Published online May 14, 2013. doi: 10.3748/wjg.v19.i18.2811
Revised: March 1, 2013
Accepted: March 21, 2013
Published online: May 14, 2013
Processing time: 147 Days and 22.7 Hours
AIM: To evaluate whether preoperative mean corpuscular volume (MCV) is a prognostic indicator in patients with resectable esophageal squamous cell carcinoma (ESCC).
METHODS: A total of 298 consecutive, prospectively enrolled patients with histologically diagnosed ESCC who underwent surgery with curative intent from 2001 to 2011 were retrospectively evaluated. Patients were excluded if they had previous malignant disease, distant metastasis at the time of primary treatment, a history of neoadjuvant treatment, had undergone non-radical resection, or had died of a non-tumor-associated cause. Survival status was verified in September 2011. Pathological staging was performed based on the 2010 American Joint Committee on Cancer criteria. Preoperative MCV was obtained from blood counts performed routinely within 7 d prior to surgery. Receiver operating characteristic (ROC) curve analysis was used to determine a cutoff for preoperative MCV.
RESULTS: The 298 patients consisted of 230 males and 68 females, with a median follow-up of 30.1 mo. ROC analysis showed an optimal cutoff for preoperative MCV of 95.6 fl. Fifty-nine patients (19.8%) had high (> 95.6 fl) and 239 (80.2%) had low (≤ 95.6 fl) preoperative MCV. Preoperative MCV was significantly associated with gender (P = 0.003), body mass index (P = 0.017), and preoperative red blood cell count (P < 0.001). The predicted 1-, 3- and 5-year overall survival (OS) rates were 72%, 60% and 52%, respectively. Median OS was significantly longer in patients with low than with high preoperative MCV (27.5 mo vs 19.4 mo, P < 0.001). Multivariate analysis showed that advanced pT (P = 0.018) and pN (P < 0.001) stages, upper thoracic location (P = 0.010), lower preoperative albumin concentration (P = 0.002), and high preoperative MCV (P = 0.001) were negative prognostic factors in patients with ESCC. Preoperative MCV also stratified OS in patients with T3, N1-N3, G2-G3 and stage III tumors.
CONCLUSION: Preoperative MCV is a prognostic factor in patients with ESCC.
Core tip: Elevated mean corpuscular volume (MCV) has been shown to predict the risk of esophageal squamous cell carcinoma (ESCC). We hypothesized that pretreatment MCV could predict prognosis. In analyzing 298 patients with ESCC, we found that the optimal cut-off for preoperative MCV was 95.6 fl. Multivariate analysis showed that high (> 95.6 fl) preoperative MCV was a negative prognostic factor, along with advanced stage, upper thoracic location and lower preoperative albumin, in patients with ESCC. Median overall survival was significantly longer in patients with low (≤ 95.6 fl) than high preoperative MCV (27.5 mo vs 19.5 mo, P < 0.001).
- Citation: Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang LJ, Fu JH, Wang JY. Prognostic value of preoperative mean corpuscular volume in esophageal squamous cell carcinoma. World J Gastroenterol 2013; 19(18): 2811-2817
- URL: https://www.wjgnet.com/1007-9327/full/v19/i18/2811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i18.2811
Elevated mean corpuscular volume (MCV) has long been recognized as a biomarker for alcoholic and folate deficient patients[1-3]. Although the nature of the relationship between them remains unclear, recent reports suggested that alcohol-induced folate deficiency can lead to macrocytosis[4]. In addition, MCV was found to be higher in Asian heavy drinkers with inactive aldehyde dehydrogenase-2 (ALDH2)[5,6] and to be a marker for alcohol abuse with inactive heterozygous ALDH2[7,8], suggesting that acetaldehyde is an important contributor to macrocytosis.
Alcohol abuse, and acetaldehyde and folate deficiency, all indicative of poor physical condition, were found to increase susceptibility to esophageal carcinoma[3,9-12], as was macrocytosis[7,13]. In addition, patients with more advanced malignancies frequently present with more severe hematological anomalies[14,15]. These findings led us to hypothesize that pretreatment MCV may predict the prognosis of patients with esophageal carcinoma. We therefore analyzed the association between preoperative MCV and different clinicopathological parameters, as well as the prognostic significance of preoperative MCV in patients with esophageal squamous cell carcinoma (ESCC).
This study was a retrospective analysis of a prospectively collected database (2001-2011) of 298 consecutive patients with histologically diagnosed ESCC who underwent surgery with curative intent at the Cancer Center of Sun Yat-Sen University, Guangzhou, China. Patients with previous malignancy, distant metastasis, neoadjuvant treatment, non-radical resection (R1/R2), or non-tumor-associated death were excluded. Tumors were pathologically staged using the American Joint Committee on Cancer (2010) staging system. Patients were followed-up in the outpatient clinic every 3-6 mo during the first 3 years and every 12 mo thereafter. Demography and clinical details were extracted from the database (Table 1). Survival status was verified in September 2011 using the best available methods. The study protocol was approved by the medical ethics committee of the Cancer Center of Sun Yat-Sen University, which waived the requirement for informed consent due to the retrospective nature of the study.
| Characteristics | Case numbers | Preoperative MCV | P valuePearson’sχ2test | |
| Low | High | |||
| Age, yr (mean ± SE) | 58.2 ± 9.2 | |||
| ≤ 65 | 231 | 184 (79.7) | 47 (20.3) | |
| > 65 | 67 | 55 (82.1) | 12 (17.9) | 0.660 |
| Gender | ||||
| Male | 230 | 176 (76.5) | 54 (23.5) | |
| Female | 68 | 63 (92.6) | 5 (7.4) | 0.003 |
| BMI, kg/m2 (mean ± SE) | 22.3 ± 3.2 | |||
| ≤ 20 | 65 | 46 (70.8) | 19 (29.2) | |
| > 20 and ≤ 25 | 180 | 144 (80.0) | 36 (20.0) | |
| > 25 | 53 | 49 (92.5) | 4 (7.5) | 0.017 |
| Smoking index | 440.1 ± 483.1 | |||
| ≤ 400 | 171 | 141 (82.5) | 30 (17.5) | |
| > 400 | 127 | 98 (77.2) | 29 (22.8) | 0.257 |
| Preoperative RBC, × 1012/L (mean ± SE) | 4.5 ± 0.6 | |||
| ≤ 4.01 | 56 | 31 (55.4) | 25 (44.6) | |
| > 4.0 | 242 | 208 (86.0) | 34 (14.0) | < 0.001 |
| Preoperative albumin, g/L (mean ± SE) | 42.9 ± 4.6 | |||
| ≤ 432 | 149 | 115 (77.2) | 34 (22.8) | |
| > 43 | 149 | 124 (83.2) | 25 (16.8) | 0.191 |
| pT status, UICC7th (mean ± SE) | ||||
| T1 | 33 | 31 (93.9) | 2 (6.1) | |
| T2 | 52 | 44 (84.6) | 8 (15.4) | |
| T3 | 213 | 164 (77.0) | 49 (23.0) | 0.051 |
| N0 | 138 | 116 (84.1) | 22 (15.9) | |
| N1 | 89 | 67 (75.3) | 22 (24.7) | |
| N2 | 51 | 42 (82.4) | 9 (17.6) | |
| N3 | 20 | 14 (70.0) | 6 (30.0) | 0.250 |
| Histologic grade | ||||
| G1 | 94 | 71 (75.5) | 23 (24.5) | |
| G2 | 156 | 127 (81.4) | 29 (18.6) | |
| G3 | 48 | 41 (85.4) | 7 (14.6) | 0.324 |
| pTNM stage (UICC7th) | ||||
| Stage I | 37 | 32 (86.5) | 5 (13.5) | |
| Stage II | 120 | 100 (83.3) | 20 (16.7) | |
| Stage III | 141 | 107 (75.9) | 34 (24.1) | 0.191 |
| Tumor location | ||||
| Upper | 48 | 37 (77.1) | 11 (22.9) | |
| Middle | 150 | 125 (83.3) | 25 (16.7) | |
| Lower | 100 | 77 (77.0) | 23 (23.0) | 0.393 |
Preoperative MCV was determined from preoperative blood counts, performed routinely within 7 d prior to surgery, using a Beckman Counter blood analyzer (version STKS, Beckman Counter Inc., Fullerton, CA, United States). The cut-off for preoperative MCV was defined by receiver operating characteristic (ROC) curve analysis, with the point maximizing the area under the curve being selected.
All statistical analysis were performed using the SPSS 19.0 software package (SPSS, Inc., Chicago, IL, United States). The ROC curve was generated and analyzed using MedCalc statistical software package 11.0.1 (MedCalc Software bvba, Mariakerke, Belgium). Correlations between preoperative MCV and clinicopathological characteristics were assessed using the Pearson’s χ2 test. Overall survival (OS) was defined as the interval from the date of surgery to the date of death, or last follow-up. Multivariate Cox regression analysis was performed for all parameters found to be significant by the univariate analysis. Survival was analyzed using the Kaplan-Meier method, and differences between curves were assessed by the Log-Rank test. Statistical significance was defined as a P value < 0.05.
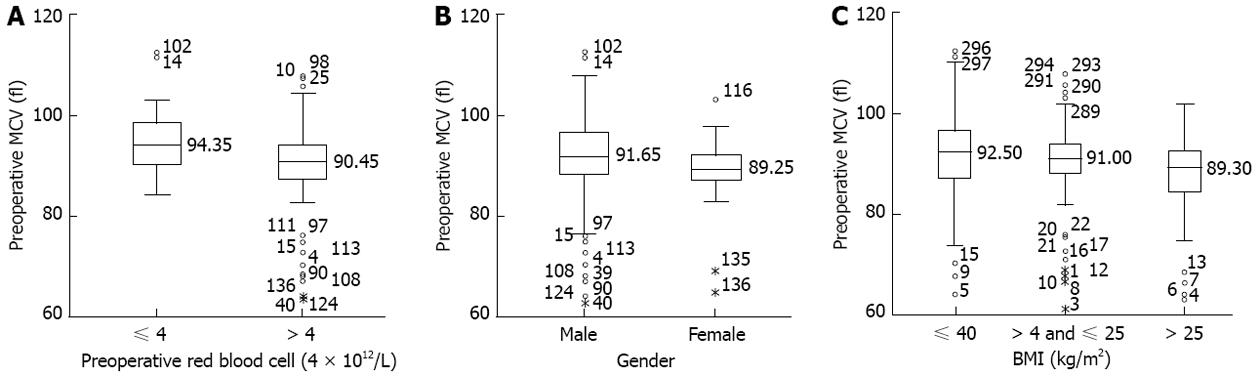
The 298 patients consisted of 230 males and 68 females, with a median preoperative MCV of 91.0 fl (range: 61.4-112.4 fl). ROC curve analysis showed that the optimal cut-off point maximizing (0.588) was 95.6 fl (P = 0.0123), with a sensitivity of 0.867 and a specificity of 0.324. Using this cut-off, 59 patients (19.8%) had high (> 95.6 fl) and 239 (80.2%) had low (≤ 95.6 fl) preoperative MCV. The correlations between preoperative MCV and clinicopathologic parameters are summarized in Table 1. Preoperative MCV was significantly associated with gender (P = 0.003), body mass index (BMI) (P = 0.017), and preoperative red blood cell (RBC) count (P < 0.001; Figure 1).
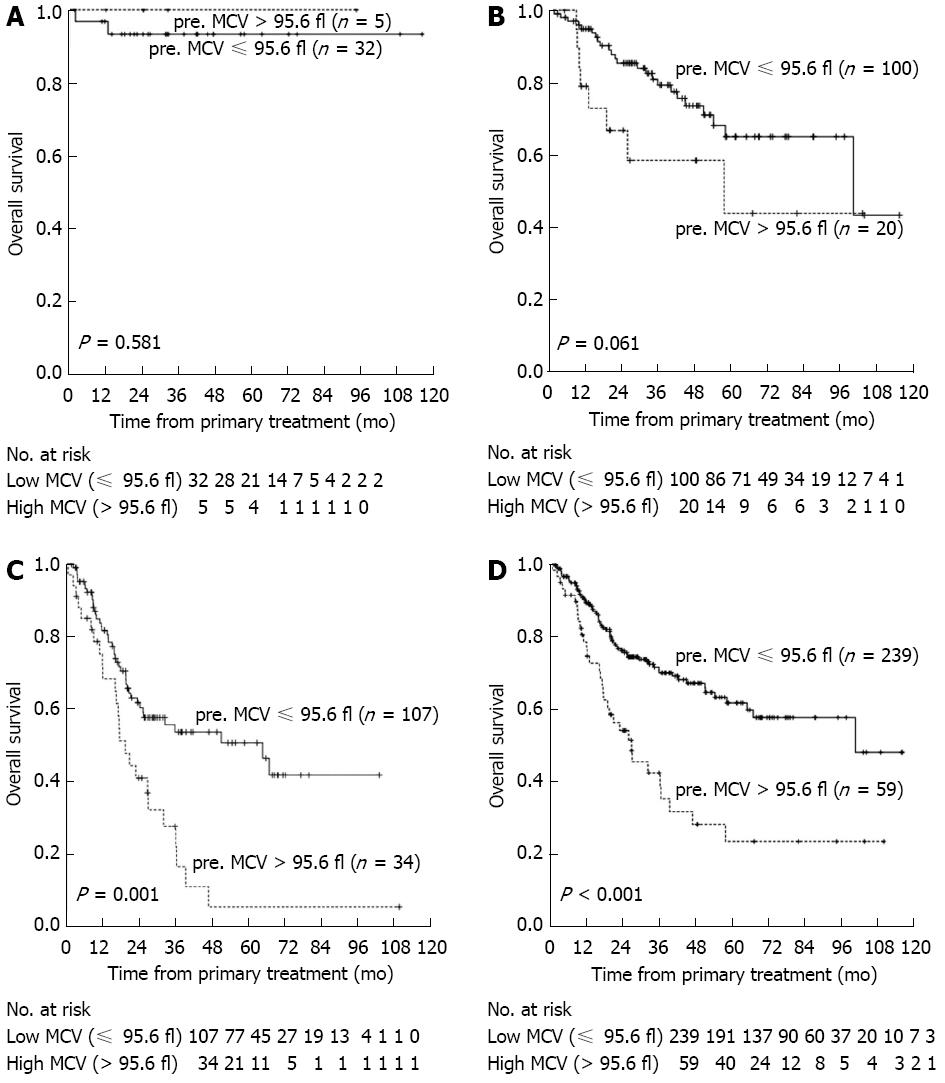
Over a median follow-up of 30.1 mo, 102 of the 298 patients (34.2%) died of cancer-related causes, whereas the other 196 (65.8%) survived. The median survival time was 25.8 mo (range: 1.6-116.1 mo), and the predicted 1-, 3- and 5-year OS rates after primary surgery were 72%, 60%, and 52% respectively. Median OS was significantly longer in patients with low than high preoperative MCV (27.5 mo vs 19.4 mo, P < 0.001; Figure 2).
To determine factors independently prognostic of patient survival, we analyzed OS using a Cox proportional hazards model. All parameters found to be potentially significant in univariate analysis were included in a multivariate analysis. We found that pT status (P = 0.018), pN status (P < 0.001), tumor location (P = 0.010), preoperative albumin concentration (P = 0.002), and preoperative MCV (P = 0.001) were significantly prognostic of survival in this patient cohort (Table 2). When we analyzed the effect of preoperative MCV on OS in patients classified by clinicopathological factors, preoperative MCV was predictive of OS in patients with T3 (P < 0.001), N1-N3 (P < 0.001), G2-G3 (P < 0.001), and stage III (P = 0.001) tumors (Figure 2 and Table 3).
| Factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value1 | HR (95%CI) | P value1 | |
| Age, yr | 1.007 (0.985-1.029) | 0.527 | ||
| Gender (male vs female) | 0.744 (0.464-1.196) | 0.222 | ||
| Smoking index ( ≤ 400 vs > 400) | 1.391 (0.940-2.060) | 0.099 | 1.302 (0.874-1.940) | 0.194 |
| BMI, kg/m2 ( ≤ 20 vs > 20; ≤ 25 vs > 25) | 0.878 (0.638-1.207) | 0.422 | ||
| Preoperative MCV, fl ( ≤ 95.6 vs > 95.6) | 2.495 (1.644-3.787) | < 0.001 | 2.108 (1.372-3.241) | 0.001 |
| Preoperative RBC, × 1012/L ( ≤ 4 vs > 4) | 0.685 (0.433-1.082) | 0.105 | 0.835 (0.507-1.350) | 0.462 |
| Preoperative albumin, g/L | 0.954 (0.919-0.990) | 0.012 | 0.938 (0.900-0.977) | 0.002 |
| pT status (pT1 vs pT2 and pT3) | 1.641 (1.147-2.348) | 0.007 | 1.589 (1.084-2.327) | 0.018 |
| pN status (pN0 vs pN1, pN2 and pN3) | 1.954 (1.603-2.382) | < 0.001 | 1.957 (1.602-2.392) | < 0.001 |
| Histologic grade (G1 vs G2 and G3) | 0.961 (0.714-1.293) | 0.791 | ||
| Tumor location (upper thoracic vs middle thoracic and lower thoracic) | 0.798 (0.605-1.051) | 0.109 | 0.692 (0.522-0.916) | 0.01 |
| Variable | Case numbers | Overall survival (mo)(mean ± SE) | P valueLog-Rank test |
| All cohort | < 0.001 | ||
| Low | 239 | 33.7 ± 24.7 | |
| High | 59 | 25.8 ± 24.2 | |
| pT status | |||
| T1-T2 | 0.075 | ||
| Low | 75 | 39.1 ± 27.1 | |
| High | 10 | 27.6 ± 22.7 | |
| T3 | < 0.001 | ||
| Low | 164 | 31.2 ± 23.2 | |
| High | 49 | 25.4 ± 24.7 | |
| pN status | |||
| N0 | 0.464 | ||
| Low | 116 | 40.0 ± 26.0 | |
| High | 22 | 35.5 ± 28.7 | |
| N1-N3 | < 0.001 | ||
| Low | 123 | 27.9 ± 22.0 | |
| High | 37 | 20.0 ± 19.2 | |
| Histologic grade | |||
| G1 | 0.211 | ||
| Low | 71 | 32.9 ± 27.6 | |
| High | 23 | 27.4 ± 24.3 | |
| G2-G3 | < 0.001 | ||
| Low | 168 | 34.1 ± 23.4 | |
| High | 36 | 24.8 ± 24.4 | |
| pTNM stage | |||
| Stage I | 0.581 | ||
| Low | 32 | 37.9 ± 27.1 | |
| High | 5 | 37.6 ± 32.6 | |
| Stage II | 0.061 | ||
| Low | 100 | 39.6 ± 25.4 | |
| High | 20 | 31.1 ± 27.7 | |
| Stage III | 0.001 | ||
| Low | 107 | 27.0 ± 21.6 | |
| High | 34 | 20.9 ± 19.8 |
Hematologic parameters have been reported to correlate significantly with prognosis in patients with advanced malignant disease[15-18]. MCV is considered a sensitive indicator of alcohol abuse and folate deficiency[1,2,4,6,19]. Recently, MCV was found to be a biomarker for alcohol abuse accompanied by inactive heterozygous ALDH2, and also allowed for the prediction of ESCC risk[8]. To our knowledge, however, no previous study has assessed the relationship between MCV and the prognosis of patients with ESCC.
Using ROC curve analysis, we found that a cut-off of 95.6 fl was a statistically significant predictor of OS. Moreover, high (> 95.6 fl) MCV was significantly correlated with male gender, lower BMI, and RBC ≤ 4 × 1012/L. Folate deficiency has been shown to inhibit red cell maturation, as well as increasing erythrocyte fragility, resulting in increased hemolysis and lower RBC count, which consequently results in macrocytosis[20]. Lower BMI may accompany poor nutritional status, which was associated with elevated MCV[3,21]. The significant correlation between high MCV and male gender may be related to the association between macrocytosis and alcohol abuse, since overdrinking is much more frequent in males than in females[6-8]. Furthermore, MCV tended to be associated with pT status (P = 0.051), consistent with findings showing that preoperative MCV may provide a complementary advantage in assessing tumor invasiveness[17].
Although TNM stage is the best predictor of survival in cancer patients, OS may differ widely in patients with the same TNM stage tumors who receive the same treatment, suggesting that other, as yet undetermined factors may affect prognosis. Since preoperative hematologic parameters have been predictive of patient prognosis[15,22-25], we performed univariate and multivariate analyses of factors predictive of OS in patients with ESCC. We found that pathological stage, tumor location, preoperative albumin concentration, and preoperative MCV were prognostic factors in our patient cohort.
We also found that OS was significantly shorter in patients with upper-thoracic cancer than those with middle and lower-thoracic esophageal cancer. A study of 605 patients with ESCC also found that median OS was significantly shorter in patients with upper thoracic cancer than in those with middle and lower thoracic tumors (45.9 mo vs 82.2 and 93.8 mo; P < 0.001)[26]. Due to their anatomical location, carcinomas of the upper thoracic esophagus often result in early invasion of adjacent structures and extensive lymph node metastasis[27]. The prognostic significance of preoperative albumin concentration may be due to it being a sensitive indicator of nutrition, liver function, and metabolic response to disease; thus patients with lower albumin concentrations may present with poorer physical status, decreasing both their response and tolerance to treatment[28,29]. Similar findings were reported in patients with adenocarcinoma of the gastric cardia[30].
Although we found that preoperative MCV was prognostic in patients with ESCC, there is no evidence that MCV has a direct effect on tumor progression or patient prognosis. MCV, however, is a marker of internal folate concentration. Folate acts to transfer one-carbon moieties, thus playing a central role in DNA synthesis, replication, repair, and methylation[31]. Folate deficiency leads to aberrant DNA methylation, which has been reported to be a predictor of clinical outcome in patients with esophageal cancer[32]. A recent study of 125 ESCC patients who underwent surgical resection showed that median OS was significantly longer in patients with high than with low/moderate folate intake (4.59 years vs 3.06 years; P = 0.007)[33]. Similar results were reported in patients with advanced gastric cancer who were treated with chemotherapy[34].
Another factor linking MCV with prognosis in ESCC is macrocytosis, which may be an indicator of malnutrition, a negative prognostic factor in various human cancers[21,35,36]. In addition, crystal osmotic pressure was shown to be a major regulator of red cell volume in internal environments[37]. Dysphagia, a frequently observed symptom in patients with advanced esophageal cancer, restricts intake, thus reducing serum concentrations of electrolytes, glucose, and amino acids. This, in turn, may decrease crystal osmotic pressure, leading to red cell dilation. Our finding, that preoperative MCV was related to pT stage and BMI, was consistent with results suggesting that increased MCV was associated with tumor invasiveness and nutritional status[3,17]. Thus, taken together, these results suggest that preoperative MCV may be a marker reflecting internal folate concentration, nutritional status, and tumor invasiveness, thus comprehensively predicting prognosis in patients with ESCC. MCV assays are also convenient and inexpensive to perform, allowing for wide clinical application and suggesting that they may be crucial in preoperative assessment.
To further evaluate the prognostic significance of preoperative MCV, we performed subgroup analysis in patients with ESCC. We found that MCV resulted in the stratification of OS in patients with T3, N1-N3, G2-G3, and stage III tumors, but not in patients with T1-T2, N0, G1, or stage I/II tumors. These findings, however, may be due to the small sample size of these subgroups. Moreover, the relatively good prognosis in patients with T1/2, N0, G1, and stage I/II tumors may mask the significance of preoperative MCV.
This study has limitations and potential biases. Due to its retrospective nature, records of alcohol consumption by patients were incomplete and folic acid concentrations were not tested in most patients. Furthermore, we could not determine whether preoperative MCV was a better predictor of OS than conventional prognostic factors. Finally, our small sample size may reflect a selection bias to some extent.
In conclusion, in patients with resectable ESCC, OS was significantly longer in patients with low (≤ 95.6 fl) than high (> 95.6 fl) preoperative MCV. Additional studies, however, are required to validate our results.
Surgical resection remains the treatment of choice for patients with localized esophageal carcinoma. Routine preoperative blood tests of red blood cells, white blood cells, and platelet counts can help estimate surgical risk. Significant hematologic variations frequently observed in patients with advanced malignant diseases may predict prognosis.
Elevated mean corpuscular volume (MCV) has long been recognized as a biomarker for alcohol abuse and folate deficiency. In addition, MCV was reported to be higher in Asian heavy drinkers with inactive aldehyde dehydrogenase-2 (ALDH2), and was found to be a marker of alcohol abuse in individuals with inactive heterozygous ALDH2, suggesting that acetaldehyde may be an important contributor to macrocytosis. A recent study showed that macrocytosis was a risk factor for esophageal carcinoma.
The authors observed a correlation between macrocytosis and prognosis in patients with esophageal carcinoma. Overall survival was significantly shorter in patients with elevated MCV than those with lower MCV. Utilizing receiver operating characteristic curve analysis, the authors determined an optimal cut-off point for MCV, which was both reasonable and objective.
These results suggest that preoperative MCV may be used to predict prognosis in patients with esophageal cancer. Routine blood tests should be performed shortly before surgery in these patients, and those with elevated MCV, especially greater than 95.6 fl, should be carefully evaluated to assess the risks and feasibility of surgery.
MCV, representing the mean volume of a single red blood cell, is determined by indirect calculation. Clinically, this parameter is often used in the differential diagnosis of various type of anemia.
This is an article on an unusual topic. The value of MCV has been known up to now as risk factor for esophageal carcinoma, but it is not known as prognostic factor.
P- Reviewers Mann O, Kopacova M S- Editor Zhai HH L- Editor Rutherford A E- Editor Li JY
| 1. | Herbert V, Zalusky R, Davidson Cs. Correlation of folate deficiency with alcoholism and associated macrocytosis, anemia, and liver disease. Ann Intern Med. 1963;58:977-988. [PubMed] |
| 2. | Unger KW, Johnson D. Red blood cell mean corpuscular volume: a potential indicator of alcohol usage in a working population. Am J Med Sci. 1974;267:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | World MJ, Ryle PR, Jones D, Shaw GK, Thomson AD. Differential effect of chronic alcohol intake and poor nutrition on body weight and fat stores. Alcohol Alcohol. 1984;19:281-290. [PubMed] |
| 4. | de la Vega MJ, Santolaria F, González-Reimers E, Alemán MR, Milena A, Martínez-Riera A, González-García C. High prevalence of hyperhomocysteinemia in chronic alcoholism: the importance of the thermolabile form of the enzyme methylenetetrahydrofolate reductase (MTHFR). Alcohol. 2001;25:59-67. [PubMed] |
| 5. | Nomura F, Itoga S, Tamura M, Harada S, Iizuka Y, Nakai T. Biological markers of alcoholism with respect to genotypes of low-Km aldehyde dehydrogenase (ALDH2) in Japanese subjects. Alcohol Clin Exp Res. 2000;24:30S-33S. [PubMed] |
| 6. | Hashimoto Y, Nakayama T, Futamura A, Omura M, Nakahara K. Erythrocyte mean cell volume and genetic polymorphism of aldehyde dehydrogenase 2 in alcohol drinkers. Blood. 2002;99:3487-3488. [PubMed] |
| 7. | Yokoyama M, Yokoyama A, Yokoyama T, Hamana G, Funazu K, Kondo S, Yamashita T, Yoshimizu H, Nakamura H. Mean corpuscular volume and the aldehyde dehydrogenase-2 genotype in male Japanese workers. Alcohol Clin Exp Res. 2003;27:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Yokoyama A, Yokoyama T, Muramatsu T, Omori T, Matsushita S, Higuchi S, Maruyama K, Ishii H. Macrocytosis, a new predictor for esophageal squamous cell carcinoma in Japanese alcoholic men. Carcinogenesis. 2003;24:1773-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Homann N, Stickel F, König IR, Jacobs A, Junghanns K, Benesova M, Schuppan D, Himsel S, Zuber-Jerger I, Hellerbrand C. Alcohol dehydrogenase 1C*1 allele is a genetic marker for alcohol-associated cancer in heavy drinkers. Int J Cancer. 2006;118:1998-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001;61:3272-3275. [PubMed] |
| 11. | Zhang J, Zotz RB, Li Y, Wang R, Kiel S, Schulz WA, Wen D, Chen Z, Zhang L, Wang S. Methylenetetrahydrofolate reductase C677T polymorphism and predisposition towards esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. J Cancer Res Clin Oncol. 2004;130:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wang LD, Guo RF, Fan ZM, He X, Gao SS, Guo HQ, Matsuo K, Yin LM, Li JL. Association of methylenetetrahydrofolate reductase and thymidylate synthase promoter polymorphisms with genetic susceptibility to esophageal and cardia cancer in a Chinese high-risk population. Dis Esophagus. 2005;18:177-184. [PubMed] |
| 13. | Yokoyama T, Yokoyama A, Kato H, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev. 2003;12:1227-1233. [PubMed] |
| 14. | Lee YT, Van Peenen HJ, Watson FR. Chemical and hematological screening in patients with malignant and non-malignant conditions. J Surg Oncol. 1980;15:43-51. [PubMed] |
| 15. | Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Banerjee RN, Narang RM. Haematological changes in malignancy. Br J Haematol. 1967;13:829-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Ruka W, Rutkowski P, Kaminska J, Rysinska A, Steffen J. Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol. 2001;12:1423-1432. [PubMed] |
| 18. | Stock W, Hoffman R. White blood cells 1: non-malignant disorders. Lancet. 2000;355:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Chalmers DM, Levi AJ, Chanarin I, North WR, Meade TW. Mean cell volume in a working population: the effects of age, smoking, alcohol and oral contraception. Br J Haematol. 1979;43:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Weiser G, Kohn C, Vachon A. Erythrocyte volume distribution analysis and hematologic changes in two horses with immune-mediated hemolytic anemia. Vet Pathol. 1983;20:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Bruckner HW, Lavin PT, Plaxe SC, Storch JA, Livstone EM. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA. 1982;247:1004-1006. [PubMed] [DOI] [Full Text] |
| 23. | Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer. 1970;25:135-140. [PubMed] |
| 24. | Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Tominaga M, Okunaga R, Shibata K, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Li H, Zhang Q, Xu L, Chen Y, Wei Y, Zhou G. Factors predictive of prognosis after esophagectomy for squamous cell cancer. J Thorac Cardiovasc Surg. 2009;137:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Wang HW, Kuo KT, Wu YC, Huang BS, Hsu WH, Huang MH, Wang LS. Surgical results of upper thoracic esophageal carcinoma. J Chin Med Assoc. 2004;67:447-457. [PubMed] |
| 28. | Haupt W, Holzheimer RG, Riese J, Klein P, Hohenberger W. Association of low preoperative serum albumin concentrations and the acute phase response. Eur J Surg. 1999;165:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Danielsen PL, Agren MS, Jorgensen LN. Platelet-rich fibrin versus albumin in surgical wound repair: a randomized trial with paired design. Ann Surg. 2010;251:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, Huang MH, Huang BS. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123-1128. [PubMed] |
| 32. | Sato F, Meltzer SJ. CpG island hypermethylation in progression of esophageal and gastric cancer. Cancer. 2006;106:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Lu C, Xie H, Wang F, Shen H, Wang J. Diet folate, DNA methylation and genetic polymorphisms of MTHFR C677T in association with the prognosis of esophageal squamous cell carcinoma. BMC Cancer. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Shitara K, Muro K, Ito S, Sawaki A, Tajika M, Kawai H, Yokota T, Takahari D, Shibata T, Ura T. Folate intake along with genetic polymorphisms in methylenetetrahydrofolate reductase and thymidylate synthase in patients with advanced gastric cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1311-1319. [PubMed] |
| 35. | Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, Cervera E, Mohar-Betancourt A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Espinosa E, Feliu J, Zamora P, González Barón M, Sánchez JJ, Ordón ez A, Espinosa J. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 1995;12:67-76. [PubMed] |
| 37. | Porath-Furedi A. The mutual effect of hydrogen ion concentration and osmotic pressure on the shape of the human erythrocyte as determined by light scattering and by electronic cell volume measurement. Cytometry. 1983;4:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |