INTRODUCTION
The endoplasmic reticulum (ER) plays a central role in the synthesis and modification of secretary and membrane proteins in all eukaryotic cells[1-3]. Under normal conditions, these proteins are correctly folded and assembled in the ER. However, when cells are exposed to disturbed environment, such as overproduction of ER proteins, viral infection, and glucose deprivation, these proteins accumulate as unfolded or misfolded forms in the ER lumen and, consequently, cause ER stress. To maintain cellular homeostasis, cells induce some adaptive responses to ER stress. One of them is the unfolded protein response (UPR), which up-regulates the transcription of various genes to increase the protein-folding and protein-degradation activity in the ER[4-7]. Inositol requiring enzyme-1 (IRE1) is an ER-located type I transmembrane protein with a kinase domain and RNase domain in the cytosolic region and has a unique function of relieving ER stress in cells. When the amino-terminal luminal region senses perturbations in the ER environment, via trans-autophosphorylation and activation of its RNase domain, IRE1 induces unconventional splicing of mRNA coding, a specific transcription factor for activating the UPR[8-10]. IRE1 is highly conserved from yeast to humans, and two IRE1 paralogues have been reported in mammals: IRE1α and IRE1β[11-13]. Upon activation by ER stress, IRE1 performs an unconventional cytoplasmic splicing of XBP1 pre-mRNA, thus allowing the synthesis of active XBP1, which activates UPR target genes to restore the homeostasis of the ER[10,14,15]. The spliced XBP1 mRNA is translated into a functional transcription factor to up-regulate gene expression for ER quality control. IRE1 is also reported to activate proapoptotic JNK signaling under ER stress conditions[16].
IRE1α is also known to be expressed ubiquitously in fetal and adult mice and to be essential for mammalian developmental processes[17]. Therefore, IRE1α inactivation results in widespread developmental defects, leading to embryonic death after 12.5 d of gestation in mice[18]. However, the cause of this embryonic lethality is not fully understood. These lines of evidence suggest that IRE1α has a unique function in mammalian developmental processes, but it has been hitherto unclear in which tissues and how IRE1α functions during embryogenesis.
Xenopus lavies is an excellent model system for studying organ development[19]. However, little information is available about the function of the IRE1/XBP1 pathway during embryogenesis, although loss of function studies revealed that IRE1 or XBP1 is absolutely required for embryonic development of C. elegans[20], Drosophila[21] or mouse[22]. In Xenopus, although the transcripts of two isoforms of IRE1, IRE1α and IRE1β show similar spatial expression in pre-neurula embryos, they are also differentially expressed following the onset of neurulation[23]. IRE1α is localized to the nervous system and mesoderm or endoderm-derived organs, such as pronephros and pancreas. However, the role of IRE1α in organogenesis is still unclear. In the present study, both gain and loss of function analyses revealed that IRE1α is required for gut development in Xenopus.
MATERIALS AND METHODS
Embryos
Wild type Xenopus lavies eggs were obtained by injecting 1000 IU of human chorionic gonadotrophin into the dorsal lymph sacs of adult females 6-8 h before egg collection. Eggs were fertilized in vitro with minced testes, dejellied with 2% cysteine hydrochloride (pH 7.8-8.0) 30 min after fertilization, and cultured in 0.1 × MBSH (8.8 mmol/L NaCl, 0.24 mmol/L NaHCO3, 0.1 mmol/LKCl, 0.082 mmol/L MgSO4, 0.041 mmol/L CaCl2, 0.033 mmol/L Ca(NO3)2, 1 mmol/L HEPES, pH 7.4). Staging of Xenopus lavies embryos was according to Nieuwkoop and Faber (1967).
Plasmids and constructs
IRE1α ORF was amplified from a cDNA pool consisting of st.1, st.8, st.10, st.15, st.20 and st.28 cDNAs and subcloned to pCS2+ vector. The construct was named pCS2+-IRE1α. To make expression constructs, complete coding region and the N-terminal region (aa 1-479) were amplified from the pCS2+-IRE1α using PCR and subcloned to the BamHI-EcoRV sites on pCS2+-GR. The resulting constructs were designated as pCS2+IRE1α-GR and pCS2+IRE1αΔC-GR.
In vitro RNA synthesis, antisense morpholino oligonucleotides and microinjection
Plasmids pCS2+IRE1α, pCS2+IRE1α-GR and pCS2+IRE1αΔC-GR were linearized with NotI. Capped mRNA for microinjection was synthesized with SP6 mMessage mMachineTM kit (Ambion) and cleaned up with RNeasy kit (Qiagen). The antisense morpholino oligonucleotide (Gene Tools) used for IRE1α functional knockdown (IRE1α MO) was: 5’-AAGAGAACCGCCAGAGGC GCCATG T-3’; and an antisense morpholino oligonucleotide XBP1(C) MO designed to inhibit the cytoplasmic splicing of xXBP1 was: 5’-GACATCTGGGCCTGCTC CTGC TGCA-3’. One and half nanogram of IRE1α, 1 ng of IRE1α-GR mRNA, 1 ng of IRE1αΔC-GR mRNA, and 50 ng of IRE1α MO or XBP1(C)MO were injected into four blastomeres at 4-cell stage for scoring the phenotype, whole-mount in situ hybridization and marker gene analysis. For the activation of the GR-fusion proteins, dexamethasone (Sigma) was prepared as 5 mmol/L stock solutions in 100% ethanol and applied to the control and mRNA injected embryos at desired stages in a concentration of 10 μmol/L in 0.1 × MBS. Embryos were kept in dexamethasone up to stage 41. To rescue the effect of blocking xXBP1 splicing by IRE1α MO, 1 ng of IRE1α-GR mRNA was co-injected with 50 ng of MO.
In vitro translation
In vitro protein translation was performed with TNT coupled Reticulocyte Lysate Systems (Promega) to test the efficiency of IRE1α MO for blocking protein translation. One μg of pCS2+IRE1α plasmid was used either alone or together with 20 μg IRE1α MO for in vitro translation. Translation products were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and chemiluminescent detection.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed according to the protocol described elsewhere[24]. The digoxigenin-labeled antisense probes were prepared as follows: pDrive-IRE1α cut with HindIII, transcribed with T7 RNA polymerase; pCS2+XIRE1β cut with XbaI, transcribed with T3 RNA polymerase; pBS-Xbra cut with SalI, transcribed with T7 RNA polymerase; and pCS2+XSox17α cut with ClaI, transcribed with T7 RNA polymerase.
Protein extraction and Western blotting
Embryos were homogenized in RIPA lysis buffer (20 mmol/L Tris, pH 8; 2 mmol/L EDTA, pH 8; 0.5% NP-40; 25 mmol/L β glycerophosphate; 100 mmol/L NaF; 100 mmol/L PMSF and phosphatase inhibitor cocktail). Lysates were centrifuged at 4 °C, 15 000 ×g, for 20 min, and the supernatants were added to 5 × SDS loading buffer. Proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane (Millipore, Billerica, MA). Immunoblotting membranes were blocked with 5% milk in TBST. After several washes in TBST, membranes were incubated overnight with the first antibody [anti-IRE1α antibody (Santa Cruz Biotechnology, Santa Cruz, CA), 1:500]. Loading controls of presumably constantly expressed proteins such as β-actin were used; however, their variability and increase in development precluded their use[25]. Detection was then done with HRP-labeled secondary antibodies and enhanced chemiluminescence (ECL).
RESULTS
Expression of xIRE1α in Xenopus lavies during development
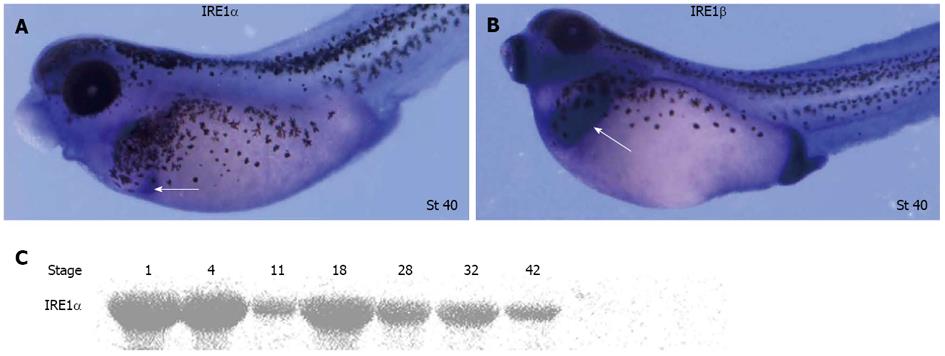
Two forms of IRE1 genes, IRE1α and IRE1β, exist in Xenopus lavies. Whole-mount in situ hybridization revealed that IRE1α and IRE1β were expressed in a similar pattern from egg to gastrulation. In tailbud embryos, IRE1α was detected in a domain that probably represented the dorsal pancreas anlage. IRE1β was only observed in the hatching gland and cement gland until the hatched tad-pole stage[23]. To further explore the spatial expression patterns of IRE1α and IRE1β in Xenopus embryos at later stages, whole-mount in situ hybridizations were carried out. During tadpole stages, relatively high expression of IRE1α was observed in the pancreas (Figure 1A), and significant transcription of IRE1β was observed in the liver (Figure 1B). IRE1α protein could be detected at all developmental stages analyzed (Figure 1C).
Figure 1 Expression pattern of xIRE1 in Xenopus lavies during development.
A, B: Whole-mount in situ hybridizations revealed relatively high expression of inositol-requiring enzyme (IRE) 1α in pancreas (white arrow in A) and relatively high expression of IRE1β in liver (white arrow in B); C: Western blotting revealed temporal expression of IRE1α during Xenopus embryogenesis.
IRE1α gain of function
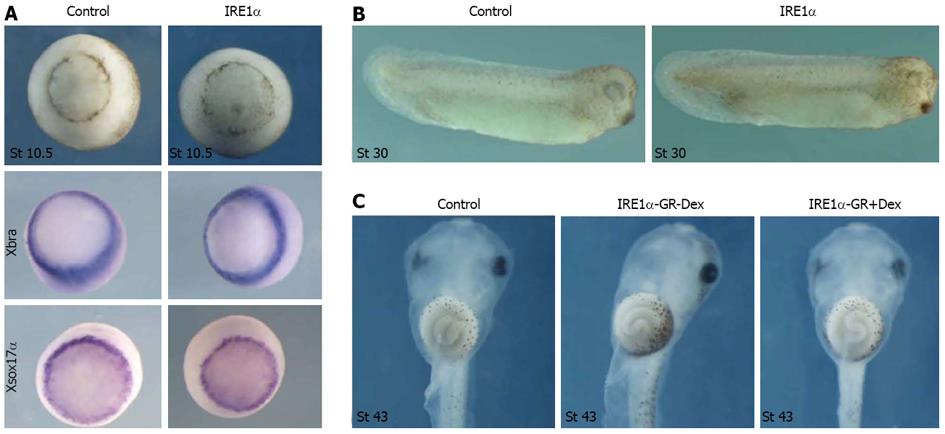
Different doses of IRE1α mRNA were injected into all blastomeres at 4-cell stage. Even at a dose of 1.5 ng, injected embryos at tailbud stage were nearly normal (Figure 2B). Whole-mount in situ hybridization of embryos injected with IRE1α mRNA revealed that the expression of the pan-mesodermal marker gene Xbra and the endodermal gene Xsox17α at stage 10.5 was not significantly changed as compared to uninjected control embryos (Figure 2A). To test the function of IRE1α in the later stage of development, the mRNA encoding a dexamethasone inducible variant of IRE1α, referred to as IRE1α-GR, was injected into all four blastomeres at 4-cell stage Xenopus embryos. For the activation of the GR-fusion proteins, dexamethasone treatment was administered at embryonic stage 27. No apparent change of phenotype was observed even at tadpole stage (Figure 2C). Therefore, gain of function of IRE1α did not lead to an apparent change of phenotype and we did not observe a significant change of mesodermal and endodermal marker gene expression.
Figure 2 Overexpression of inositol-requiring enzyme 1α affected neither mesoderm and endoderm formation nor gut development.
A: Overexpression of inositol-requiring enzyme (IRE) 1α did not change the phenotype and the expression of Xbra and Xsox17α at stage 10.5; B: IRE1α mRNA injected embryos at tailbud stage were nearly normal; C: Embryos injected with IRE1α-GR mRNA did not display any defect until tadpole stage.
IRE1α MO blocking IRE1α translation
To further explore the function of xIRE1α during embryonic development, we performed a loss of function (LOF) analysis using an antisense morpholino oligonucleotide (IRE1α MO) directed against xIRE1α. In vitro protein translation was performed with TNT coupled Reticulocyte Lysate Systems to test the efficiency of IRE1α MO for blocking protein translation. As shown in Figure 3, IRE1α MO totally blocked IRE1α translation.
Figure 3 Inositol-requiring enzyme 1α morpholino oligonucleotide blocks inositol-requiring enzyme 1α translation in an in vitro transcription/translation assay.
In this assay, xIRE1α was effectively transcribed/translated from a pCS2+xIRE1α construct. However, translation was dramatically decreased by addition of IRE1α morpholino oligonucleotide (MO). IRE: Inositol-requiring enzyme.
IRE1α loss of function
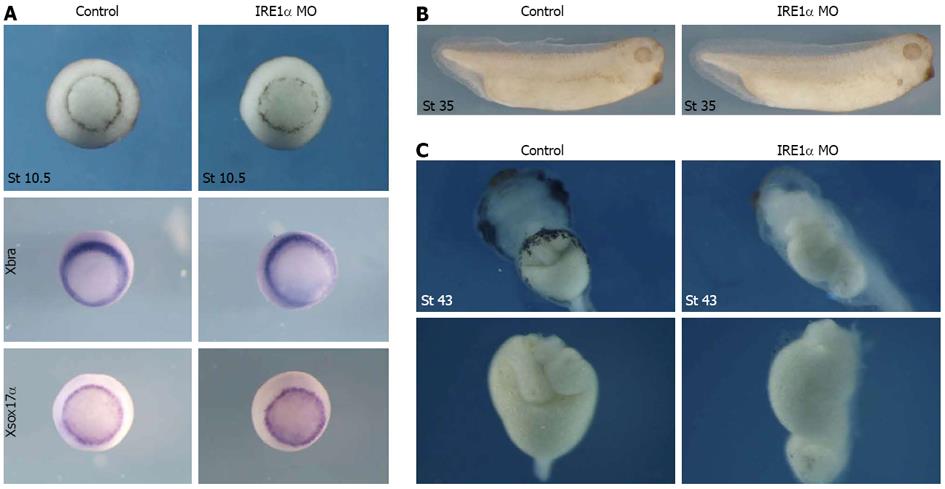
The IRE1α MO-injected embryos were morphologically normal before the tailbud stages (Figure 4B) and we did not observe a significant change of mesodermal and endodermal marker gene expression (Figure 4A), but after stage 40, about 80% of the MO (50 ng)-injected embryos exhibited dramatic gut defects in which the guts did not coil, while other structures outside the gastrointestinal tract developed normally (Figure 4). To test if the phenotypes were specifically caused by the knockdown of IRE1α, a rescue experiment was performed by co-injection of IRE1α-GR mRMA with MO. The results indicated that the phenotype could be rescued (Figure 5). Taken together, these data indicate that IRE1α is required for proper gut development in Xenopus.
Figure 4 Xenopus inositol-requiring enzyme 1α is required for gut development.
A: Knockdown of x IRE1α did not change the phenotype and the expression of Xbra and Xsox17α at stage 10.5; B: The inositol-requiring enzyme (IRE) 1α morpholino oligonucleotide (MO)-injected embryos were morphologically normal before the tailbud stages; C: IRE1α knockdown upon injection of 50 ng of MO resulted in a gut defective phenotype. Surgically-resected guts from embryos were shown in C under panel. Coiled structure of gut was not detected in IRE1α MO-injected embryos at stage 43. MO injection was repeated 5 times in a total of 278 embryos.
Figure 5 Rescue of xIRE1α knockdown with IRE1α-GR mRNA.
Gut defective phenotype caused by 50 ng of morpholino oligonucleotide (MO) could be rescued by co-injection of 1 ng of inositol-requiring enzyme (IRE) 1α-GR mRNA. Co-injection was done 3 times in a total of 258 embryos.
Deletion mutant of IRE1α
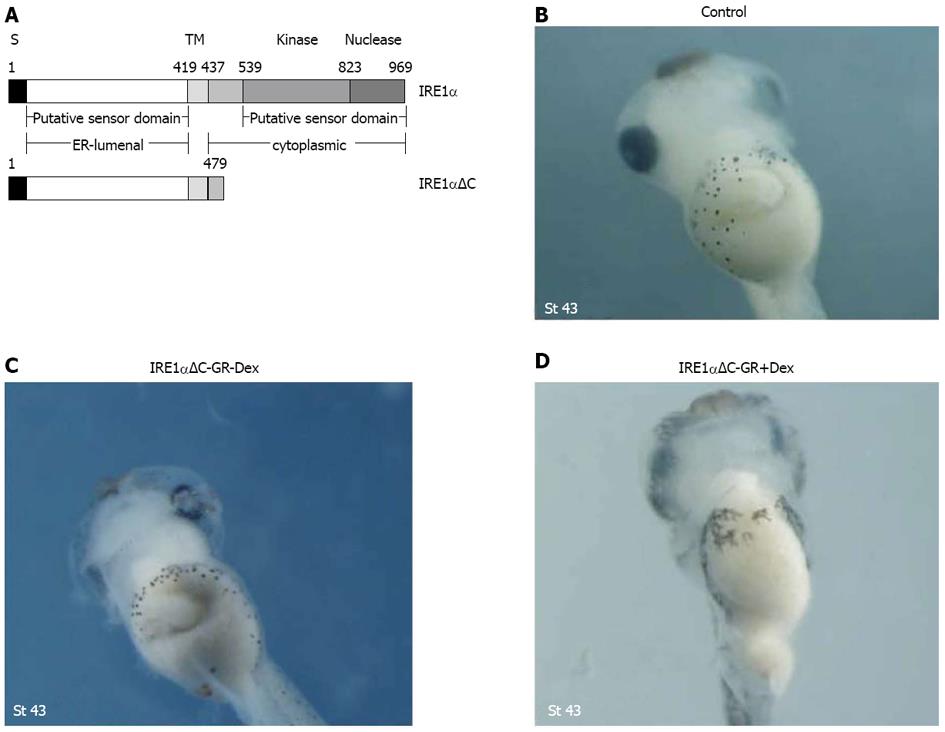
IRE1α is a Ser/Thr protein kinase and endoribonuclease that, upon activation, initiates the unconventional splicing of the mRNA of XBP1[12]. The deletion mutant was constructed consisting of the N-terminal part without the C-terminal kinase and RNase domains (Figure 6A). Injection of IRE1αΔC-GR mRNA caused similar morphological alterations with gut malformation by interfering with the function of endogenous xIRE1α (Figure 6B-D). These data further confirmed the notion that IRE1αΔC represents a dominant-negative form of IRE1α.
Figure 6 Injection of IRE1αΔC-GR mRNA caused morphological alterations with gut malformation.
A: Diagram depicting the construction of deletion mutants. S and TM denote the signal peptide and the transmembrane domain; B-D: Gut defects were also observed with a dominant-negative mutant, IRE1αΔC, lacking the cytoplasmic kinase and RNase domains.
XBP1 loss of function
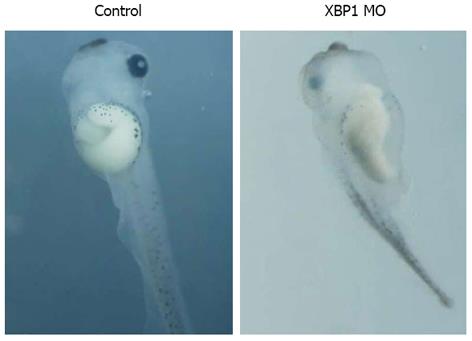
IRE1α is the most evolutionarily conserved branch of the UPR, and upon activation, initiates the unconventional splicing of the mRNA encoding the transcriptional factor XBP1 to attenuate ER stress by mediating UPR. In other words, IRE1α mediates XBP1 splicing. IRE1α MO could knockdown IRE1α expression, and then XBP1 splicing would be repressed. To test if knockdown of XBP1 might cause similar phenotype with IRE1α knockdown, 50 ng of XBP1(C)MO was injected into four blastomeres at 4-cell stage for scoring the phenotype. As shown in Figure 7, injection of XBP1(C)MO caused similar phenotype with gut-coiling defect.
Figure 7 XBP1 knockdown affected gut development.
Injection of XBP1(C) morpholino oligonucleotide (MO) caused similar phenotype with gut-coiling defect at stage 43.
DISCUSSION
In eukaryotic cells, ER is responsible for the early steps in the maturation of most proteins in the secretory pathway, such as folding of the newly synthesized polypeptide chains and post-translational modifications that are essential for protein function[26,27]. Nascent polypeptides are translocated to the ER lumen in an unfolded state, where they are processed for folding. However, the function of ER will be disrupted when the inflow of unfolded polypeptide chains exceeds the folding or processing capacity of the ER[28,29]. This ER stress in turn leads to the activation of a series of adaptive pathways known as UPR to maintain ER homeostasis[30]. The adaptive process, UPR, has at least two distinct components[4]. The first consists of the rapid and transient attenuation of new protein synthesis and can be considered to be an attempt on the part of the cell to limit the load on the folding apparatus in the ER. The second component consists of the upregulation of expression of genes whose products promote protein folding in the ER and degradation of malfolded proteins. The latter UPR signaling pathways are transduced by three ER resident transmembrane proteins IRE1, PERK and ATF6 upon activation[14], among which IRE1 functions as an endoribonuclease (RNase) to process XBP1 pre-mRNA to a mature form. Spliced XBP1 is a transcriptional activator that plays a fundamental role in the activation of a wide variety of UPR target genes[31,32]. Two mammalian homologues of yeast IRE1 have been identified: IRE1α and IRE1β. IRE1α is expressed in most cells and tissues, with highest levels of expression in the pancreas and placenta[17]. IRE1β expression is prominent only in intestinal epithelial cells[13].
Accumulation of malfolded proteins in the ER occurs under many pathophysiological conditions, in addition, it also takes place during embryonic development. During embryogenesis of vertebrates, such as Xenopus, germ layer induction, pattern formation, and morphogenetic movement are known to be mediated by secreted proteins, including fibroblast growth factors (FGFs)[33], TGF-β/nodal/BMPs, and Wnts[34,35]. Therefore, dysfunction of the ER should also interfere with the secretion of these proteins and consequently disrupt early embryonic development[36].
Although the effect of ER stress on cellular physiology has been extensively investigated, little is known so far about how it affects early embryonic development. The previous study has verified the conservation of IRE1/XBP1 pathway in Xenopus embryos and the importance of IRE1β for mesoderm formation in Xenopus embryos[23]. In this study, we demonstrated that (1) IRE1α mainly expressed in pancreas at tadpole stages and IRE1α protein could be detected at all developmental stages analyzed (Figure 1); (2) although it did not cause overt phenotypes upon overexpression (Figure 2), specific knockdown of IRE1α (Figure 4) or XBP1 (Figure 7) led to a gut-coiling defect, and injection of xIRE1αΔC-GR mRNA caused similar morphological alterations with gut malformation (Figure 6); and (3) IRE1α-GR mRNA can rescue IRE1 knockdown phenotypes (Figure 5).
In conclusion, our loss and gain of function data support the notion that IRE1α is required for gut development in Xenopus embryos. Our results demonstrate that homeostasis of ER and xIRE1α functions are required for gut development in Xenopus embryos. We infer from our results that IRE1 regulates gut development through IRE1α-XBP1 pathway.
COMMENTS
Background
Inositol-requiring enzyme 1α (IRE1α) is an endoplasmic reticulum (ER)-located type I transmembrane protein with a kinase domain and RNase domain in the cytosolic region. IRE1α induces the unconventional splicing of XBP1 mRNA under ER stress condition. However, a XBP1-independent IRE1α function also exists. IRE1α is expressed ubiquitously in fetal and adult mice and is essential for mammalian developmental processes. However, the function of IRE1α in specific organs and tissues remains incompletely understood.
Research frontiers
IRE1α is confirmed to be essential during mammalian development. However, the IRE1α conventional knockout mice showed embryonic lethality, and it has been reported that during development IRE1α is required for B-cell differentiation, placental development and embryonic viability. And the function of IRE1α in specific organs and tissues deserves to be illustrated.
Innovations and breakthroughs
Compared with previous studies, this study used the Xenopus lavies as animal model to study the function of IRE1α during their development. After overexpressing and knockdown of the IRE1α, the phenotype could be analyzed. And the results showed that at early stage, IRE1α did not play a significant role in germ layer formation, however, at the stage of organogenesis, knockdown of IRE1α or XBP1 caused gut-coiling defect. The results suggested that IRE1α is not required for germ layer formation, but for gut development in Xenopus lavies and it may function via XBP1-dependent pathway.
Applications
The results in this study showed that IRE1α does play a role in organogenesis, however, two questions remain to be answered: which organs or tissues in gut are affected and what is the underlying mechanism.
Peer review
This is an interesting article. The authors show in a systematic manner that the role of IRE1a in the gut development of Xenopus. They also show that it acts through the XBP1 processing.