivity associated with IBS was established by intracolonic instillation of dilute AA between P8 and P21. In our model, dilute AA treatment of pups had no effect on the growth of rats. As adults, these rats showed no identifiable peripheral pathology, which was in line with the characteristics of IBS. These rats exhibited higher mean AWR scores and EMG activity at all distension pressures compared with controls. These findings suggest that neonatal AA treatment induced long-lasting visceral hypersensitivity without significant inflammation in the colon, which was in agreement with previous findings[17]. Therefore, this model can better reflect the chronic hyperesthetic state of IBS and is applicable to the study of visceral hypersensitivity.
Nesfatin-1 is a recently discovered 82-amino-acid satiety peptide and has a predicted molecular mass of 9.7 kDa. Nesfatin-1, injected intracerebroventricularly or peripherally in rats, reduced food intake in a dose-dependent manner[2,18]. As yet, however, the nesfatin-1 receptor has not been identified. Shimizu et al[18] reported that NUCB2 can be potentially processed into an active derivative, nesfatin-1. However, this mature peptide has not been detected in protein extracts from rat brain[2]. Likewise, the western blots of all tissues and cells studied here failed to show a band at 9.7 kDa[19,20]. It has been confirmed that nesfatin-1 exists in the blood of rodents and humans using a sandwich-type ELISA, but normal values have not yet been established for nesfatin-1[21]. Nesfatin-1 can cross the blood-brain barrier without saturation[22,23], therefore the measurement of nesfatin-1 in the blood may partly reflect its level in the brain. The present study showed that the average serum nesfatin-1 level was significantly higher in neonatal AA-treated rats than in control rats, suggesting nesfatin-1 may be associated with a visceral hypersensitivity state in model rats.
Furthermore, we found that model rats receiving ICV injection of anti-nesfatin-1/NUCB2 showed decreased mean AWR scores and EMG activity at 20, 40, 60 and 80 mmHg compared with model rats receiving vehicle injection. Results suggest that ICV injection of nesfatin-1 antibody may neutralize endogenous nesfatin-1 and therefore dramatically attenuate visceral hypersensitivity in model rats.
Taken together, these results strongly suggest that nesfatin-1 may be involved in visceral hypersensitivity in model rats. To date, the evidence for the role of a brain CRF/CRF1 signaling system in the modulation of visceral sensitivity in rodents has been based on the study of visceral hypersensitivity in acute models of stress[24]. In this model, our results also showed that ICV administration of α-helical CRF9-41 and NBI-27914 caused a significant decrease in mean AWR scores and EMG activity at 20, 40, 60 and 80 mmHg in comparison with ICV administration of vehicle, which was consistent with the previous studies[25,26]. These data demonstrate that brain CRF/CRF1 signaling pathways may be involved in visceral hypersensitivity in the present study.
Previous studies have shown that nesfatin-1 was colocalized with CRF in the PVN[5]. ICV administration of nesfatin-1 increased the incidence of c-Fos expression in CRF neurons, and nesfatin-1 increased cytosolic Ca2+ concentration in the CRF-immunoreactive neurons isolated from the PVN[27]. Taken together with our present study, these results suggested that nesfatin-1 may be implicated in brain CRF/CRF1 signaling pathways, which then contribute to visceral hypersensitivity in model rats.
In conclusion, nesfatin-1 may be associated with the visceral hypersensitivity state of IBS, and this may be mediated at least in part by brain CRF/CRF1 signaling pathways.
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder in clinical practice, but the pathophysiology of IBS has not been completely elucidated. Visceral hypersensitivity is common in IBS patients and has recently been considered as a biological marker for IBS. Nesfatin-1 is a recently discovered 82-amino-acid satiety peptide. Nesfatin-1 is co-localized with corticotropin releasing factor (CRF) in the hypothalamic paraventricular nucleus (PVN). Furthermore, intracerebroventricular administration of nesfatin-1 induced c-Fos expression in CRF neurons, and nesfatin-1 increased cytosolic Ca2+ concentrations in single CRF neurons in the PVN. It is now well established that the brain CRF/CRF1 signaling system modulates pain responses. These observations suggest that nesfatin-1 may be involved in the autonomic regulation of visceral sensation.
Visceral hypersensitivity is a topic of intense research in gastrointestinal disorders. The research hotspot in terms of the visceral hypersensitivity mechanism is the signaling system and the exact sites mediating modulation.
The authors found that the average serum nesfatin-1 level was significantly higher in neonatal acetic acid-treated rats than in control rats, suggesting nesfatin-1 may be associated with a visceral hypersensitivity state in IBS-like model rats. Currently, no reports have been published discussing the role of nesfatin-1 in IBS. The purpose of the present study, therefore, was to investigate the effect of nesfatin-1 on visceral sensitivity in IBS and the possible underlying mechanisms of this action.
The study results suggest that nesfatin-1 may be associated with the visceral hypersensitivity state of IBS, and this may be mediated, at least in part, by brain CRF/CRF1 signaling pathways. This may provide new targets for the treatment of IBS.
The authors investigated serum nesfatin-1 in a rat model of visceral hypersensitivity associated with IBS to explore the role of nesfatin-1 in the pathogenesis of IBS-like visceral hypersensitivity. The study found that nesfatin-1 may be associated with visceral hypersensitivity in model rats and may be implicated in brain CRF/CRF1 signaling pathways. Overall, the study has been well designed and the results are of great scientific significance.
P- Reviewers Liu S, Zheng HC S- Editor Huang XZ L- Editor Cant MR E- Editor Xiong L
| 1. | Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709-712. [PubMed] |
| 3. | Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911-4919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088-5094. [PubMed] |
| 6. | Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-557. [PubMed] |
| 7. | Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1-12. [PubMed] |
| 9. | Mousa SA, Bopaiah CP, Richter JF, Yamdeu RS, Schäfer M. Inhibition of inflammatory pain by CRF at peripheral, spinal and supraspinal sites: involvement of areas coexpressing CRF receptors and opioid peptides. Neuropsychopharmacology. 2007;32:2530-2542. [PubMed] |
| 10. | Qian AH, Liu XQ, Yao WY, Wang HY, Sun J, Zhou L, Yuan YZ. Voltage-gated potassium channels in IB4-positive colonic sensory neurons mediate visceral hypersensitivity in the rat. Am J Gastroenterol. 2009;104:2014-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] |
| 12. | Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135:2075-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Martínez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29-35. [PubMed] |
| 14. | Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21-28. [PubMed] |
| 15. | Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-G524. [PubMed] |
| 16. | Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73-82. [PubMed] |
| 17. | Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-1777. [PubMed] |
| 18. | Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223-2228. [PubMed] |
| 23. | Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372-2381. [PubMed] |
| 24. | Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071-4088. [PubMed] |
| 25. | Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321-1330. [PubMed] |
| 26. | Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958-964. [PubMed] |
| 27. | Yoshida N, Maejima Y, Sedbazar U, Ando A, Kurita H, Damdindorj B, Takano E, Gantulga D, Iwasaki Y, Kurashina T. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY). 2010;2:775-784. [PubMed] |