Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3354
Revised: March 30, 2013
Accepted: April 27, 2013
Published online: June 7, 2013
Processing time: 172 Days and 16.2 Hours
Solitary fibrous tumors are predominantly benign and are most commonly found in the thoracic cavity and pleura; while reports exist in the literature of malignant solitary fibrous tumors and those located in extrathoracic organs, these cases are considered extremely rare. Herein, a case is reported of a malignant solitary fibrous tumor involving the liver that was diagnosed and treated in a 62-year-old woman. The patient presented with complaints of upper abdominal pain and unintentional weight loss. Computed tomography scan of the abdomen revealed a remarkably large mass, measuring 15 cm × 10 cm × 20 cm, which appeared to be unrelated to any particular organ. The intraoperative finding of a wide communication with the left liver suggested hepatic origin, and served as an indicator for tumor resection via left hemihepatectomy. The diagnosis of solitary fibrous tumor and its malignant nature was confirmed by histological and immunohistochemical examination of the resected tissues. Hepatic solitary fibrous tumor is very rare, and surgery remains the mainstay of treatment. Due to limited reports of such tumors in the literature, little can be said about the benefit of adjuvant therapy and prognosis for the rare cases with malignant histological findings.
Core tip: Solitary fibrous tumors are predominantly benign and most commonly found in the thoracic cavity and pleura, but occasionally show malignant characteristics and occur in extrathoracic organs. A malignant solitary fibrous tumor involving the liver that was diagnosed in a 62-year-old woman and treated by surgical resection is reported here. Solitary fibrous tumor is very rare in a hepatic location, and surgery is the mainstay of treatment. Due to limited reports of such tumors in the literature, little can be said about the benefit of adjuvant therapy and prognosis for the rare cases with malignant histological findings.
- Citation: Jakob M, Schneider M, Hoeller I, Laffer U, Kaderli R. Malignant solitary fibrous tumor involving the liver. World J Gastroenterol 2013; 19(21): 3354-3357
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3354
Solitary fibrous tumor (SFT) is a rare mesenchymal neoplasm. Whereas most of the SFTs reported in the literature have occurred in the thoracic cavity and pleura, cases of SFTs involving extrathoracic organs exist[1-3]. The majority of SFTs are benign and patients commonly present as asymptomatic; however, excessive size or involvement of vital structures may lead to paraneoplastic and local symptoms[1,2,4,5]. Surgery remains the treatment of choice, and little is known about the benefits of adjuvant therapy for those rare cases with histological findings that indicate malignancy[6]. Herein, we report a very rare case of a malignant SFT involving the liver.
A 62-year-old woman presented at our hospital with complaints of upper abdominal pain persisting for several days and unintended weight loss that had occurred over the past month approximately. The patient’s surgical history included resection of the transverse colon in 1984 to remove an adenocarcinoma, for which the results of follow-up examinations were unremarkable.
Physical examination upon admission revealed cachexia and a palpable large, firm mass in the epigastrium region that was roughly the size of a coconut. Laboratory tests showed normal complete blood cell count and slight thrombocytosis (525 g/L vs normal range: 150-400 g/L), as well as normal levels of all liver function markers except for C-reactive protein, which was elevated (29 mg/L vs normal range: 0-5 mg/L).
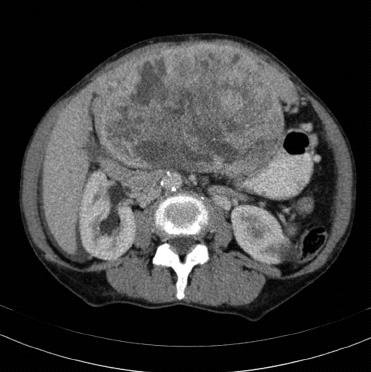
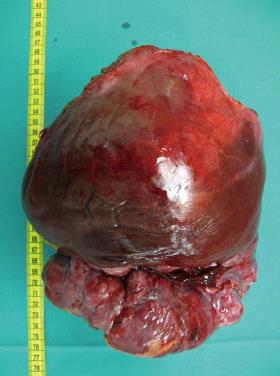
Computed tomography (CT) scan revealed a remarkably large single circumscribed intraabdominal tumor (Figure 1) without evidence of lymphatic metastases; the imaging analysis provided no indication of tumor relation to any particular organ. The presence of distant metastases was excluded by findings from additional CT scanning of the thoracic cavity. A median laparotomy was performed. The intraoperative findings of a wide communication between the tumor and the left liver and absence of penetration into adjacent organs suggested hepatic origin, and a left hemihepatectomy was carried out. The gross specimen was a large, lobulated, sharply demarcated tumor with obvious intratumoral necrotic areas (Figure 2).
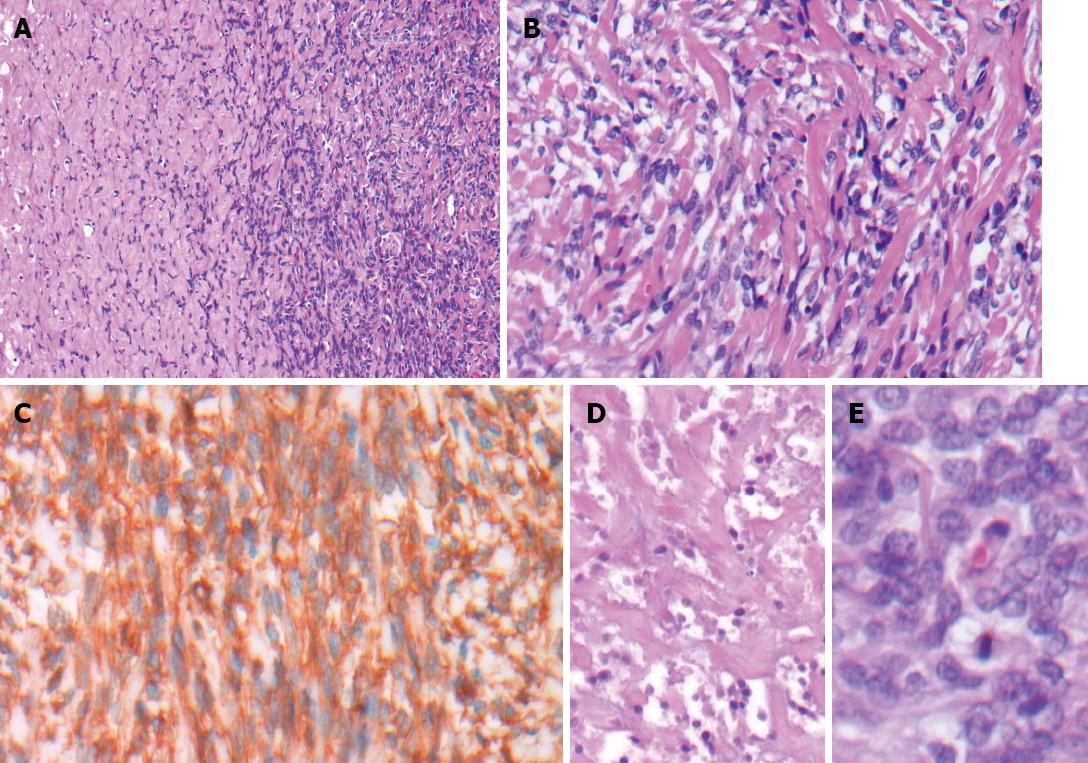
Histological examination revealed the specimen to be a well-circumscribed mesenchymal tumor adjacent to the hepatic parenchyma and capsule. The tumor was composed of short spindly and ovoid cells arranged in a random pattern amidst a variable collagenous background with hemangiopericytoma-like thin-walled ectatic blood vessels (Figure 3). In addition, areas of high cellularity with nuclear crowding, cytological atypia, tumor necrosis, and increased mitotic rate (up to six mitotic figures per 10 high-power fields) were observed. Immunohistochemical analysis showed positivity for CD34, CD99 and Bcl-2, and negativity for cytokeratin, CD117, desmin, S-100 and actin. The diagnosis was made of a malignant SFT involving the liver.
The postoperative course was uneventful, although the recovery duration was slightly protracted because of the patient’s poor nutritional status.
SFTs are most commonly found in the thoracic cavity and pleura, but have also been reported in various extrathoracic organs, including the upper respiratory tract, orbits, soft tissue, abdomen, and breast[1-3]. Very rarely do SFTs involve the liver, and the incidence of these tumors is unknown[7]. The majority of hepatic SFT diagnoses have been in adults (mean age: 57.5 years) and there appears to be a strong bias towards the female sex (2:1)[4].While most cases present as asymptomatic, some cases, such as our patient described herein, show abdominal fullness and a palpable mass[5]. Other symptoms that have been described include paraneoplastic hypoglycemia, fatigue, and weight loss[4]. From a biochemical perspective, the association of neoplasms with thrombocytosis and elevated C-reactive protein level is well known[8,9], and the presence of a tumor may be suspected when these findings are present. However, definitive diagnosis of SFT is based on a characteristic panel of histological and immunohistochemical features. Histologically, SFT is composed of spindly to ovoid cells, possibly of fibroblastic origin, which are arranged in a haphazard “patternless” pattern and intimately intertwined by collagen fibers of various thicknesses, and associated with numerous hemangiopericytoma-like dilated thin-walled blood vessels. Immunohistochemically, SFT is positive for CD34, CD99 and Bcl-2[10]. The diagnosis of SFT was made in the present case according to these characteristic immunohistochemical findings and histological features.
Whereas most of the SFTs reported in the literature have been benign (> 80%), their malignant potential is largely unknown[2]. About 10%-15% of SFTs behave aggressively. Although no strict correlation has been found between the SFTs’ morphological features and malignancy, the current World Health Organization (WHO) classification criteria of soft tissue tumors is used to identify malignant SFT; these criteria include hypercellularity, cytological atypia, tumor necrosis, high mitotic rate (four or more mitotic figures per 10 high-power fields), and/or infiltrative margins[10,11]. The resected tumor specimenfrom our case showed features of high cellularity with nuclear crowding, moderate to marked cellular atypia, up to six mitotic figures per 10 high-power fields and tumor necrosis, fulfilling the WHO criteria for malignant SFT. The imaging features previously reported for other benign and malignant SFTs involving the liver seem to overlap, and there is no distinctive radiological criterion for diagnosing malignancy, excepting the presence of distant metastases[12].
Malignant SFTs involving the liver are currently treated by surgical resection, with the aim of obtaining a margin-negative specimen[6]. Because the number of cases reported to date is small, evidence of the benefit of adjuvant therapy is lacking and its use remains controversial[2,13]. As the biological behavior of these tumors and the patients’ prognosis after treatment have not been well defined[5], careful follow-up is mandatory.
In conclusion, malignant SFT involving the liver is very rare. Nevertheless, it should be considered as a potential differential diagnosis when a single large hepatic mass is present. Definitive diagnosis is based on histopathological and immunohistochemical findings, andsurgery remains the mainstay of treatment. The rarity of these cases and limited reports in the literature preclude any significant speculation regarding the benefit of adjuvant therapy and patient prognosis.
P- Reviewer Xian L S- Editor Zhai HH L- Editor A E- Editor Li JY
| 1. | Neeff H, Obermaier R, Technau-Ihling K, Werner M, Kurtz C, Imdahl A, Hopt UT. Solitary fibrous tumour of the liver: case report and review of the literature. Langenbecks Arch Surg. 2004;389:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Peng L, Liu Y, Ai Y, Liu Z, He Y, Liu Q. Skull base metastases from a malignant solitary fibrous tumor of the liver. A case report and literature review. Diagn Pathol. 2011;6:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross-sectional imaging spectrum. Radiographics. 2011;31:393-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Moran CA, Ishak KG, Goodman ZD. Solitary fibrous tumor of the liver: a clinicopathologic and immunohistochemical study of nine cases. Ann Diagn Pathol. 1998;2:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Sun K, Lu JJ, Teng XD, Ying LX, Wei JF. Solitary fibrous tumor of the liver: a case report. World J Surg Oncol. 2011;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Terkivatan T, Kliffen M, de Wilt JH, van Geel AN, Eggermont AM, Verhoef C. Giant solitary fibrous tumour of the liver. World J Surg Oncol. 2006;4:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Vennarecci G, Ettorre GM, Giovannelli L, Del Nonno F, Perracchio L, Visca P, Corazza V, Vidiri A, Visco G, Santoro E. Solitary fibrous tumor of the liver. J Hepatobiliary Pancreat Surg. 2005;12:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Heikkilä K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 199] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Guillou L, Fletcher JA, Fletcher CD, Mandahl N. Extrapleural solitary fibrous tumor and hemangiopericytoma. WHO classification of tumours: Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press 2002; 86-90. |
| 11. | Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 442] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 12. | Fuksbrumer MS, Klimstra D, Panicek DM. Solitary fibrous tumor of the liver: imaging findings. AJR Am J Roentgenol. 2000;175:1683-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Nath DS, Rutzick AD, Sielaff TD. Solitary fibrous tumor of the liver. AJR Am J Roentgenol. 2006;187:W187-W190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |