Published online Jan 14, 2013. doi: 10.3748/wjg.v19.i2.209
Revised: September 26, 2012
Accepted: November 14, 2012
Published online: January 14, 2013
Processing time: 145 Days and 5.2 Hours
AIM: To investigate the effect of local intestinal perfusion with hypertonic saline (HTS) on intestinal ischemia-reperfusion injury (IRI) in both ex vivo and in vivo rat models.
METHODS: All experiments were performed on male Wistar rats anesthetized with pentobarbital sodium given intraperitoneally at a dose of 60 mg/kg. Ex vivo vascularly perfused rat intestine was subjected to 60-min ischemia and either 30-min reperfusion with isotonic buffer (controls), or 5 min with HTS of 365 or 415 mOsm/L osmolarity (HTS365mOsm or HTS415mOsm, respectively) followed by 25-min reperfusion with isotonic buffer. The vascular intestinal perfusate flow (IPF) rate was determined by collection of the effluent from the portal vein in a calibrated tube. Spontaneous intestinal contraction rate was monitored throughout. Irreversible intestinal injury or area of necrosis (AN) was evaluated histochemically using 2.3.5-triphenyltetrazolium chloride staining. In vivo, 30-min ischemia was followed by either 30-min blood perfusion or 5-min reperfusion with HTS365mOsm through the superior mesenteric artery (SMA) followed by 25-min blood perfusion. Arterial blood pressure (BP) was measured in the common carotid artery using a miniature pressure transducer. Histological injury was evaluated in both preparations using the Chui score.
RESULTS: Ex vivo, intestinal IRI resulted in a reduction in the IPF rate during reperfusion (P < 0.05 vs sham). The postischemic recovery of the IPF rate did not differ between the controls and the HTS365mOsm group. In the HTS415mOsm group, postischemic IPF rates were lower than in the controls and the HTS365mOsm group (P < 0.05). The intestinal contraction rate was similar at baseline in all groups. An increase in this parameter was observed during the first 10 min of reperfusion in the control group as compared to the sham-treated group, but no such increase was seen in the HTS365mOsm group. In controls, AN averaged 14.8% ± 5.07% of the total tissue volume. Administration of HTS365mOsm for 5 min after 60-min ischemia resulted in decrease in AN (5.1% ± 1.20% vs controls, P < 0.01). However, perfusion of the intestine with the HTS of greater osmolarity (HTS415mOsm) failed to protect the intestine from irreversible injury. The Chiu score was lower in the HTS365mOsm group in comparison with controls (2.4 ± 0.54 vs 3.2 ± 0.44, P = 0.042), while intestinal perfusion with HTS415mOsm failed to improve the Chiu score. Intestinal reperfusion with HTS365mOsm in the in vivo series secured rapid recovery of BP after its transient fall, whereas in the controls no recovery was seen. The Chiu score was lower in the HTS365mOsm group vs controls (3.1 ± 0.26 and 3.8 ± 0.22, P = 0.0079 respectively,), although the magnitude of the effect was lower than in the ex vivo series.
CONCLUSION: Brief intestinal postischemic perfusion with HTS365mOsm through the SMA followed by blood flow restoration is a protective procedure that could be used for the prevention of intestinal IRI.
- Citation: Kornyushin O, Galagudza M, Kotslova A, Nutfullina G, Shved N, Nevorotin A, Sedov V, Vlasov T. Intestinal injury can be reduced by intra-arterial postischemic perfusion with hypertonic saline. World J Gastroenterol 2013; 19(2): 209-218
- URL: https://www.wjgnet.com/1007-9327/full/v19/i2/209.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i2.209
Acute mesenteric ischemia (AMI) is a life-threatening vascular emergency with a mortality rate as high as 60%-80%[1]. It can be caused by local arterial obstruction, impairment of venous outflow, or a systemic hemodynamic disorder resulting in severe intestinal hypoxic injury. The rather non-specific clinical presentation of AMI can be an obstacle to its early recognition, thus delaying the proper treatment, namely revascularization[2]. However, if AMI can be diagnosed unambiguously prior to the development of intestinal infarction, either embolectomy or the intra-arterial infusion of thrombolytic agents can materially improve the outcome[3]. Unfortunately, even after timely revascularization, the positive effect can be compromised, at least in part, by the development of reperfusion injury, including edema, the no-reflow phenomenon, and the progressive death of mucosal cells[4]. In experimental models, intestinal ischemia-reperfusion injury (IRI) has been shown to be alleviated by a variety of pre- and postconditioning procedures[5,6], while various antioxidants, anesthetics, vasodilators, anti-inflammatory agents, and cytokine receptor blockers can also be helpful[7,8]. Furthermore, the systemic administration of hypertonic saline (HTS) has given clearly positive results[9,10]. The protective effect of HTS in these experiments is believed to be based on the following mechanisms: arteriolar vasodilation, osmotic stress-induced inhibition of inflammatory cells, and prevention of intestinal edema[10-12]. It is to be noted that the application of either HTS or some of the above-mentioned drugs may have certain limitations, such as an increased risk of electrolyte imbalance[12] and the development of adverse drug reactions, in either case as a result of systemic administration. It has been hypothesized, therefore, that perfusion of the intestine with HTS through the superior mesenteric artery (SMA) might prove to be a more advantageous strategy, given its greater efficacy in delivering the hypertonic solution to the target.
The present study was an experimental investigation of the effect of local intra-arterial HTS administration during the early reperfusion phase on the functional and structural manifestations of intestinal injury. To the best of our knowledge, this approach has not yet been attempted.
2.3.5-triphenyltetrazolium chloride (TTC) was obtained from Sigma-Aldrich (St. Louis, MO, United States). Pentobarbital sodium was purchased from Apoteket (Umea, Sweden). Bovine serum albumin was obtained from Biowest (Nuaille, France). Dextran 60 000 was obtained from Biochemist (Saransk, Russian Federation). Other chemicals used for the preparation of both Krebs-Henseleit buffer and HTS were purchased from Acros Organics (Geel, Belgium).
All experiments were performed on male Wistar rats weighting 250-300 g. The animals were fed regular chow, and water was available ad libitum. The procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the local Ethics Committee. The animals were anesthetized with pentobarbital sodium given intraperitoneally at a dose of 60 mg/kg. Core body temperature was maintained at 37.0 ± 0.5 °C by a feedback-controlled heating pad (TCAT-2LV controller, PHYSITEMP Instruments Inc., United States).
Isolated perfused small intestine preparation: The intestine was isolated and perfused according to a previously described technique[13]. Briefly, the abdominal cavity was opened by a midline incision. To prevent blood coagulation, 50 units of heparin sodium were injected into the inferior vena cava. The abdominal aorta was exposed from the level of the celiac trunk (CT) to the aortic bifurcation. The intestinal segment between the pyloric sphincter and the distal portion of the ileum was cut using an electrocautery unit. The branches of the SMA that supply the cecum and proximal part of the colon were exposed, ligated, and cut, followed by the removal of the proximal colon. The intestinal lumen was rinsed with warm (37 °C) saline. The aorta was cannulated through its infrarenal segment with subsequent advancement of the cannula tip to the SMA level. The aorta was then ligated immediately above the CT, and perfusion of the small intestine with oxygenated (95% O2 and 5% CO2) Krebs-Henseleit buffer (KHB) was initiated through the SMA and CT at a pressure of 80 mmHg. KHB solution was modified to contain high molecular weight compounds (1.0% bovine serum albumin and 3.0% dextran 60 000) plus (all in mmol/L) 118.5 NaCl, 25.0 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.5 CaCl2, 5.0 glucose, 2.0 lactate, 0.2 pyruvate. The portal vein was cannulated to ensure outflow of the perfusion fluid. After ligation of the splenic and gastric vessels, the exposed part of the intestine was placed into the water-jacketed bath filled with normal saline to maintain the temperature at 37 °C during the experiment. All surgical procedures described were performed within 50-60 min and were followed by a 20-min stabilization period.
Experimental protocol: After stabilization, ischemia of the intestine was induced by cessation of the perfusate flow for 60 min, followed by reperfusion. During the initial 5-min reperfusion, the perfusion was resumed with the modified KHB of either normal (physiological) or increased osmolarity (315 vs 365 or 415 mOsm/L, designated as HTS365mOsm or HTS415mOsm, respectively), in each case delivered under a pressure of 80 mmHg, followed by perfusion with normotonic KHB for 25 min. The experiments were randomized and allocated to the following 4 groups: (1) Sham (n = 6): the intestine was continuously perfused for 110 min; (2) Controls (n = 6): 60-min intestinal ischemia, followed by 30-min reperfusion with normotonic KHB (osmolarity 315 mOsm/L); (3) HTS365mOsm (n = 5): 60-min intestinal ischemia, followed by 5-min reperfusion with HTS having an increased osmolarity of 365 mOsm/L (KHB supplemented with 50 mmol NaCl), followed by 25-min reperfusion with normotonic KHB; and (4) HTS415mOsm (n = 6): the same sequence of procedures as in the previous group, but using HTS with osmolarity increased to 415 mOsm/L.
Assessment of vascular intestinal perfusate flow rate: The vascular intestinal perfusate flow (IPF) rate was determined each 5 min during both the stabilization and reperfusion periods by collection of the effluent from the portal vein in a calibrated tube.
Determination of luminal intestinal flow: The outflow from the intestinal lumen was determined in all experiments during the stabilization period. Luminal flow exceeding 0.5 mL/min is indicative of pathological intestinal secretion, which is usually due to ischemic injury of the intestine during surgical manipulations. Thus, the experiments with luminal flow exceeding 0.5 mL/min were excluded from the final analysis.
Histochemical analysis: We used the technique of visualizing the area of necrosis (AN) with 2.3.5-triphenyltetrazolium chloride (TTC), a method generally considered to be a “gold standard” for AN delineation in the heart in other studies, including our own[14,15]. Briefly, immediately after the end of reperfusion into the SMA, 10 mL of 0.5% TTC solution were administered for 30 s. Fifteen minutes after that, 3 equally spaced segments of the intestine with a total length of 3 cm were excised and fixed in 4% buffered formaldehyde solution for 24 h at room temperature. The samples were cut into 7-8 transverse slices, each 1.5 mm thick, which were photographed for further analysis using Adobe Photoshop CS. TTC-positive tissue, brick red in color, was considered to be viable, in contrast to the necrotic tissue, which had a pale appearance. The AN volume in each sample was calculated by multiplying the TTC-negative area by the slice thickness. The AN volume of the whole intestinal segment was calculated by summing the AN volumes of the slices and was expressed as the percentage of AN in relation to the total volume of the intestinal tissue being analyzed.
Evaluation of spontaneous intestinal motility: According to the original technique described by Hoffman et al[16], this parameter is assessed by recording the movement of fecal pellets down the intestine. We used a different approach to register the spontaneous intestinal contraction rate. In brief, a pair of soft rubber rings, hand-crafted from a black tube with a diameter close to that of the intestine, were cut at one point and carefully placed around the intestine to signify equally spaced anatomical locations throughout the experiments. Sixty-second video recordings (5 frames per second) were taken every 10 min during the stabilization and reperfusion periods and were digitized with VirtualDub software (Avery Lee, United States). Based on the distances between the rings, the values were plotted against time, followed by a Fourier transform and probability plotting of the frequency distribution. The data were presented as the rates of low-frequency intestinal contractions (Hz).
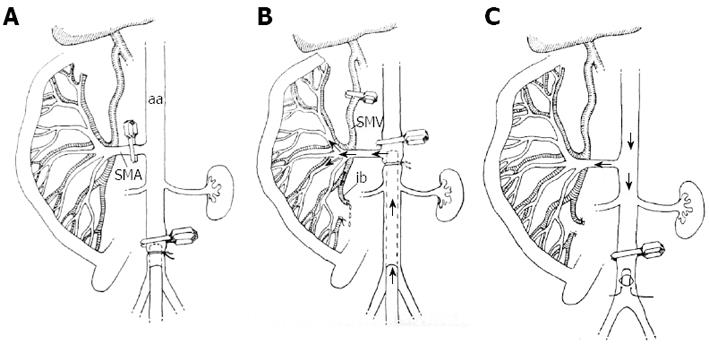
For the investigation of the effect of HTS perfusion on the intestinal IRI, we developed a technique for perfusion through the SMA, which, in our opinion, offers some advantages over the systemic administration of HTS used before. The infrarenal aorta was occluded with a microclip and cannulated with an 18G polyethylene cannula (Figure 1A). The micro clip was then removed and placed on the aorta between the SMA and the CT, after which the cannula was advanced up to the SMA level, where its tip was fixed by ligation (Figure 1B). The next step included 2 manipulations: the occlusion of the distal part of the superior mesenteric vein (SMV) and the incision of the ileocecal SMV branch to secure the outflow of the perfusate from the intestinal circulation. The oxygenated HTS maintained at 37 °C was delivered through the SMA with an infusion pump (SPACE infusion system, B. Brown, Germany) at a constant rate of 15 mL/min. The flow rate was selected on the basis of our pilot experiments showing that the mean blood flow in the SMA of animals of comparable weight, having blood pressure (BP) in the range between 100 and 120 mmHg, was approximately 10% higher than 15 mL/min. Any appearance of blood in the perfusate was checked by visual inspection. At the end of the perfusion, the ileocecal SMV branch was ligated and the main trunk of the SMV was reopened. The aortic cannula was removed and the aortic incision closed with a suture (Figure 1C), so that blood flow was restored in both the aorta and the mesenteric vascular bed.
Experimental protocol: The animals were randomly allocated into 2 groups: (1) Controls (n = 6): intestinal ischemia by SMA occlusion for 30 min, followed by 30-min reperfusion with blood; and (2) HTS365mOsm (n = 5): intestinal ischemia by SMA occlusion for 30 min, followed by 5-min reperfusion with HTS365mOsm, followed by 25-min reperfusion with blood.
Blood pressure measurement: Arterial BP was measured in the common carotid artery using a miniature pressure transducer (Baxter, United States), to be monitored using PhysExp X4 software (Cardioprotect Ltd., St. Petersburg, Russian Federation). BP values were registered under the following conditions: at baseline, prior to SMA occlusion, 15 and 30 min after SMA occlusion, and at the 15th and 30th min of reperfusion.
Histological studies: At the end of reperfusion, intestinal samples were taken and routinely fixed in 10% buffered formaldehyde solution, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin. The slides were analyzed under a light microscope by the pathologist blinded to the treatment mode used in each group. The results were scored from 0 to 5 according to the scale of Chiu et al[17]. The method is based on the well established relationship between the grade of intestinal injury, if any, and its structural manifestation (grade 0: normal villi; grade 1: development of subepithelial space; grade 2: moderate lifting of subepithelial layer; grade 3: massive epithelial lifting with a few tips denuded; grade 4: denuded villi with lamina propria and dilated capillaries exposed; grade 5: digestion and disintegration of lamina propria with ulceration). In the present study, we rigorously followed this gradation as applied in similar trials by several groups[10,18,19].
Data were analyzed with SPSS 11.0 (SPSS Inc. Software, Chicago, IL, United States). All values are expressed as mean ± SD. Differences in the intestinal motility and IPF rate were tested by repeated-measures analysis of variance, followed by a Tukey post-hoc test. The non-parametric Mann-Whitney U test was used to determine the inter-group differences in AN value and histopathological score. P≤ 0.05 were considered significant.
Exclusions: Two animals were excluded from the final analysis (one in the control group and one in the HTS415mOsm group) because the luminal flow exceeded 0.5 mL/min.
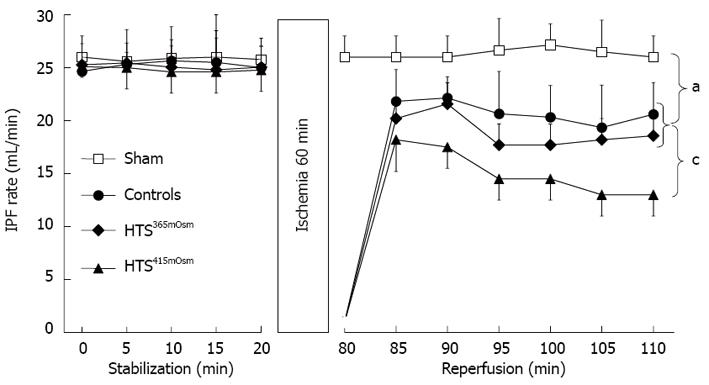
Intestinal perfusate flow rate: There were no inter-group differences in the IPF rate during the stabilization period (Figure 2). The IPF values remained unchanged over the entire 110-min perfusion period in the sham-treated group. In contrast, intestinal ischemia-reperfusion resulted in a reduction in the IPF rate during reperfusion (P < 0.05 vs sham). This reduction could have been due to IRI in the intestinal microcirculatory bed, resulting in no-reflow. The postischemic recovery of the IPF rate did not differ between the controls and the HTS365mOsm group. In the HTS415mOsm group, postischemic IPF rates were lower than in the controls and the HTS365mOsm group (P < 0.05).
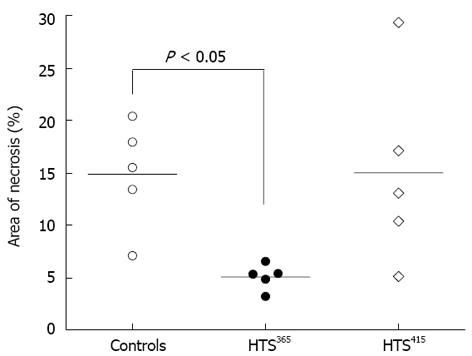
Area of necrosis: TTC-negative intestinal tissue was not present in the samples obtained from the sham-treated group. In the controls, AN averaged 14.8% ± 5.07% of the total tissue volume within the analyzed intestinal segment (Figure 3). Administration of HTS365mOsm for 5 min after 60-min ischemia resulted in decrease in AN (5.1% ± 1.20% vs controls, P < 0.01). However, perfusion of the intestine with the HTS of greater osmolarity (HTS415mOsm) failed to protect the intestine from irreversible injury (AN = 15.0% ± 9.12% vs controls, P > 0.05).
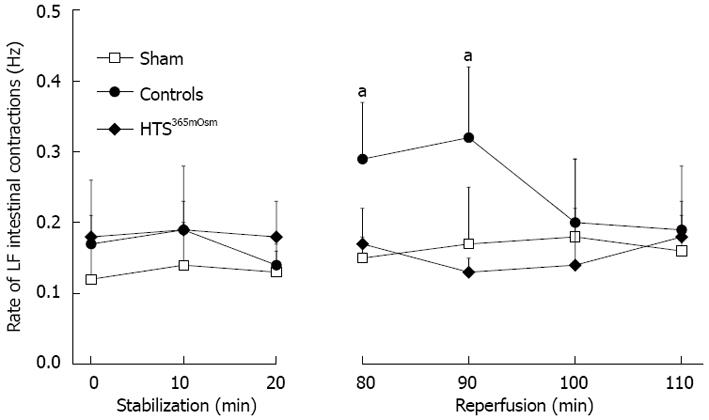
Intestinal motility: The rate of intestinal contraction-relaxation pulses, which was used as a measure of intestinal motility in the present study, was similar at baseline in all groups (Figure 4). An increase in this parameter was observed during the first 10 min of reperfusion in the control group as compared to the sham-treated group, but no such increase was seen in the HTS365mOsm group.
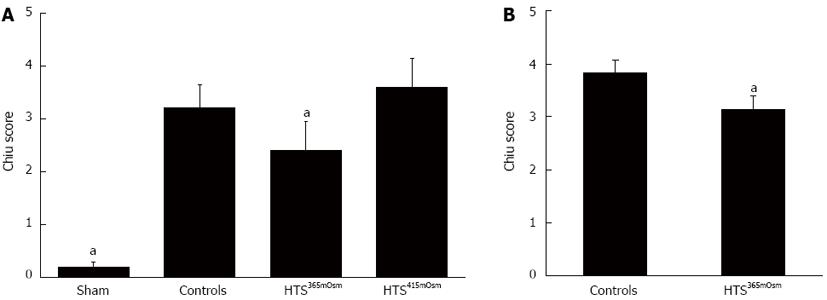
Histological data: Histological data from the intestinal samples obtained after 30-min reperfusion ex vivo are shown in Figure 5A. Sham-treated animals had normal or near-normal histology. The Chiu score was lower in the HTS365mOsm group in comparison with controls (2.4 ± 0.54 vs 3.2 ± 0.44, P = 0.042, Figure 5A), while intestinal perfusion with HTS415mOsm failed to improve the Chiu score (3.6 ± 0.54 vs controls, P > 0.05).
Exclusions: Although the experimental model suggested is highly invasive and complex, the extensive training prior to the beginning of the main experimental series has minimized the number of animals excluded due to technical reasons. Only one rat was excluded in the control group because of the severe bleeding and low BP.
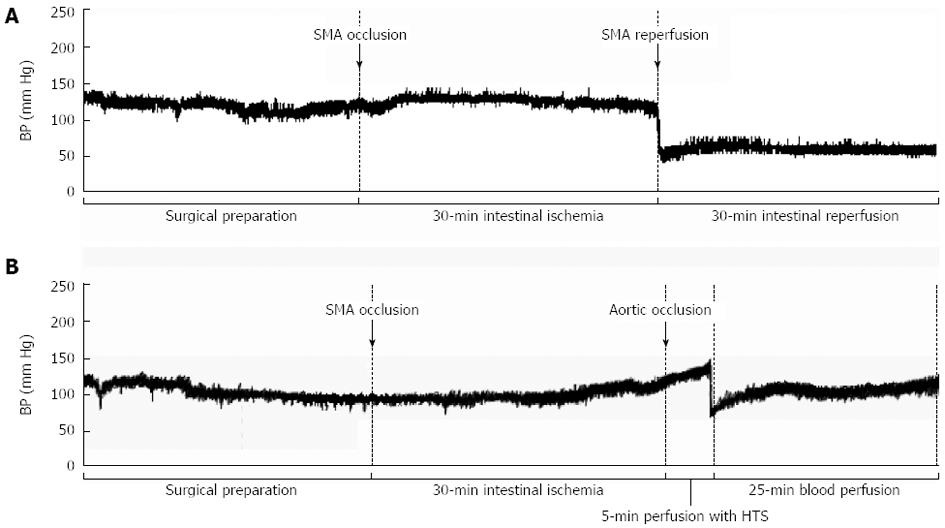
Blood pressure values during intestinal perfusion in vivo: Baseline BP values did not differ between the controls and the HTS365mOsm group (Table 1, Figure 6). SMA occlusion did not cause appreciable changes in BP level in either group. Aortic occlusion during the 5-min SMA perfusion period in the HTS365mOsm group resulted in a trend to BP elevation. Reinstitution of blood flow in the aorta caused a temporary decrease in BP that lasted for only 2-3 min before it returned to the baseline values (Table 1). In contrast, SMA reperfusion in controls caused a decrease in BP level that persisted during the entire reperfusion period (Table 1, Figure 6A).
Histology: Histological data from the intestinal samples obtained after 30-min reperfusion in vivo are shown in Figure 5B. The Chiu score was lower in the HTS365mOsm group vs controls (3.1 ± 0.26 and 3.8 ± 0.22, P = 0.0079, respectively, Figure 5B), although the magnitude of the effect was lower than in the ex vivo series.
The results of this study indicate that the technique of isolated in vivo vascular perfusion of the intestine is reproducible and reliable. Using this approach, intestinal perfusion with HTS365mOsm over the initial 5 min of reperfusion resulted in significant protection of the intestine from IRI, as demonstrated using morphological data (per both TTC and Chiu et al[17]). In order to evaluate both the experimental and also the possible clinical implications of this strategy, it seems appropriate to review in detail the relevant findings from systemic HTS administration. A salvation effect on the intestinal IRI, as well as limitation of remote organ injury, were observed in rats that were subjected to 60-min SMA occlusion followed by 6-h reperfusion, when intravenous administration of HTS was performed during the last 5 min of ischemia[10]. In parallel, an improvement in intestinal transit time, a reduction in mucosal injury, and limitation of intestinal myeloperoxidase activity were demonstrated using the same model; all effects were considered to be dependent on heme oxygenase-1 activity[18]. In addition, HTS infusion performed within the first 5 min of reperfusion after 120 min of SMA occlusion in pigs resulted in a better recovery of both mean arterial blood pressure and intestinal microvascular perfusion in comparison with conventional isotonic resuscitation[9]. In a somewhat different rat model of intestinal IRI, this time induced by hemorrhagic shock followed by blood reinfusion, the administration of HTS for 25 min after the return of shed blood into circulation resulted in better post-resuscitation blood flow and improved endothelial function of the intestinal vasculature[12]. It also suppressed the apoptotic death of intestinal epithelial cells[20] and prevented intestinal mucosal injury[21]. As to the remote effects of systemic HTS treatment, a more complete and stable restoration of BP[12] as well as the prevention of systemic oxidative stress[21] have been observed. However, in general, systemic HTS administration has several intrinsic drawbacks, including an increased risk of hypernatremia, plasma hyperosmolarity, cardiac arrhythmias, and heart failure[12]. Apart from being free of the limitations inherent to systemic HTS administration, the technique of local HTS delivery seems to have 2 more additional advantages over a variety of protocols attempted previously[22,23]. First, the delivery of perfusate directly into the SMA, with outflow from the opened vein, completely prevents the otherwise inevitable generalized spread throughout the systemic circulation of the metabolites and bacterial toxins accumulated during ischemia. Second, the pharmacological agents recommended for strengthening the protective effect of HTS[24,25]-which could thus be potentially helpful to HTS administration via a local route-could be used at higher doses and therefore with higher efficiency, analogously with the strategy applied for regional perfusion in chemotherapy for certain neoplasms[26].
The question might arise as to whether the HTS osmolarity value used in the present study was optimal. While the osmolarity of HTS for hypertonic resuscitation averages approximately 7.5% NaCl, in our local delivery model we tested solutions with much lower osmolarities. Previous studies have tried values ranging from normal (300-310 mOsm/L) to nearly 400 mOsm/L for local organ anti-ischemic protection, particularly the heart; the optimal osmolarity was found to be 334 mOsm/L[27]. Interestingly, in ex vivo experiments, osmolarity values above 400 mOsm/L apparently produced a negative effect; that is, HTS delivery resulted in a significant deterioration in postischemic IPF rate recovery as compared to controls (Figure 2). Although different mechanisms might be involved in the observed effect, the most likely are endothelial injury and impaired microvascular function; however, this matter requires further investigation. Based on these findings, in this study a solution with a lower osmolarity (HTS365mOsm) was preferred and was found to be effective for the preservation of intestinal viability, not only ex vivo, but also in the in vivo model. A higher HTS osmolarity value than that optimal for the heart could be justified by the higher hydrophilicity of the intestinal tissue, which would thus also require more potent anti-edema protection. It should be noted, however, that further experimentation will be needed in order to determine more precisely the optimal HTS parameter(s) for the anti-IRI protection of intestinal tissue.
Although not fully understood, the mechanism of the intestine protection with HTS may include such factors as dilatation of precapillary arterioles[12], inhibition of neutrophil-mediated reperfusion injury[11], or prevention of intestinal edema due to reperfusion-induced water influx into the cells[28]. At the molecular level, the major factor underlying the removal of excess water from the cells has been shown to be the up-regulation of aquaporin 4 within the plasma membrane of the mucosal cells[29].
As mentioned before, protective effects of local HTS delivery on reperfusion injury have also been found in the heart, suggesting that common mechanisms may be involved. The concept of controlled cardiac reperfusion was originally proposed in the late 1980s to protect the myocardium from IRI by modifying the composition and/or delivery rate of the perfusion fluid[30]. One of the major modifications was the increased osmolarity of the perfusate, which was shown to decrease the incidence of reperfusion-induced arrhythmias, to attenuate tissue injury, and to reduce edema in the isolated hearts of pig and rabbit[27,31]. This promising approach is still awaiting implementation in clinical practice, initially in open heart surgery.
Local infusion of saline before reperfusion was also found to be protective in the brain. It was found that flushing the ischemic area of the brain with isotonic saline prior to reperfusion resulted in a reduced infarct volume and improved cerebral blood flow in the rat model[32]. Since normotonic saline was used in this study, the authors attributed its protective effect to the local clearance of proinflammatory cytokines and metabolic end-products.
In the present study, the spontaneous intestinal motility was assessed with a technique that has not yet been employed in the experiments simulating IRI. The observation of significant augmentation of the intestinal contraction rate over the first 10 min of intra-arterial perfusate reflow after 60-min in vitro ischemia (Figure 4) is in general agreement with data showing a transient increase in both the contraction rate and baseline tension observed upon reoxygenation of the jejunal rings after they were subjected to 60-min in vitro hypoxia[33]. In both situations, the increased motor activity of the intestine within the early postischemic period might be explained, in gross analogy with the postischemic heart response[34], by sudden oxygen-evoked re-energizing of the cells immediately after severe hypoxia. The subsequent excessive activation of contractile machinery might, in turn, contribute to the progression of mucosal injury, most likely due to the adverse effect of hypermotility. Its complete prevention with HTS perfusion (Figure 4) may point to the solution of the relevant clinical problem.
One obvious limitation of our in vivo model is the need to advance the perfusion cannula through the infrarenal aorta, rather than directly into the SMA. As a result, the kidneys and hindlimbs were exposed to an additional 5 min of ischemia. However, this limitation is imposed by the small size of the animal and the need to restore blood flow in the mesenteric vascular bed. In order to make the results comparable, control animals also received 5-min occlusion of the infrarenal aorta. Future studies are needed to verify the protective effect of local HTS infusion in large animal models. For instance, the study might be repeated in pigs using an endovascular approach with percutaneous puncture in the femoral artery and balloon occlusion of the SMA.
As to further extrapolations useful for clinical practice, the findings from the present study imply that intra-arterial HTS administration could contribute to the prevention, or at least amelioration, of 2 seemingly inevitable negative effects that would otherwise arise; that is, if “unprotected” reperfusion is applied. The first of these is reperfusion-induced intestinal damage, while the second includes whole-body disturbances, such as the systemic inflammatory response syndrome[4], hemodynamic impairments[35], and remote organ injuries[36], all of which are associated with the massive washout of metabolites and bacterial toxins from the ischemia-affected intestine into the systemic circulation. Our in vivo model could lead to the development of a novel therapy that combines local perfusion of the ischemic region with mechanical restoration of the blood flow and/or the administration of pharmacological agents. As to the clinical translation of the model, it might be speculated that perfusion of the SMA in patients with AMI could be performed after laparotomy, either via a catheter directly placed in the SMA or during percutaneous intervention. The perfusate outflow could be ensured through the punctured SMV. It should be noted that the suggested technique delayed restoration of blood flow to the intestine by 5 min; however, it is unlikely that perfusion with an oxygen- and glucose-enriched solution could cause any ischemic/hypoxic injury to the intestine during this period. It is also of importance to determine the optimal osmolarity value of the HTS, since the results of the present study have demonstrated the negative effect of HTS with excessive osmolarity, i.e., 415 mOsm/L.
In conclusion, the results of our experiments show that isolated intestinal perfusion with HTS at the beginning of the reperfusion period can appreciably protect the intestine from IRI. Despite the positive effect of intra-arterial HTS delivery, aimed in the present study at establishing the validity of this approach in principle, its further improvement by optimization of the particular parameters during all steps of the procedure will be required before clinical trials can be contemplated.
Acute mesenteric ischemia (AMI) is a life-threatening vascular emergency with high mortality rate. The major treatment approach for AMI is rapid intestinal revascularization. Unfortunately, even after timely revascularization, its positive effect can be compromised, at least in part, by the development of intestinal reperfusion injury.
Systemic administration of hypertonic saline (HTS) was shown to be protective in the experimental models of AMI. In the present study, the authors investigated the effect of local intra-arterial HTS administration during the early reperfusion phase on the functional and structural manifestations of intestinal injury.
The authors showed for the first time that isolated intestinal perfusion with HTS at the beginning of the reperfusion period can appreciably protect the intestine from ischemia-reperfusion injury. In addition, the technique of isolated in vivo vascular perfusion of the intestine is suggested and experimentally validated.
This in vivo model could lead to the development of a novel therapy that combines local perfusion of the ischemic intestine with mechanical restoration of the blood flow and/or the administration of pharmacological agents.
Intestinal ischemia-reperfusion injury (IRI) develops because of the temporal complete or partial cessation of blood supply to the intestine. HTS is crystalloid or colloid solution with osmolarity value exceeding physiological. Potential benefits of HTS in the settings of AMI include prevention of edema, improvement in the microcirculation, and washout of potentially toxic metabolites.
This paper investigated the effect of local intestinal perfusion with HTS on intestinal IRI in both ex vivo and in vivo rat models, to find that brief intestinal postischemic perfusion with HTS365mOsm through the SMA followed by blood flow restoration was a protective procedure that could be used for the prevention of intestinal IRI. It is well written and organized.
P- Reviewer Augustin G S- Editor Xiong L L- Editor A E- Editor Zhang DN
| 1. | Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Berland T, Oldenburg WA. Acute mesenteric ischemia. Curr Gastroenterol Rep. 2008;10:341-346. [PubMed] |
| 3. | Arthurs ZM, Titus J, Bannazadeh M, Eagleton MJ, Srivastava S, Sarac TP, Clair DG. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698-704; discussion 704-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Takeshita M, Tani T, Harada S, Hayashi H, Itoh H, Tajima H, Ohnishi I, Takamura H, Fushida S, Kayahara M. Role of transcription factors in small intestinal ischemia-reperfusion injury and tolerance induced by ischemic preconditioning. Transplant Proc. 2010;42:3406-3413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Liu KX, Li YS, Huang WQ, Chen SQ, Wang ZX, Liu JX, Xia Z. Immediate postconditioning during reperfusion attenuates intestinal injury. Intensive Care Med. 2009;35:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [PubMed] [DOI] [Full Text] |
| 8. | Cámara CR, Guzmán FJ, Barrera EA, Cabello AJ, Garcia A, Fernández NE, Caballero E, Ancer J. Ketamine anesthesia reduces intestinal ischemia/reperfusion injury in rats. World J Gastroenterol. 2008;14:5192-5196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Jonas J, Heimann A, Strecker U, Kempski O. Hypertonic/hyperoncotic resuscitation after intestinal superior mesenteric artery occlusion: early effects on circulation and intestinal reperfusion. Shock. 2000;14:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Gonzalez EA, Kozar RA, Suliburk JW, Weisbrodt NW, Mercer DW, Moore FA. Conventional dose hypertonic saline provides optimal gut protection and limits remote organ injury after gut ischemia reperfusion. J Trauma. 2006;61:66-73; discussion 73-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Shukla A, Hashiguchi N, Chen Y, Coimbra R, Hoyt DB, Junger WG. Osmotic regulation of cell function and possible clinical applications. Shock. 2004;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Zakaria el R, Tsakadze NL, Garrison RN. Hypertonic saline resuscitation improves intestinal microcirculation in a rat model of hemorrhagic shock. Surgery. 2006;140:579-587; discussion 587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Gardemann A, Watanabe Y, Grosse V, Hesse S, Jungermann K. Increases in intestinal glucose absorption and hepatic glucose uptake elicited by luminal but not vascular glutamine in the jointly perfused small intestine and liver of the rat. Biochem J. 1992;283:759-765. [PubMed] |
| 14. | Galagudza M, Vaage J, Valen G. Isoflurane and other commonly used anaesthetics do not protect the isolated buffer perfused mouse heart from ischemia-reperfusion injury. Clin Exp Pharmacol Physiol. 2006;33:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Csonka C, Kupai K, Kocsis GF, Novák G, Fekete V, Bencsik P, Csont T, Ferdinandy P. Measurement of myocardial infarct size in preclinical studies. J Pharmacol Toxicol Methods. 2010;61:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal Motility Monitor (GIMM). J Vis Exp. 2010;2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1420] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 18. | Attuwaybi B, Kozar RA, Gates KS, Moore-Olufemi S, Sato N, Weisbrodt NW, Moore FA. Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma. 2004;56:749-758; discussion 758-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Guzmán-de la Garza FJ, Cámara-Lemarroy CR, Alarcón-Galván G, Cordero-Pérez P, Muñoz-Espinosa LE, Fernández-Garza NE. Different patterns of intestinal response to injury after arterial, venous or arteriovenous occlusion in rats. World J Gastroenterol. 2009;15:3901-3907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Lu YQ, Gu LH, Huang WD, Mou HZ. Effect of hypertonic saline resuscitation on heme oxygenase-1 mRNA expression and apoptosis of the intestinal mucosa in a rat model of hemorrhagic shock. Chin Med J (Engl). 2010;123:1453-1458. [PubMed] |
| 21. | Powers KA, Zurawska J, Szaszi K, Khadaroo RG, Kapus A, Rotstein OD. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery. 2005;137:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Pang KS, Yuen V, Fayz S, te Koppele JM, Mulder GJ. Absorption and metabolism of acetaminophen by the in situ perfused rat small intestine preparation. Drug Metab Dispos. 1986;14:102-111. [PubMed] |
| 23. | Scholtka B, Stümpel F, Jungermann K. Acute increase, stimulated by prostaglandin E2, in glucose absorption via the sodium dependent glucose transporter-1 in rat intestine. Gut. 1999;44:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Shih HC, Huang MS, Lee CH. Estrogen augments the protection of hypertonic saline treatment from mesenteric ischemia-reperfusion injury. Shock. 2011;35:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Shih HC, Huang MS, Lee CH. Magnolol attenuates the lung injury in hypertonic saline treatment from mesenteric ischemia reperfusion through diminishing iNOS. J Surg Res. 2012;175:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Sanki A, Kroon HM, Kam PC, Thompson JF. Isolated limb perfusion and isolated limb infusion for malignant lesions of the extremities. Curr Probl Surg. 2011;48:371-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Weng ZC, Nicolosi AC, Detwiler PW, Hsu DT, Schierman SW, Goldstein AH, Spotnitz HM. Effects of crystalloid, blood, and University of Wisconsin perfusates on weight, water content, and left ventricular compliance in an edema-prone, isolated porcine heart model. J Thorac Cardiovasc Surg. 1992;103:504-513. [PubMed] |
| 28. | Radhakrishnan RS, Radhakrishnan HR, Xue H, Moore-Olufemi SD, Mathur AB, Weisbrodt NW, Moore FA, Allen SJ, Laine GA, Cox CS. Hypertonic saline reverses stiffness in a Sprague-Dawley rat model of acute intestinal edema, leading to improved intestinal function. Crit Care Med. 2007;35:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Radhakrishnan RS, Shah SK, Lance SH, Radhakrishnan HR, Xue H, Radhakrishnan GL, Ramaswamy US, Walker PA, Uray KS, Laine GA, Stewart RH, Cox CS. Hypertonic saline alters hydraulic conductivity and up-regulates mucosal/submucosal aquaporin 4 in resuscitation-induced intestinal edema. Crit Care Med. 2009;37:2946-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Okamoto F, Allen BS, Buckberg GD, Young H, Bugyi H, Leaf J. Reperfusate composition: interaction of marked hyperglycemia and marked hyperosmolarity in allowing immediate contractile recovery after four hours of regional ischemia. J Thorac Cardiovasc Surg. 1986;92:583-593. [PubMed] |
| 31. | Careaga G, Argüero R, Chavez-Negrete A, Valero G, Portilla E, Garcia RM, Mendoza L, Angulo L, Miranda Y. Control of myocardial reperfusion injury with hypertonic-hyperosmotic solution in isolated rabbit heart. Eur Surg Res. 1995;27:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Ding Y, Li J, Rafols JA, Phillis JW, Diaz FG. Prereperfusion saline infusion into ischemic territory reduces inflammatory injury after transient middle cerebral artery occlusion in rats. Stroke. 2002;33:2492-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Bielefeldt K, Conklin JL. Intestinal motility during hypoxia and reoxygenation in vitro. Dig Dis Sci. 1997;42:878-884. [PubMed] |
| 34. | Abdallah Y, Gkatzoflia A, Gligorievski D, Kasseckert S, Euler G, Schlüter KD, Schäfer M, Piper HM, Schäfer C. Insulin protects cardiomyocytes against reoxygenation-induced hypercontracture by a survival pathway targeting SR Ca2+ storage. Cardiovasc Res. 2006;70:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Walensi M, de Groot H, Schulz R, Hartmann M, Petrat F. Mesenteric ischemia-reperfusion injury: Clearly improved hemodynamics but only minor protection of the rat small intestine by (sub)therapeutic heparin sodium and enoxaparin doses. J Surg Res. 2013;179:e57-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Varga J, Tóth S, Staško P, Tóth S, Bilecová-Rabajdová M, Ostró A, Veselá J. Intestinal ischemia-reperfusion injury - the histopathological status of remote vital organs in acute and subacute phases. Ann Transplant. 2012;17:11-20. [PubMed] |