Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3263
Revised: April 12, 2013
Accepted: April 28, 2013
Published online: June 7, 2013
Processing time: 182 Days and 17.7 Hours
AIM: To assess the stage and size of rectal tumours using 1.5 Tesla (1.5T) magnetic resonance imaging (MRI) and three-dimensional (3D) endosonography (ERUS).
METHODS: In this study, patients were recruited in a phase I/II trial of neoadjuvant chemotherapy for biopsy-proven rectal cancer planned for surgical resection with or without preoperative radiotherapy. The feasibility and accuracy of 1.5T MRI and 3D ERUS were compared with the histopathology of the fixed surgical specimen (pathology) to determine the stage and size of the rectal cancer before and after neoadjuvant chemotherapy. A Philips Intera 1.5T with a cardiac 5-channel synergy surface coil was used for the MRI, and a B-K Medical Falcon 2101 EXL 3D-Probe was used at 13 MHz for the ERUS. Our hypothesis was that the staging accuracy would be the same when using MRI, ERUS and a combination of MRI and ERUS. For the combination, MRI was chosen for the assessment of the lymph nodes, and ERUS was chosen for the assessment of perirectal tissue penetration. The stage was dichotomised into stage I and stage II or greater. The size was measured as the supero-inferior length and the maximal transaxial area of the tumour.
RESULTS: The staging feasibility was 37 of 37 for the MRI and 29 of 36 for the ERUS, with stenosis as a limiting factor. Complete sets of investigations were available in 18 patients for size and 23 patients for stage. The stage accuracy by MRI, ERUS and the combination of MRI and ERUS was 0.65, 0.70 and 0.74, respectively, before chemotherapy and 0.65, 0.78 and 0.83, respectively, after chemotherapy. The improvement of the post-chemotherapy staging using the combination of MRI and ERUS compared with the staging using MRI alone was significant (P = 0.046). The post-chemotherapy understaging frequency by MRI, ERUS and the combination of MRI and ERUS was 0.18, 0.14 and 0.045, respectively, and these differences were non-significant. The measurements of the supero-inferior length by ERUS compared with MRI were within 1.96 standard deviations of the difference between the methods (18 mm) for tumours smaller than 50 mm. The agreement with pathology was within 1.96 standard deviations of the difference between imaging and pathology for all tumours with MRI (15 mm) and for tumours that did not exceed 50 mm with ERUS (22 mm). Tumours exceeding 50 mm in length could not be reliably measured by ERUS due to the limit in the length of each recording.
CONCLUSION: MRI is preferable to use when assessing the size of large or stenotic rectal tumours. However, staging accuracy is improved by combining MRI with ERUS.
Core tip: To the best of our knowledge, the possibility of increasing the accuracy of the staging of rectal cancer by combining the strengths of magnetic resonance imaging (MRI) and endosonography (ERUS) in the same patient has not been reported. In neoadjuvantly treated rectal cancer, a combination of lymph node assessment by MRI and assessment of perirectal tissue penetration by ERUS improved the staging accuracy, with stage II as the cut-off. Furthermore, this study showed that ERUS could replace MRI in the measurement of the transaxial area of all non-stenotic tumours and in the measurement of the length of non-stenotic tumours up to 50 mm.
- Citation: Swartling T, Kälebo P, Derwinger K, Gustavsson B, Kurlberg G. Stage and size using magnetic resonance imaging and endosonography in neoadjuvantly-treated rectal cancer. World J Gastroenterol 2013; 19(21): 3263-3271
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3263
Radical surgery is the curative treatment option for rectal cancer. The preoperative work-up generally includes an assessment of the stage and size of the rectal tumour to optimise the treatment plan. This work-up ranges from early tumours possibly eligible for local excision to advanced cancers for which long-term radiochemotherapy is indicated prior to full-scale resection. To decrease recurrence, preoperative short-course radiotherapy is generally indicated in stages II-III, and long-course radiochemotherapy is usually indicated in “ugly” tumours with threatened surgical margins[1]. New strategies have been tested, including the use of preoperative chemotherapy, regardless of any radiotherapy, to potentially downstage and downsize the tumour. This approach has been tried, and further studies are on-going[2,3]. Furthermore, local excision is normally only indicated in T1 tumours with a diameter of less than 30 mm, and Zlobec et al[4] found that a diameter greater than 34 mm predicts late T-stage. These preoperative treatment strategies demand improved staging and enhanced responses for evaluating neoadjuvant therapy using accurate and accessible size measurement methods.
The main instruments for the preoperative assessment of rectal tumours are magnetic resonance imaging (MRI) and endorectal ultrasound (ERUS). Many groups have concluded that MRI is superior to ERUS in the staging of rectal tumours[5-7]. However, Bipat et al[8] found that ERUS was more accurate than MRI for perirectal tissue invasion and that the assessment of lymph nodes and invasion of adjacent organs was comparable in a meta-analysis that included all stages. Some studies have shown that MRI is preferable in advanced and stricturing tumours and in the assessment of lymph node involvement, whereas ERUS is advantageous in assessing the wall penetration of the early stages of rectal cancer[9-14]. Many of these studies used 1 Tesla (1T) MRI and two-dimensional (2D) ERUS[15,16], and the results may be improved in newer models of the MRI and ERUS equipment. Increased specificity has been reported with 3T MRI machines compared with 1T or 1.5T[17]. However, Maas et al[18] did not find that 3T MRI improved the accuracy compared with 1.5T in borderline T2-T3 rectal cancer. Diffusion-weighted MRI appears promising, but the specificity in lymph node detection is still limited[19]. Compared with 2D ERUS, the development of 3D ERUS has resulted in increased accuracy, but a problem remains regarding the detection of lymph nodes and the margins of the mesorectal fascia[20]. Earlier studies of the measurement of the rectal tumour size have primarily involved MRI[21]. Torkzad et al[22] showed that rectal tumours can be measured on MRI images, with results that correlated well with the histopathology results, and Nougaret et al[23] showed that MRI volumetry may predict early responders to neoadjuvant therapy. Murad-Regadas et al[24] found that size measurement by ERUS was useful in the selection of possible sphincter-saving surgery after neoadjuvant therapy. In many parts of Western Europe, MRI is preferred for the assessment of rectal cancer, except in the disease’s early stage, but in other parts of the world, ERUS is still the method of choice for the initial evaluation of rectal cancer at all stages[16,20,25]. Even in parts of Western Europe, this issue was not settled in 2012[7,26]. Many authors regard the methods as complementary[12,14,27,28]. Some authors have argued that a combination of both MRI and ERUS may increase the accuracy of the preoperative staging[13,14,27]. The restaging of advanced rectal cancer after neoadjuvant therapy has been studied for MRI[15,29,30] and ERUS[15,31,32]. In this context, MRI appears more promising, but there is a problem with accuracy for both methods.
In this study, 1.5T MRI and 3D ERUS were compared with histopathology of the fixed surgical specimen (pathology) to determine the stage and size of the rectal cancer before and after neoadjuvant chemotherapy. Our hypothesis was that the staging accuracy would be the same when using MRI, ERUS and a combination of MRI and ERUS. For the combination, MRI was chosen for the assessment of the lymph nodes, and ERUS was chosen for the assessment of perirectal tissue penetration because studies have reported that these are the respective strengths of the two methods[11,27,28,33]. Furthermore, we hypothesised that the MRI and ERUS results would be in agreement with regard to the tumour size measurement. The aims of the study were to evaluate the feasibility and accuracy of MRI and ERUS in staging and to measure the supero-inferior length and maximal transaxial area before and after neoadjuvant chemotherapy compared with postoperative histopathology.
The present study was conducted in cooperation with a prospective phase I/phase II study of neoadjuvant treatment with pemetrexed in rectal cancer set at a university hospital in Sweden (Alimta trial)[2]. Briefly, the study included 37 patients with rectal cancer who all received three cycles of chemotherapy prior to surgery. They underwent MRI scans before and after chemotherapy. Radiotherapy followed by resection surgery was conducted as established by national guidelines. The study concluded that the treatment concept was feasible, with limited risks of side-effects and a majority of the patients experiencing symptom relief and downsizing of the tumour.
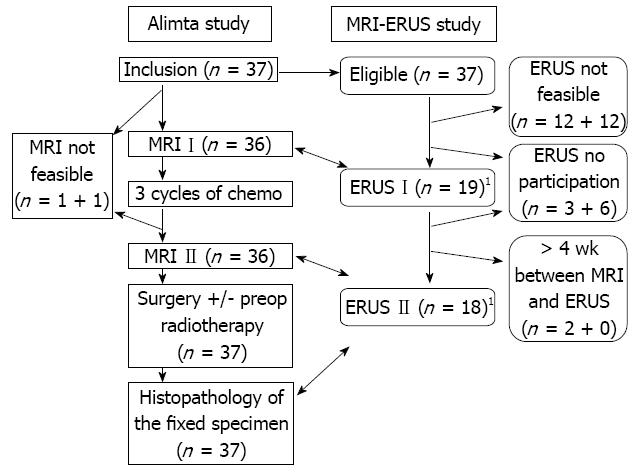
All patients who participated in the Alimta trial were asked to participate in the associated MRI-ERUS study. The demographic data of the included patients are shown in Table 1. The intention was to match the scheduled investigations using MRI with ERUS before and after chemotherapy to enable a comparison of the staging and size measurements, including paired analyses. All surgically removed specimens were routinely sent for histopathological examination, which also provided a comparison between MRI, ERUS and the fixed specimen in terms of both stage and size. The supero-inferior length was compared between MRI, ERUS and the fixed specimen. The maximal transaxial area in a dimension perpendicular to the long axis of the rectum at the site of the cancer was compared between MRI and ERUS. The MRI and ERUS were performed as detailed below. When the reports included more than one stage, the more advanced stage was chosen in the analysis. The order in which the MRI and ERUS were conducted was not controlled. Investigations with a time lapse between the MRI and ERUS greater than four weeks were not included in the analysis. The study is schematically presented in Figure 1. The protocol was approved by the local hospital ethics committee. Patients provided written consent to participate in the investigation.
| Age, median (IQ range) | 60 (60-66) |
| Gender (male/female) | 17/4 |
| Surgery (AR/APR/Hartman) | 13/8/0 |
| Laparoscopy (yes/no) | 10/11 |
| Differentiation grade (G1/2/3/4) | 0/19/0/0 (2 not classified) |
| T-stage (T1/2/3/4) | 2/8/9/2 |
| N-stage (N0/1/2) | 12/6/3 |
| Assessed nodes, median (IQ range) | 15 (14-18) |
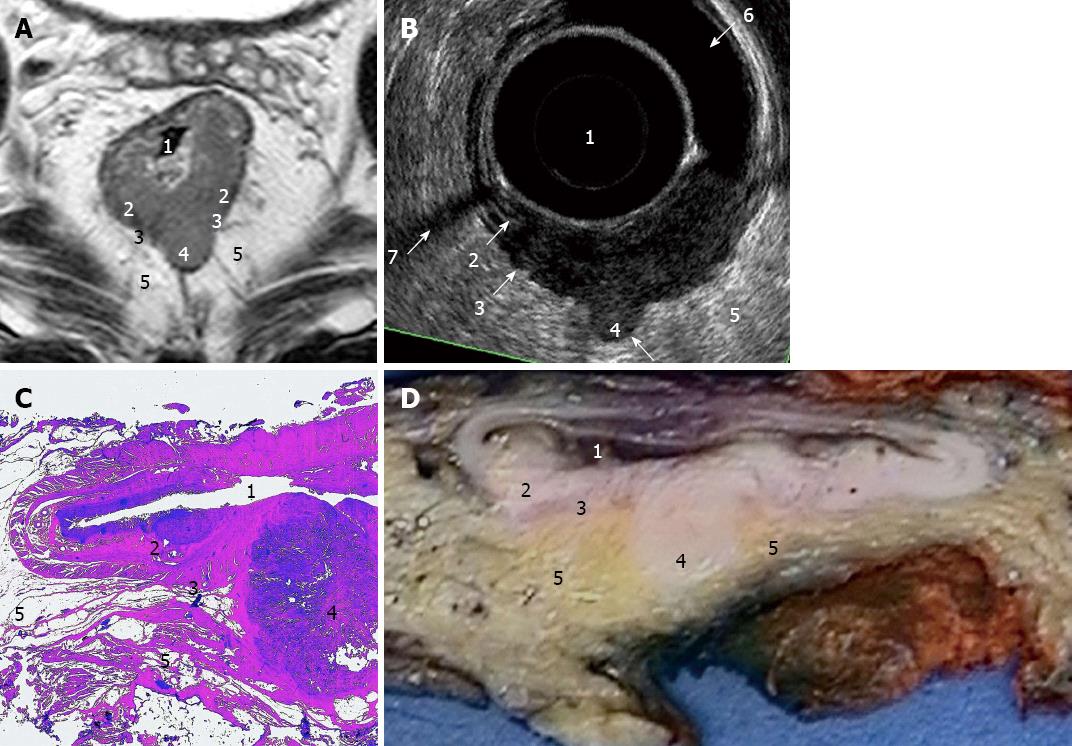
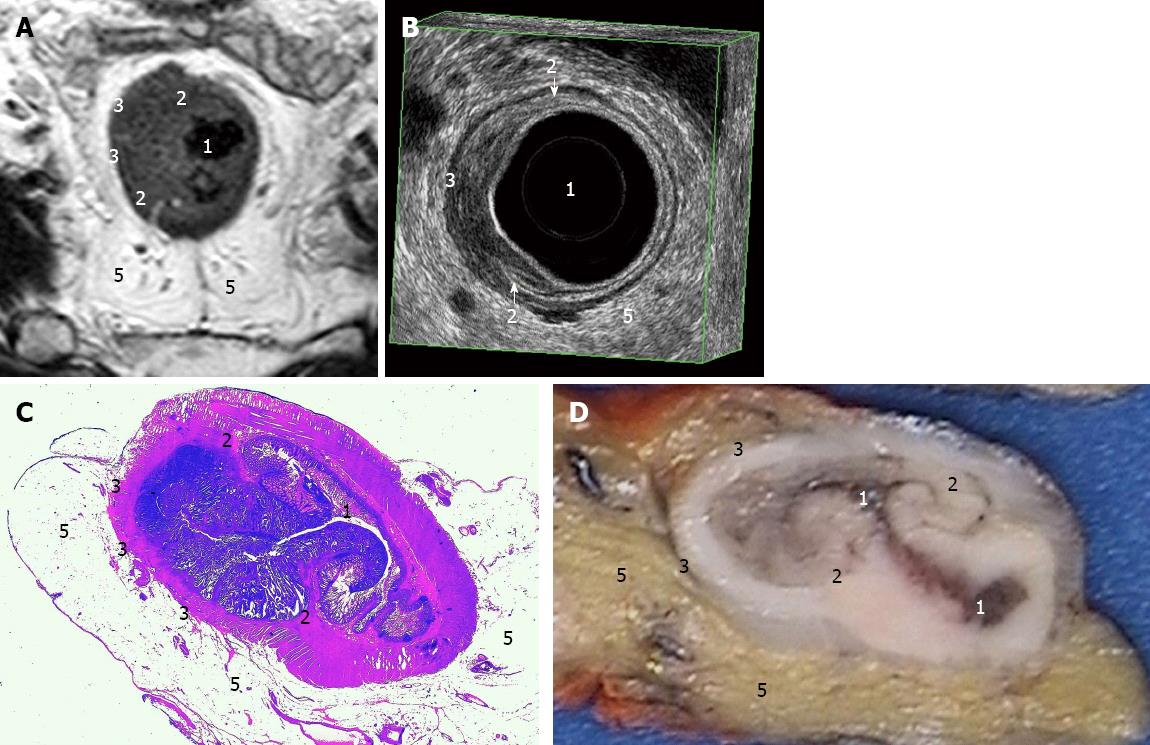
Philips Intera 1.5T was used together with a cardiac 5-channel synergy surface coil (Philips) for an optimal signal. Antispasmolytic drugs, 40 mg of buscopan and 1 mg of glucagon, were administered before the start of the examination. There was no bowel preparation of the patient. The protocol included three T2-weighted turbo spin-echo sequences in the sagittal, oblique axial and oblique coronal planes. The following conditions were used: slice thickness 3 mm, gap 0.3 mm, turbofactor between 20-22, field-of-view (FOV) 20-22 cm, 30-36 slices and in-plane resolution 0.67-0.7 mm. The echo time (TE) was 90 ms, and the repetition time (TR) of 2800-3400 ms was dependent on the sequence and number of signals acquired (NSA) 4. The scan time varied between 4-6 min per sequence. At the end, an additional oblique axial contrast-enhanced 3D T1-weighted gradient echo sequence with fat sat in the venous phase (Thrive, Philips, Eindhoven, The Netherlands) was used with the following conditions: 2 mm slices, FOV 26 cm, NSA 2, TR 9.1 ms, TE 4.5 ms, flip 10 degrees, scan time 1.49 min, and voxel size 0.81 mm. On average, each investigation lasted 40 min, during which the patients were required to lie still. The MRI images were interpreted by one radiologist (Kälebo P). The measurements were performed using a software function and recorded in millimeter. The TN staging (Figures 2A and 3A), supero-inferior length and maximal transaxial area in a dimension perpendicular to the long axis of the rectum at the site of the cancer were registered.
A B-K Medical Falcon 2101 EXL, 3D-Probe, 13 MHz was used for the ERUS. The ERUS was performed by one of two investigators who had at least four years of experience. The preparation included an enema. The investigation was conducted in the left lateral position using a probe covered by a condom filled with water and introduced through a rigid proctoscope. The maximum length of one recording was 60 mm. The measurements were recorded in mm. The recordings were saved in the 3D-mode (Figures 2B and 3B).
The histopathological assessments of the resected surgical specimens were performed after fixation. The specimens were immersed in 4% formaldehyde for 48-72 h. The specimens were then cut (Figures 2D and 3D) and again immersed in formaldehyde for another 12 h before dehydration overnight Then, the specimens were embedded in paraffin and sectioned. Routine staining was used (Figures 2C and 3C). The slides were analysed by specialist pathologists according to the everyday protocol utilized in the pathology department. The diameter in three directions and the TN staging were recorded. The histology report did not include an area measurement.
The SPSS 18.0 statistics software was used for the analyses. The tumour stage (I-IV) was dichotomised into stage I and stages II-IV because this was a cut-off for the indication for radiotherapy at the time of the study. Accuracy was defined as the number of correct positive and negative predictions divided by the total. Sensitivity was defined as the number of correct predictions of stage II or higher divided by the number of stage II or higher based on pathology. Specificity was defined as the number of correct predictions of stage I or lower divided by the number or stage I or lower based on pathology. Understaging was defined as the number of incorrect predictions, given as stages that were too low (or too early), divided by the total. Staging with MRI and ERUS was compared with histopathology, which was regarded as the gold standard. The measurement correlations among MRI, ERUS and histopathology were shown in Bland-Altman plots, and a related samples comparison was applied to the correct stage predictions, lengths and areas by MRI, ERUS and histopathology using the non-parametric related samples Wilcoxon Signed Rank Test.
Of the 37 eligible patients, 19 were assessed for size before chemotherapy, and 18 were assessed for size after chemotherapy using both MRI and ERUS within a period of four weeks (Figure 1). Another 10 patients were assessed for stage before chemotherapy, and five were assessed for stage after chemotherapy using both MRI and ERUS. The stage estimates are summarised in Table 2. A comparison of the supero-inferior length of the resected specimen by histopathology was conducted with the 18 post-chemotherapy measurements using MRI and ERUS. The size estimates are summarised in Table 3.
| MRI-pre | ERUS-pre | Combo-pre | MRI-post | ERUS-post | Combo-post | |
| Feasibility of stage | 1.00 | 0.81 | ||||
| Feasibility of size | 0.97 | 0.74 | ||||
| Accuracy of differentiating stages I/II | 0.65 | 0.70 | 0.74 | 0.65 | 0.78 | 0.83 |
| Sensitivity of differentiating stages I/II | 0.73 | 0.87 | 0.87 | 0.73 | 0.80 | 0.93 |
| Specificity of differentiating stages I/II | 0.5 | 0.38 | 0.50 | 0.50 | 0.75 | 0.62 |
| Kappa value of differentiating stages I/II | 0.23 | 0.26 | 0.40 | 0.23 | 0.53 | 0.58 |
| Overstaging of differentiating stages I/II | 0.17 | 0.22 | 0.17 | 0.17 | 0.087 | 0.13 |
| Understaging of differentiating stages I/II | 0.17 | 0.087 | 0.087 | 0.17 | 0.13 | 0.048 |
| Accuracy of perirectal penetration | 0.48 | 0.70 | 0.52 | 0.78 | ||
| Accuracy of lymph node detection | 0.74 | 0.56 | 0.74 | 0.74 |
| Modality | Before chemotherapy | After chemotherapy | Specimen | ||
| Length | Area | Length | Area | Length | |
| MRI | 47 (36-68) | 641 (283-1345) | 44 (19-68) | 616 (156-1431) | Not applicable |
| ERUS | 46 (29-59) | 680 (290-2110) | 41 (15-56) | 595 (100-1380) | Not applicable |
| Histopathology | Not applicable | Not applicable | Not applicable | Not applicable | 34 (12-70) |
The staging feasibility was 37 of 37 for MRI and 29 of 36 for ERUS. The feasibility of the size assessment was 36 of 37 for MRI and 25 of 34 for ERUS. The inability to lie still for approximately 40 min was the reason for noncompliance in the MRI and stenosis or pain on examination was the reason for noncompliance in the ERUS. In a number of patients, air pockets within the bowel lumen and remnants of bowel contents compromised the quality of ERUS but not of MRI (Figure 2A and B). The assessment of size demanded higher quality examinations than the assessment of stage for both MRI and ERUS. For example, relative stenosis could allow a sufficient view to assess a tumour as stage II or higher but did not allow for a precise assessment of size.
The staging accuracy of the perirectal tissue penetration was 0.48 and 0.52 by MRI and 0.70 and 0.78 by ERUS before and after chemotherapy, respectively. The staging accuracy of the lymph node metastases was 0.74 and 0.74 by MRI and 0.56 and 0.74 by ERUS before and after chemotherapy, respectively.
The accuracy of the TNM staging, with stage II as the cut-off, by MRI, ERUS and the combined MRI and ERUS examinations was 0.65, 0.70 and 0.74, respectively, before chemotherapy and 0.65, 0.78 and 0.83, respectively, after chemotherapy. Thus, the post-chemotherapy staging by MRI alone was improved by a combination of MRI assessment of the lymph nodes and ERUS assessment of the perirectal tissue penetration (P = 0.046, Wilcoxon signed rank test). The post-chemotherapy understaging frequency by MRI, ERUS and the combined MRI and ERUS exams was 0.18, 0.14 and 0.045, respectively, but the differences were not significant.
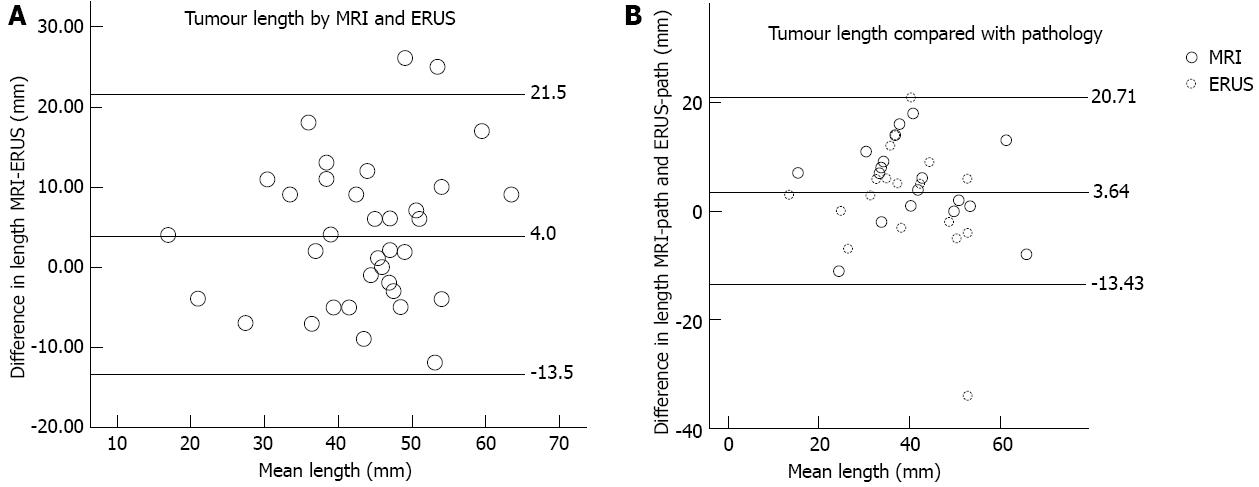
For tumours smaller than 50 mm, measurements of the supero-inferior length by ERUS compared with MRI were within 1.96 standard deviations of the difference between the methods (18 mm). For all tumours, agreement with the histopathology of the resected specimen after fixation was within 1.96 standard deviations of the difference between imaging and pathology for MRI (15 mm), and agreement with the histopathology of the resected specimen after fixation was within 1.96 standard deviations of the difference between imaging and pathology for ERUS (22 mm) for tumours that did not exceed 50 mm. There was an overestimation of the supero-inferior length by MRI (P = 0.016) compared with histopathology and ERUS/histopathology (P = 0.021). The ERUS measurement of tumour downsizing did concur (P < 0.05) with the MRI size assessments. The measurements of length are shown by Bland-Altman plots in Figure 4.
The measurements of the maximal transaxial area by ERUS compared with MRI were within 1.96 standard deviations of the difference between the methods (684 mm2) for all tumours. An area measurement was not obtained by histopathology.
The aim of this study was to assess the feasibility and accuracy of 1.5T MRI and 3D-ERUS compared with histopathology to determine the stage and size of rectal tumours. The staging feasibility was 37 of 37 for MRI but only 29 of 36 for ERUS. The accuracy of the staging with stage II as the cut-off was improved by combining the results of the lymph node assessments by MRI with the results of the perirectal tissue penetration by ERUS. Overstaging and understaging were observed in 17% of the patients by MRI both before and after chemotherapy. Overstaging could be explained by downstaging due to neoadjuvant therapy, but this does not explain understaging. Understaging with stage II as the cut-off means that the patients are likely to be incorrectly denied preoperative radiotherapy. Overstaging, in contrast, means that the patients will likely be given unnecessary radiotherapy. If a combination of MRI and ERUS could increase the accuracy in patients on the cut-off edge between stages I and II, performing both examinations might be worthwhile, even if this approach increases the number of investigations.
The staging accuracy of both modalities was lower in this study than those reported in many other studies[33]. Still, only a fair agreement between MRI and histopathology was reported by Tytherleigh et al[34], and Harewood[35] showed that the ERUS staging was lower outside the initial studies. Ashraf reported that ERUS staging in “real world” practice in the United Kingdom is much lower than that shown in many studies[36]. These authors have concluded that the accuracy of MRI or ERUS can be expected to be lower in clinical practice than often reported from dedicated institutions. Another possible explanation for the low accuracy could be that the neoadjuvant chemotherapy treatment caused a desmoplastic reaction, making correct staging more difficult. A reduction in accuracy has been noted after neoadjuvant radiochemotherapy[13,32]. Another reason may be the overrepresentation of T2-T3 tumours, which are difficult to distinguish from each other using both MRI and ERUS[14,18].
One central issue in preoperative staging is the possible presence of tumour-affected lymph nodes. This issue is important in standard resection surgery because the risk of node involvement affects the use of short-course preoperative radiotherapy before surgery[1]. Preoperative staging of lymph node involvement is certainly central when considering local resections and could be of great value when assessing patients who could benefit from a neoadjuvant chemotherapy regime. Several groups have found that MRI is the preferred method for lymph node involvement[37], whereas ERUS is the preferred method for wall and perirectal tissue penetration[8,33]. However, Halefoglu et al[12] reported that MRI can be better in more advanced stages, even in cases of wall penetration. Some authors have suggested that the two methods complement each other, which concurs with the findings of the present study[12,14,27,28]. As previously reported, MRI tends to be superior in the assessment of the lymph nodes, whereas ERUS tends to be superior in the assessment of the penetration of the bowel wall and perirectal tissue[11,27,28,33]; however, the possibility of increasing accuracy by combining the strengths of each modality in the same patient has not yet been reported to the best of our knowledge.
MRI has several advantages for use in the measurement of tumour size. Its feasibility in stenosing tumours and in patients who are sensitive to pain on introduction of the probe was important as in approximately 1/4 of the cases, these factors made ERUS measurements impossible. Furthermore, the probe used for ERUS provided a view that was 60 mm in supero-inferior length at most. Tumours larger than this could not be reliably measured with ERUS because of the necessity for multiple recordings. Thus, MRI had a better agreement with histopathology in the tumours with a supero-inferior length greater than 50 mm. The measurements of the supero-inferior tumour length by ERUS compared with MRI agreed within 1.96 standard deviations (18 mm) for tumours up to 50 mm. Accepting, at most, an 18 mm deviation, ERUS can only replace MRI in the measurement of supero-inferior length for tumours up to approximately 50 mm in length. A deviation by 18 mm seems large, but even MRI deviated up to 15 mm compared with pathology. The measurement of the maximum transaxial area agreed within 1.96 standard deviations of the difference between the two methods (684 mm2) for all tumours. For ERUS, the transaxial area appears more reproducible than the supero-inferior length for large tumours.
Both MRI and ERUS overestimated the tumour length compared with histopathology. This difference is not explained by specimen, shrinkage as studies have indicated that the specimen slightly increases in size (up to 8%) due to fixation[38]. A more likely explanation could be that both MRI and ERUS overestimate length due to desmoplastic reactions, which may not be distinguished from the tumour itself[28]. Another explanation could be the short-course preoperative radiotherapy; however, this explanation is less likely because Brown et al[38] found no stage effect. A theoretical cause of size difference could also be a further anti-tumoural effect from the chemotherapy administered in the Alimta study.
This study focused on the accuracy of the staging and size measurements. There were limitations to this study, including the low number of patients. However, this study does involve a special group of patients and includes information on the examinations before and after treatment, as well as histopathological data. Its other strengths are that all of the MRI measurements were performed by one radiologist and that the ERUS was performed by one of two senior surgeons. The measurement of the size of the rectal tumours by MRI compared with ERUS has not been extensively described previously. The increase in the staging accuracy using a combination of MRI and ERUS is interesting and could warrant further studies.
For staging rectal tumours, 1.5T MRI was more feasible than 3D ERUS because of a higher yield in stenotic tumours. The level of accuracy did not differ in the tumours assessed using both methods. A combination of lymph node assessment by MRI and assessment of perirectal tissue penetration by ERUS improved the staging accuracy, with stage II as the cut-off. For the measurement of tumour size, MRI is more feasible and accurate when large and stenotic tumours are included. However, in non-stenotic tumours, ERUS could replace MRI in the measurement of the transaxial area of all tumours and in the measurement of the length of tumours up to 50 mm.
We thank the Gothenburg Medical Association, the Lions Cancerfond Väst and the Björnsson Foundation for financial support. We would also like to express our gratitude to study nurse Hillevi Björkqvist for assisting in the collection and registration of data and samples and to biomedical scientist Karin Blomqvist for helping in the preparation of the pathology images.
Magnetic resonance imaging (MRI) and endorectal ultrasound (ERUS) are the most widely used methods to assess the stage and size of rectal cancer to select the patients who benefit from neoadjuvant therapy. Lymph node metastasis, which is an indication for neoadjuvant therapy, is difficult to detect in early stages. Therefore, all patients with T3-tumours are often given neoadjuvant radiotherapy because a high rate of non-detectable lymph node metastases can be anticipated. Accurate preoperative assessment of both lymph node involvement and wall penetration is vital in this context. Likewise, reliable measurements of tumour size are important to monitor the effects of neoadjuvant therapy.
Several studies have shown that MRI is more accurate in staging of lymph nodes and the relation of the tumour margins to the surrounding structures, while ERUS is more accurate in staging of early wall penetration. Measurement of tumour size using either MRI or ERUS has been described but not in comparison to each other.
In neoadjuvantly treated rectal cancer, a combination of lymph node assessment by MRI and assessment of perirectal tissue penetration by ERUS improved the staging accuracy, with stage II as the cut-off. Furthermore, this study showed that ERUS could replace MRI in the measurement of the transaxial area of all non-stenotic tumours and in the measurement of the length of non-stenotic tumours up to 50 mm.
To increase staging accuracy, MRI and ERUS may be combined in the same patient, paying more attention to the strengths of each method. A combined examination may especially be considered in tumours at the cut-off between stage I and II for more correct use of preoperative radiotherapy. To measure tumour size, MRI is better suited in large and stenotic tumours, while ERUS is a sufficient alternative in small tumours passable by proctoscopy.
MRI with 1.5 Tesla strength of the magnetic field is widely used in assessment of rectal cancer. Three-dimensional (3D) ERUS saves a 3D image, which may be assessed in several planes. 2D-versions allow assessment in one plane only. Neoadjuvant chemo- and/or radio-therapy is given before surgery to decrease the risk of tumour recurrence.
The paper compares imaging of neoadjuvantly treated rectal cancer using MRI and ERUS. Among weaknesses are a relatively small number of patients with many assessments lost. The strength of the paper is the application of everyday clinical practice in imaging of rectal cancer.
P- Reviewer Arbab AS S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Blomqvist L, Glimelius B. The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol. 2008;47:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Derwinger K, Kodeda K, Swartling T, Kälebo P, Carlsson G, Gustavsson B. A phase I/II study of neoadjuvant chemotherapy with Pemetrexed (Alimta) in rectal cancer. Eur J Surg Oncol. 2011;37:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 4. | Zlobec I, Minoo P, Karamitopoulou E, Peros G, Patsouris ES, Lehmann F, Lugli A. Role of tumor size in the pre-operative management of rectal cancer patients. BMC Gastroenterol. 2010;10:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Akasu T, Iinuma G, Takawa M, Yamamoto S, Muramatsu Y, Moriyama N. Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann Surg Oncol. 2009;16:2787-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Moll R. Diagnosis of rectal cancer: pro MRI. Dtsch Med Wochenschr. 2012;137:2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 720] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 9. | Karantanas AH, Yarmenitis S, Papanikolaou N, Gourtsoyiannis N. Preoperative imaging staging of rectal cancer. Dig Dis. 2007;25:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Daniels IR, Fisher SE, Heald RJ, Moran BJ. Accurate staging, selective preoperative therapy and optimal surgery improves outcome in rectal cancer: a review of the recent evidence. Colorectal Dis. 2007;9:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Engelen SM, Beets GL, Beets-Tan RG. Role of preoperative local and distant staging in rectal cancer. Onkologie. 2007;30:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal ultrasonography versus phased-array magnetic resonance imaging for preoperative staging of rectal cancer. World J Gastroenterol. 2008;14:3504-3510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Beets GL, Beets-Tan RG. Pretherapy imaging of rectal cancers: ERUS or MRI? Surg Oncol Clin N Am. 2010;19:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Pomerri F, Pucciarelli S, Maretto I, Zandonà M, Del Bianco P, Amadio L, Rugge M, Nitti D, Muzzio PC. Prospective assessment of imaging after preoperative chemoradiotherapy for rectal cancer. Surgery. 2011;149:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Ren JH, Guo FJ, Dai WD, Han XJ, Ma N. Study of endorectal ultrasonography in the staging of rectal cancer. Chin Med J (Engl). 2012;125:3740-3743. [PubMed] |
| 17. | Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Maas M, Lambregts DM, Lahaye MJ, Beets GL, Backes W, Vliegen RF, Osinga-de Jong M, Wildberger JE, Beets-Tan RG. T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging. 2012;37:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18:2224-2231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Cârţână ET, Pârvu D, Săftoiu A. Endoscopic ultrasound: current role and future perspectives in managing rectal cancer patients. J Gastrointestin Liver Dis. 2011;20:407-413. [PubMed] |
| 21. | Torkzad MR, Lindholm J, Martling A, Cedermark B, Glimelius B, Blomqvist L. MRI after preoperative radiotherapy for rectal cancer; correlation with histopathology and the role of volumetry. Eur Radiol. 2007;17:1566-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Torkzad M, Lindholm J, Martling A, Blomqvist L. Retrospective measurement of different size parameters of non-radiated rectal cancer on MR images and pathology slides and their comparison. Eur Radiol. 2003;13:2271-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Nougaret S, Fujii S, Addley HC, Bibeau F, Pandey H, Mikhael H, Reinhold C, Azria D, Rouanet P, Gallix B. Neoadjuvant chemotherapy evaluation by MRI volumetry in rectal cancer followed by chemoradiation and total mesorectal excision: Initial experience. J Magn Reson Imaging. 2012;Nov 13; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Murad-Regadas SM, Regadas FS, Rodrigues LV, Crispin FJ, Kenmoti VT, Fernandes GO, Buchen G, Monteiro FC. Criteria for three-dimensional anorectal ultrasound assessment of response to chemoradiotherapy in rectal cancer patients. Colorectal Dis. 2011;13:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Isbert C, Germer CT. Impact of endoscopy and endosonography on local staging of rectal carcinoma. Chirurg. 2012;83:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Klingel H. Diagnosis of rectal cancer: pro endosonography. Dtsch Med Wochenschr. 2012;137:2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Bianchi P, Ceriani C, Palmisano A, Pompili G, Passoni GR, Rottoli M, Cappellani A, Montorsi M. A prospective comparison of endorectal ultrasound and pelvic magnetic resonance in the preoperative staging of rectal cancer. Ann Ital Chir. 2006;77:41-46. [PubMed] |
| 28. | Bianchi PP, Ceriani C, Rottoli M, Torzilli G, Pompili G, Malesci A, Ferraroni M, Montorsi M. Endoscopic ultrasonography and magnetic resonance in preoperative staging of rectal cancer: comparison with histologic findings. J Gastrointest Surg. 2005;9:1222-1227; discussion 1227-1228. [PubMed] |
| 29. | Patel UB, Blomqvist LK, Taylor F, George C, Guthrie A, Bees N, Brown G. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol. 2012;199:W486-W495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 30. | Lambregts DM, Beets GL, Maas M, Kessels AG, Bakers FC, Cappendijk VC, Engelen SM, Lahaye MJ, de Bruïne AP, Lammering G. Accuracy of gadofosveset-enhanced MRI for nodal staging and restaging in rectal cancer. Ann Surg. 2011;253:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Pastor C, Subtil JC, Sola J, Baixauli J, Beorlegui C, Arbea L, Aristu J, Hernandez-Lizoain JL. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum. 2011;54:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Marone P, de Bellis M, Avallone A, Delrio P, di Nardo G, D’Angelo V, Tatangelo F, Pecori B, Di Girolamo E, Iaffaioli V. Accuracy of endoscopic ultrasound in staging and restaging patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Clin Res Hepatol Gastroenterol. 2011;35:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Tytherleigh MG, Ng VV, Pittathankal AA, Wilson MJ, Farouk R. Preoperative staging of rectal cancer by magnetic resonance imaging remains an imprecise tool. ANZ J Surg. 2008;78:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Harewood GC. Assessment of publication bias in the reporting of EUS performance in staging rectal cancer. Am J Gastroenterol. 2005;100:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Ashraf S, Hompes R, Slater A, Lindsey I, Bach S, Mortensen NJ, Cunningham C. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 607] [Article Influence: 27.6] [Reference Citation Analysis (0)] |