Published online Jan 14, 2013. doi: 10.3748/wjg.v19.i2.284
Revised: November 14, 2012
Accepted: November 24, 2012
Published online: January 14, 2013
Processing time: 130 Days and 8.3 Hours
AIM: To investigate tumor response and survival in patients with postembolization fever (PEF) and to determine the risk factors for PEF.
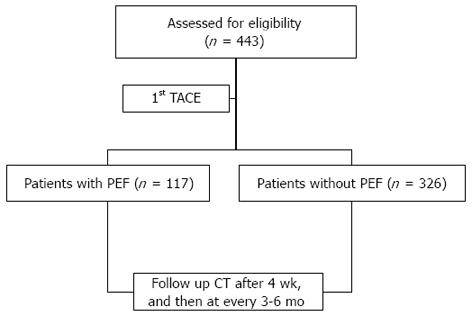
METHODS: Four hundred forty-three hepatocellular carcinoma (HCC) patients who underwent the first session of transcatheter arterial chemoembolization (TACE) between January 2005 and December 2009 were analyzed retrospectively. PEF was defined as a body temperature greater than 38.0 °C that developed within 3 d of TACE without evidence of infection. The tumor progression-free interval was defined as the interval from the first TACE to the second TACE based on mRECIST criteria. Clinical staging was based on the American Joint Committee on Cancer tumor, node, metastases (TNM) classification of malignant tumors. All patients were admitted before their 1st TACE treatment, and blood samples were obtained from all patients before and after treatment. Clinicoradiological variables and host-related variables were compared between two groups: patients with PEF vs patients without PEF. Additionally, variables related to 20-mo mortality and tumor progression-free survival were analyzed.
RESULTS: The study population comprised 370 (85.4%) men and 73 (14.6%) women with a mean age of 62.29 ± 10.35 years. A total of 1836 TACE sessions were conducted in 443 patients, and each patient received between 1 and 27 (mean: 4.14 ± 3.57) TACE sessions. The mean follow-up duration was 22.23 ± 19.6 mo (range: 0-81 mo). PEF developed in 117 patients (26.41%) at the time of the first TACE session. PEF was not associated with 20-mo survival (P = 0.524) or computed tomography (CT) response (P = 0.413) in a univariate analysis. A univariate analysis further indicated that diffuse-type HCC (P = 0.021), large tumor size (≥ 5 cm) (P = 0.046), lipiodol dose (≥ 7 mL, P = 0.001), poor blood glucose control (P = 0.034), alanine aminotransferase (ALT) value after TACE (P = 0.004) and C-reactive protein (CRP) value after TACE (P = 0.036) served as possible risk factors correlated with PEF. The ALT value after TACE (P = 0.021) and lipiodol dose over 7 mL (P = 0.011) were independent risk factors for PEF in the multivariate analysis. For the 20-mo survival, poor blood sugar control (P < 0.001), portal vein thrombosis (P = 0.001), favorable CT response after TACE (P < 0.001), initial aspartate aminotransferase (P = 0.02), initial CRP (P = 0.042), tumor size (P < 0.001), TNM stage (P < 0.001) and lipiodol dose (P < 0.001) were possible risk factors in the univariate analysis. Tumor size (P = 0.03), poor blood sugar control (P = 0.043), and portal vein thrombosis (P = 0.031) were significant predictors of survival in the multivariate analysis. Furthermore, the tumor progression-free interval was closely associated with CRP > 1 mg/dL (P = 0.003), tumor size > 5 cm (P < 0.001), tumor type (poorly defined) (P < 0.001), and lipiodol dose (> 7 mL, P < 0.001).
CONCLUSION: PEF has no impact on survival at 20 mo or radiologic response. However, the ALT level after TACE and the lipiodol dose represent significant risk factors for PEF.
- Citation: Jun CH, Ki HS, Lee HK, Park KJ, Park SY, Cho SB, Park CH, Joo YE, Kim HS, Choi SK, Rew JS. Clinical significance and risk factors of postembolization fever in patients with hepatocellular carcinoma. World J Gastroenterol 2013; 19(2): 284-289
- URL: https://www.wjgnet.com/1007-9327/full/v19/i2/284.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i2.284
Hepatocellular carcinoma (HCC) is the seventh most common carcinoma worldwide and the third most common cause of cancer-related mortality[1]. In South Korea, the age-standardized incidence rate of HCC is 46.5 per 100 000 individuals[2]. Recent advances in treatment, including liver transplantation, surgical resection, percutaneous ethanol injection therapy, radiofrequency ablation and transcatheter arterial chemoembolization (TACE) have improved the prognosis for patients with HCC[3,4]. In addition, beneficial therapeutic options that may affect long-term cure include surgical resection, liver transplantation, and percutaneous ablation[5]. However, these curative therapies are feasible only for a small subset of patients with HCC[6]. Among the noncurative therapies, only chemoembolization, the most widely used treatment for unresectable HCC, has demonstrated a positive effect on survival[7]. In contrast, TACE can be employed for any type of HCC irrespective of tumor size, location, or number[8].
However, TACE inevitably results in a hypoxic insult to the HCC and the surrounding liver tissue[9], and postembolization syndrome is common[10]. Postembolization syndrome, which consists of temporary fever, ileus, and abdominal pain, is the most common side effect of chemoembolization, affecting 60% to 80% of patients with HCC[5,11]. Postembolization fever after TACE is the most significant adverse effect, and it frequently affects the duration of hospitalization and causes the needless administration of antibiotics, although the fever is self-limited in most cases. However, few data about postembolization fever have been reported. Therefore, we evaluated the risk factors and prognostic significance of postembolization fever in patients with HCC.
Four hundred forty-three HCC patients who underwent the first session of TACE between January 2005 and December 2009 were analyzed retrospectively. The diagnosis of HCC was confirmed histologically or based on consistent findings obtained from at least two imaging techniques: ultrasonography, computed tomography (CT), magnetic resonance imaging, and/or selective hepatic arterial angiography[12,13]. Clinical staging was determined based on the American Joint Committee on Cancer tumor, node, metastases (TNM) classification of malignant tumors[14].
Clinicoradiological variables were compared between two groups (patients with PEF vs patients without PEF). The host-related variables included age, sex, viral status, cause of HCC, Child-Pugh score, Eastern Cooperative Oncology Group (ECOG) performance status, white blood cell counts, aspartate aminotransferase (AST), alanine aminotransferase (ALT), α-fetoprotein (AFP), and 20-mo mortality. The tumor-related variables included maximal tumor size, number of tumors, TNM stage, radiological findings (poorly defined or well defined), portal vein thrombosis, and CT response after 1st TACE.
All patients were admitted before their 1st TACE, and blood samples were obtained from all patients before and after treatment. Serum AFP, CRP, blood chemistry and ECOG score at admission were measured. After the 1st TACE, the patients were carefully followed. Dynamic CT was performed after 4 wk and then every 3 to 6 mo (Figure 1).
Our institutional review board did not require approval because the procedures were performed for clinical reasons. Informed consent was obtained from all patients after the nature and purpose of the TACE procedure had been fully explained.
An arterial catheter was inserted into the femoral artery using the Seldinger method and placed in the hepatic artery. Tumor-feeding vessels were superselected whenever possible, and a solution containing 10 to 40 mg of doxorubicin hydrochloride (ADM; Dong-A Pharmacy, Seoul, Korea) and 0 to 40 mL of iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France) with absorbable gelatin particles (Gelfoam; Upjohn, Kalamazoo, Michigan) was injected through the catheter (5F) or microcatheter (2.8 or 3F). The doses of doxorubicin and iodized oil were individually determined according to tumor size, tumor extent, and the patient’s underlying liver function.
For the purpose of this study, we defined postembolization fever as a body temperature greater than 38.0 °C during the 3 d after TACE. Body temperature was measured qid by nurses using an axillary thermometer. Bacterial cultures from blood and urine and chest X-rays were performed for patients who had fevers after TACE to detect any potential infectious agents. Empirical broad-spectrum intravenous antibiotics were used to treat potential infections if there was fever but were discontinued when bacterial cultures did not reveal any causative agent and the fever had subsided. Ultrasonography or CT scans were performed if the fever persisted despite the use of antibiotics to detect the possible formation of an abscess. Acetaminophen or nonsteroidal anti-inflammatory drugs were used for symptom control, if necessary.
The efficacy of TACE was evaluated by comparing the CT scans obtained before and after chemoembolization in terms of iodized oil uptake patterns in the tumor that could be considered necrotic[12] and tumor extent. The iodized oil uptake was considered compact if the oily contrast medium was clearly dispersed through all viable target tumors but was noncompact in all other cases[8]. Tumor response to TACE was defined as a compact uptake of iodized oil or at least a 30% decrease in the sum of the largest diameters of viable tumors, despite noncompact iodized oil uptake.
PEF was defined as a body temperature greater than 38.0 °C that developed within 3 d of TACE without evidence of infection. Poor blood glucose control was defined as a mean blood glucose level > 200 mg/dL. Poorly defined tumor type was defined as diffuse-type HCC, whereas well-defined tumor type was defined as nodular HCC. Tumor progression-free survival was defined as the interval during and after treatment in which a patient remained alive and the disease did not worsen (in this case, the interval from the 1st TACE to the 2nd TACE).
Comparisons were performed using the student’s t test for continuous variables and Pearson’s χ2 test. Factors that were significant in the univariate analysis were entered into a stepwise multivariate analysis to identify the most significant risk factors. The hazard function data were estimated using the Kaplan-Meier curve and compared using the log-rank test. Multivariate analyses were performed using the Cox proportional hazards model to identify prognostic factors. We performed statistical analyses using SPSS 17.0 (SPSS Inc., Chicago, United States). A P-value less than 0.05 was considered statistically significant.
The study population consisted of 370 (85.4%) men and 73 (14.6%) women with a mean age of 62.29 ± 10.35 years. A total 1836 sessions of TACE were conducted in 443 patients between January 2005 and December 2009. Each patient received between 1 and 27 (mean, 4.14 ± 3.57) sessions of TACE. The mean follow-up duration was 22.23 ± 19.6 mo (range: 0-81 mo).
One hundred seventeen episodes of postembolization fever (26.41%) occurred in 443 HCC patients after the 1st TACE session. Most of the postembolization fever episodes peaked within the first two days after TACE. The post-TACE fever was usually self-limiting, with durations ranging from 1 to 10 d (mean: 1.72 ± 1.11 d). The infectious complication rate was 0.16% (3/1836 cases). Two cases of bacteremia and one case of liver abscess developed after 1836 TACE sessions. A comparison of the TACE sessions with and without fever is presented in Table 1.
| Variables | Patients with PEF (n = 117) | Patients without PEF (n = 326) | P-value |
| Sex (M/F) | 94/23 | 276/50 | 0.309 |
| Age (yr) | 62.26 ± 10.79 | 62.16 ± 9.98 | 0.971 |
| Cause of HCC (HBV/HCV/alcohol/other) | 66/25/14/11 | 180/44/73/25 | 0.088 |
| Tumor type (well defined/poorly defined) | 57/44 | 202/90 | 0.021 |
| Portal vein thrombosis (yes/no) | 9/93 | 39/253 | 0.292 |
| Poor blood glucose control (yes/no) | 29/88 | 116/210 | 0.039 |
| Favorable tumor response | 61 (52.5%) | 155 (48.5%) | 0.516 |
| 20-mo mortality | 60 (51.7%) | 172 (53.4%) | 0.828 |
| Initial WBC/mm3 | 5969 ± 3497 | 5547 ± 2535 | 0.194 |
| Initial AST U/L | 68.87 ± 45.49 | 64.11 ± 44.79 | 0.358 |
| Initial ALT U/L | 50.84 ± 41.58 | 43.89 ± 33.16 | 0.129 |
| Initial CRP mg/dL | 1.23 ± 1.66 | 0.86 ± 0.93 | 0.116 |
| Initial AFP IU/mL | 2778 ± 8014 | 2600 ± 8656 | 0.857 |
| Child-Pugh score | 5.66 ± 0.72 | 5.77 ± 0.97 | 0.296 |
| TNM stage | 1.95 ± 1.05 | 1.83 ± 1.07 | 0.305 |
| Interval from 1st TACE to 2nd TACE (d) | 157 ± 225 | 180 ± 270 | 0.412 |
| Total TACE sessions | 1.48 ± 3.45 | 4.02 ± 3.62 | 0.230 |
A univariate analysis indicated that diffuse-type HCC (P < 0.05), large tumor size (≥ 5 cm) (P < 0.05), lipiodol dose (≥ 7 mL) (P < 0.01), poor blood glucose control (P < 0.05), ALT value after TACE (P < 0.01) and CRP value after TACE (P < 0.05) were possible risk factors correlated with postembolization fever in patients with HCC.
A multivariate analysis using logistic regression showed that the ALT value after TACE (P < 0.05) and the lipiodol dose (≥ 7 mL) (P < 0.05) were independent predictive factors of postembolization fever (Table 2).
| Variables | HR | 95%CI | P-value |
| Multivariate analysis1 | |||
| ALT value after TACE | 1.002 | 1.00-1.005 | < 0.05 |
| Lipiodol dose (< 7 mL) | 0.539 | 0.329-0.881 | < 0.05 |
| Univariate analysis2 | |||
| Poor BS control | 2.673 | 1.77-4.034 | < 0.01 |
| Portal vein thrombosis | 3.048 | 1.536-6.06 | < 0.01 |
| Poor CT response | 2.638 | 1.785-3.891 | < 0.01 |
| Initial AST | 1.006 | 1.001-1.011 | < 0.05 |
| Initial CRP | 1.348 | 1.011-1.796 | < 0.05 |
| Tumor size | 1.31 | 1.198-1.433 | < 0.01 |
| TNM | 1.555 | 1.26-1.92 | < 0.01 |
| Lipiodol dose | 1.12 | 1.058-1.185 | < 0.01 |
| Multivariate analysis3 | |||
| Tumor size | 1.252 | 1.108-1.414 | < 0.01 |
| Poor BS control | 2.442 | 1.310-4.55 | < 0.01 |
| Portal vein thrombosis | 3.344 | 1.021-10.98 | < 0.05 |
| Cox regression analysis4 | |||
| Poor CT response after TACE | 0.302 | 0.192-0.765 | < 0.0 |
| Lipiodol dose (≥ 7 mL) | 0.494 | 0.279-0.874 | < 0.05 |
PEF was not associated with 20-mo survival (P = 0.754), 10-mo survival (P = 0.524) and CT response (P = 0.461) in the univariate analysis.
The univariate analysis revealed that poor blood sugar control (P < 0.01), portal vein thrombosis (P < 0.01), favorable CT response after TACE (P < 0.01), initial AST (P < 0.05), initial CRP (P < 0.05), tumor size (P < 0.01), TNM stage (P < 0.01) and lipiodol dose (P < 0.01) were possible risk factors correlated with 20-mo mortality (Table 2). A multivariate analysis using logistic regression showed that tumor size (P < 0.01), poor blood glucose control (P < 0.01) and portal vein thrombosis (P < 0.05) were independent risk factors for 20-mo mortality (Table 2).
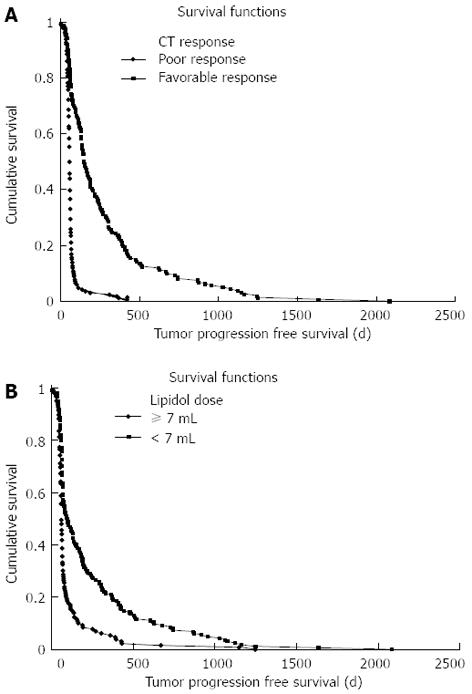
The progression-free survival in the poorly defined tumor (diffuse) type group was significantly shorter than in the well-defined (nodular) tumor type group (P < 0.01). Additionally, large size (size ≥ 5 cm, P < 0.01), no antiviral treatment (P < 0.05), poor CT response (P < 0.01), lipiodol dose (dose ≥ 7 mL, P < 0.01), antibiotic use (P < 0.05) and CRP (CRP ≥ 1 mg/dL, P < 0.01) were statistically significant factors in the univariate analysis (Figure 2A and B). In the multivariate Cox proportional hazard model for progression-free survival, CT response (P < 0.01) and lipiodol dose (≥ 7 mL) (P < 0.05) were identified as independent factors (Table 2).
TACE is one of the major treatment methods for unresectable HCC and has demonstrated survival benefits[4,10-15]. Chemoembolization acts by obstructing the hepatic artery with embolization agents, usually gelatin, and introducing antitumor agents (e.g., cisplatin, doxorubicin, and mitomycin C) emulsified in iodized oil, thereby inducing extensive necrosis in large vascularized HCC tumors[7]. TACE complications can be categorized as hepatic injuries, including deterioration of hepatic function, hepatic infarction, or intrahepatic biloma, and liver abscess; extrahepatic complications, including gastrointestinal bleeding, gallbladder or spleen infarction, and pulmonary embolism; and systemic complications, including postembolization syndrome and septicemia[16]. The most frequent complication of chemoembolization is postembolization fever, which can typically be satisfactorily alleviated with symptom treatment[11,16]. Nevertheless, PEF frequently troubles patients, family members and physicians, and few data have been published concerning postembolization fever; therefore, we investigated the risk factors and clinical significance of PEF that developed after TACE in patients with HCC.
Infectious complications are very rare because of the standard antiseptic procedures associated with TACE. Another study reported that only 0.26% of HCC patients developed liver abscesses after TACE[17]. Thus, antibiotic prophylaxis is usually not necessary in patients with HCC who are undergoing TACE[10]. Although fevers were common (27%) in this study, they were generally not caused by an infectious process because very few patients had bacterial infections (0.16%). These fevers can often be adequately controlled with antipyretics, and in most cases, antibiotics are not necessary.
The pathogenesis of PEF remains unclear and complicated. The main aspects are as follows: (1) lipiodol-induced embolisms may result in ischemia, hypoxia, and necrosis in some normal hepatic cells; (2) chemotherapeutic drugs themselves have toxicities[18]; (3) the procedure itself can lead to a considerable release of inflammatory factors[19]; and (4) such stimuli as injury and drugs can contribute to stress responses in the human body. In the present study, the occurrence of PEF was closely associated with several clinical and laboratory variables, including poor blood glucose control, large tumor size (> 5 cm), poorly defined tumor type, post-TACE CRP level, lipiodol dose higher than 7 mL, and post-TACE ALT level in a univariate analysis. However, the multiple regression analysis showed that a lipiodol dose over 7 mL and the post-TACE ALT level were independent risk factors, which is similar to the result of another recent study[20]. No difference was found between favorable CT responses and unfavorable CT responses regarding the presence of PEF, which is in concordance with another study showing that post-TACE fever was not associated with an enhanced tumor response in patients with HCC[20-22].
PEF is thought to reflect extensive tumor necrosis, thereby representing the efficacy of chemoembolization[22-24]. However, we observed no other robust association between PEF and survival in this study. Moreover, PEF did not independently affect progression-free survival, which may be an indirect indicator of treatment efficacy. These findings suggest that the previously described correlation between PEF and the extent of tissue necrosis cannot always be justified because the extent of tissue necrosis after chemoembolization is proportional to tumor mass, a factor that is independently associated with PEF.
Raoul et al[25] suggested that factors associated with poor TACE outcomes included Child-Pugh score, reduced liver function, AFP level, tumor size, tumor number, tumor type, portal vein thrombosis, multiple TACE sessions and lobar embolization. In our study, large tumor size, poor blood glucose control and portal vein thrombosis were independent risk factors for 20-mo mortality. However, unlike in other studies, the Child-Pugh score was not a significant prognostic factor in our study. It was likely that most of the patients enrolled in our study had Child A (the Child score for patients with PEF was 5.66 vs 5.77 for those without PEF), and as a result, liver function did not affect the prognosis. According to Shim et al[22], hepatitis B virus infection, modified UICC stage (Stage 1), and response to chemoembolization are independent predictive factors for the tumor progression-free interval. In good agreement with other studies, we found that the TACE response and lipiodol dose (< 7 mL) were closely associated with tumor progression-free survival. Because the lipiodol dose was dependent on tumor size, progression-free survival was closely associated with tumor size and response to TACE.
This study had several limitations, including its retrospective design. Although we performed laboratory and culture studies of blood, urine, and ascites to detect hidden infections, it was impossible to rule out all infective complications in patients with PEF. In addition, the tumor response to chemoembolization may have been overestimated in patients with poorly defined HCC, particularly those with the diffuse infiltrative type of HCC, because of the difficulty of evaluating the degree of viable tumor. Finally, it may be difficult to determine whether the development of PEF after a single session of TACE was exclusively associated with overall survival because the mortality of TACE-treated HCC patients is subject to many other factors.
In conclusion, ALT levels after TACE and lipiodol dose were independent risk factors for postembolization fever in HCC patients. However, postembolization fever after TACE had no impact on survival at 20 mo or on the radiologic response.
Postembolization fever (PEF) is thought to reflect extensive tumor necrosis and thereby represent the efficacy of transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC). The aim of this study was to assess the tumor response and survival of patients with PEF after TACE and to determine the risk factors for PEF.
TACE has improved the prognosis of patients with HCC. However, TACE inevitably results in a hypoxic insult to the HCC and the surrounding liver tissue, and postembolization fever is common. However, the clinical meaning of and the risks factor for PEF are not known.
PEF has been associated with overall survival in HCC patients. However, there is limited published data about PEF and the risk factors for PEF. Unlike other studies, their demonstrated that PEF was not associated with overall survival or radiological tumor response. Furthermore, this study demonstrated that alanine aminotransferase (ALT) levels after TACE and lipiodol doses were risk factors for PEF.
The results of this study imply that PEF is associated with lipiodol dose and ALT levels after TACE but does not affect prognosis; therefore, patients undergoing TACE who have risk factors for PEF should receive active or prophylactic antipyretics for PEF control.
Postembolization fever was defined as a body temperature greater than 38.0 °C that developed within 3 d of TACE without evidence of infection. Poorly defined tumor type was defined as diffuse-type HCC, whereas well-defined tumor type was defined as nodular HCC. Tumor progression-free survival was defined as the interval of time during and after treatment in which a patient remained alive and the disease did not worsen (in the case of this study, the interval from the 1st TACE to the 2nd TACE).
Postembolization fever is a clinically relevant problem in the treatment of patients with HCC. The authors addressed potential causes and risk factors and found that the ALT level after TACE and the lipiodol dose served as independent risk factors for PEF after TACE and that PEF had no impact on 20-mo survival in HCC patients. These results were extracted and calculated from a large group of 443 patients who were treated and observed within a 5-year period.
P- Reviewer Gangl A S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1060] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 2. | Park JW. [Hepatocellular carcinoma in Korea: introduction and overview]. Korean J Gastroenterol. 2005;45:217-226. [PubMed] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5302] [Article Influence: 182.8] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2609] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 5. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [PubMed] |
| 6. | Llovet JM, Bruix J, Gores GJ. Surgical resection versus transplantation for early hepatocellular carcinoma: clues for the best strategy. Hepatology. 2000;31:1019-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2269] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 8. | Okada S. Transcatheter arterial embolization for advanced hepatocellular carcinoma: the controversy continues. Hepatology. 1998;27:1743-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Matsuda Y, Kawata S, Nagase T, Maeda Y, Yamasaki E, Kiso S, Ishiguro H, Matsuzawa Y. Interleukin-6 in transcatheter arterial embolization for patients with hepatocellular carcinoma. Effects of serine protease inhibitor. Cancer. 1994;73:53-57. [PubMed] |
| 10. | A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 596] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704. [PubMed] |
| 13. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kanematsu T, Furuta T, Takenaka K, Matsumata T, Yoshida Y, Nishizaki T, Hasuo K, Sugimachi K. A 5-year experience of lipiodolization: selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology. 1989;10:98-102. [PubMed] |
| 15. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1986] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 16. | Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, Kim CY. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33-40. [PubMed] |
| 17. | Ong GY, Changchien CS, Lee CM, Wang JH, Tung HD, Chuah SK, Chiu KW, Chiou SS, Cheng YF, Lu SN. Liver abscess complicating transcatheter arterial embolization: a rare but serious complication. A retrospective study after 3878 procedures. Eur J Gastroenterol Hepatol. 2004;16:737-742. [PubMed] |
| 18. | Nagano M, Nakamura T, Niimi S, Fujino T, Nishimura T, Murayama N, Ishida S, Ozawa S, Saito Y, Sawada J. Substitution of arginine for cysteine 643 of the glucocorticoid receptor reduces its steroid-binding affinity and transcriptional activity. Cancer Lett. 2002;181:109-114. [PubMed] |
| 19. | Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Li CP, Chao Y, Chen LT, Lee RC, Lee WP, Yuan JN, Yen SH, Lee SD. Fever after transcatheter arterial chemoembolization for hepatocellular carcinoma: incidence and risk factor analysis. Scand J Gastroenterol. 2008;43:992-999. [PubMed] |
| 21. | Wigmore SJ, Redhead DN, Thomson BN, Currie EJ, Parks RW, Madhavan KK, Garden OJ. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer. 2003;89:1423-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Shim JH, Park JW, Choi JI, Kim HB, Lee WJ, Kim CM. Does postembolization fever after chemoembolization have prognostic significance for survival in patients with unresectable hepatocellular carcinoma? J Vasc Interv Radiol. 2009;20:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Hemingway AP, Allison DJ. Complications of embolization: analysis of 410 procedures. Radiology. 1988;166:669-672. [PubMed] |
| 24. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [PubMed] |
| 25. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |