Published online Apr 28, 2013. doi: 10.3748/wjg.v19.i16.2578
Revised: February 4, 2013
Accepted: March 6, 2013
Published online: April 28, 2013
Processing time: 185 Days and 14.4 Hours
This report describes a case of a space-occupying lesion in the right liver in a 38-year-old man who was found to have peliosis hepatis. Clinical data of this patient were presented, including medical history, laboratory test and imaging results, and postoperative pathological findings (hematoxylin and eosin staining). Review of his medical history showed that the patient had been bitten by a dog three years earlier. B-mode ultrasonography revealed an uneven echo mass in the right hemiliver, and magnetic resonance imaging scans also showed a mass in the anterior segment of the right liver. Upon surgical removal, the mass was found to be 4.0 cm × 3.8 cm × 3.8 cm in size and located in segment VI. The mass had a dark and soft appearance, with an irregular edge on intraoperative ultrasonography. Postoperative pathological findings revealed many small capsules filled with blood cells. The patient was diagnosed with peliosis hepatis based on his medical history of having been bitten by a dog, presence of mild anemia, and lack of characteristic symptoms, including fever of unknown origin, abdominal pain, and hepatosplenomegaly, combined with intraoperative and postoperative pathologic findings. The operation was successful, and after being treated with anti-infection agents, the patient had a good recovery.
Core tip: This report describes a case of a space-occupying lesion in the right liver in a 38-year-old man who was diagnosed with peliosis hepatis based on the medical history of having been bitten by a dog, presence of mild anemia, and lack of characteristic symptoms, including fever of unknown origin, abdominal pain, and hepatosplenomegaly, combined with intraoperative and postoperative pathologic findings. The operation was successful, and after being treated with anti-infection agents, the patient had a good recovery.
- Citation: Pan W, Hong HJ, Chen YL, Han SH, Zheng CY. Surgical treatment of a patient with peliosis hepatis: A case report. World J Gastroenterol 2013; 19(16): 2578-2582
- URL: https://www.wjgnet.com/1007-9327/full/v19/i16/2578.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i16.2578
Peliosis hepatis (PH) is a rare clinical disease, appearing mostly as diffuse hepatic lesions. PH is considered a benign vasogenic lesion and is characterized by the presence of cystic blood-filled cavities distributed randomly throughout the liver parenchyma. This disease is more common in adults than in children or adolescents. We herein report a case of PH in a 38-year-old man.
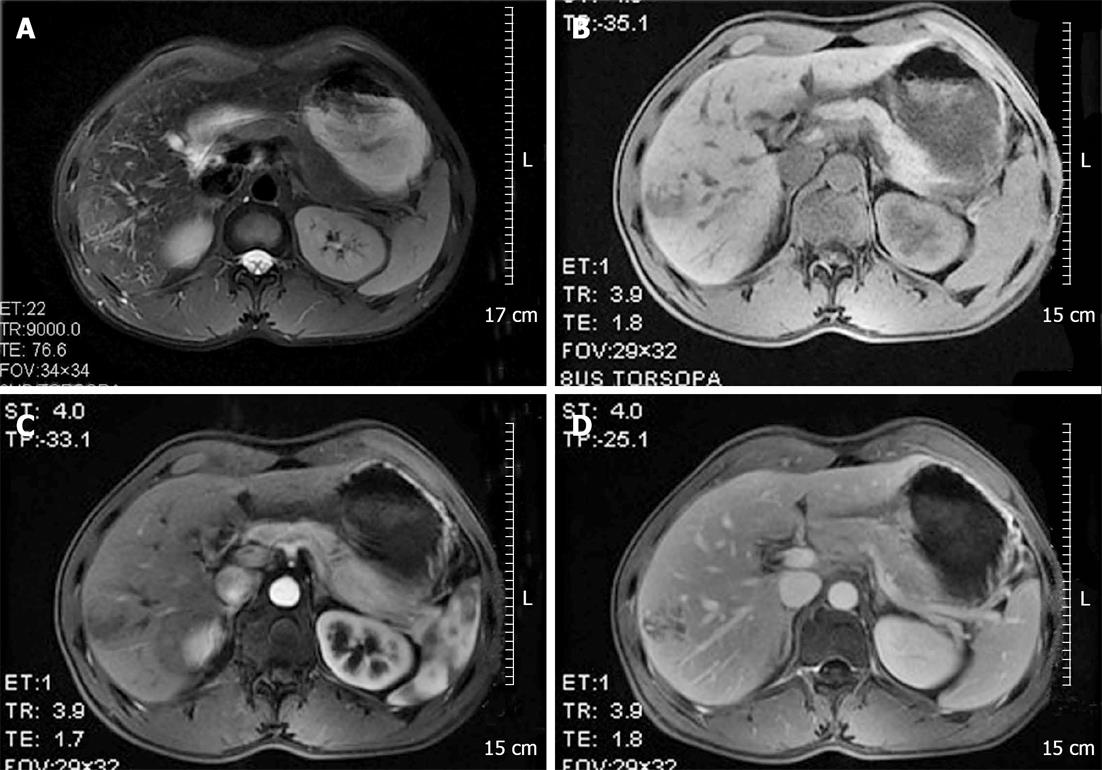
A 38-year-old man presented with a space-occupying lesion in his right liver. The patient had been bitten by a dog three years earlier, but his health was generally good. The patient did not have hepatosplenomegaly or symptoms of tuberculosis intoxication, nor did he have a history of blood transfusions or long-term use of glucocorticoids or anabolic hormones. Physical examination revealed no abnormalities. Blood tests showed that his hemoglobin level was 90 g/L, whereas his blood biochemistry, liver and kidney function tests, and alpha fetal protein, cancer embryo antigen, carbohydrate antigen 19-9 concentrations were all within normal ranges. He was positive for hepatitis B surface antibody (323.21 IU/L) and core antibody (4.25 S/C.0), but negative for other hepatitis B antigens and antibodies and negative for human immunodeficiency virus. Hepatobiliary magnetic resonance imaging (MRI) revealed a 3.8 cm × 3.1 cm mass with irregular boundaries in the anterior segment of his right liver.
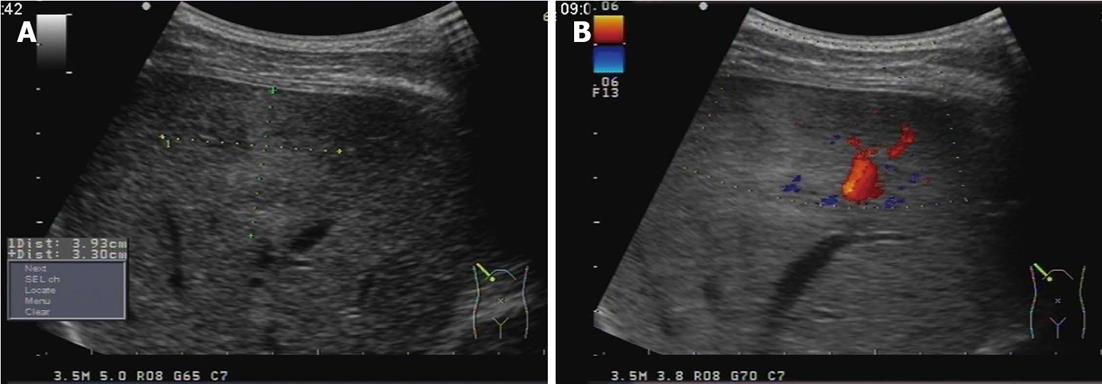
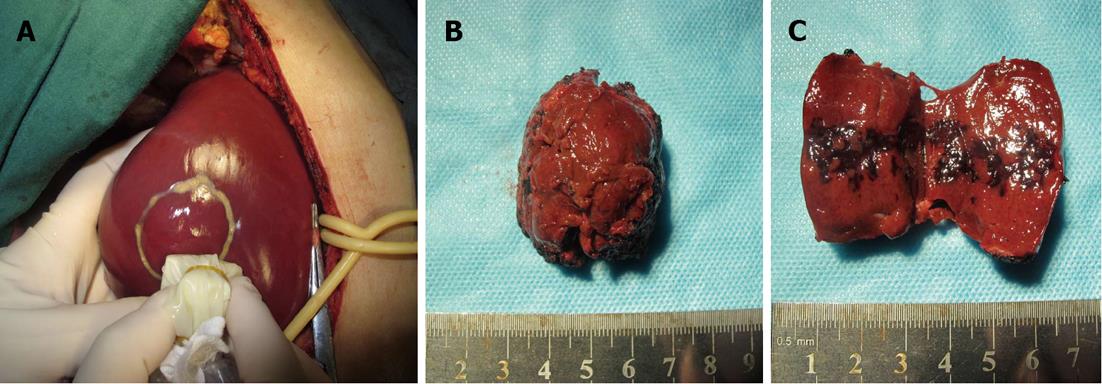
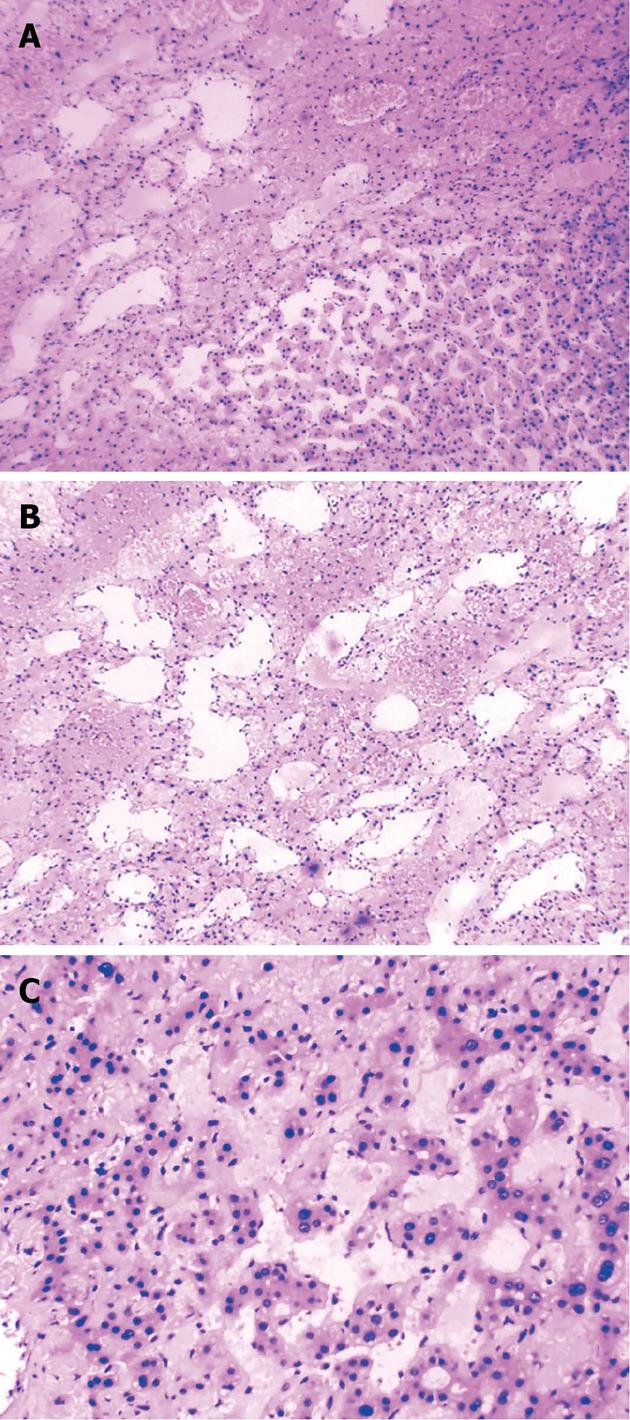
Enhanced scanning showed inhomogeneous enhancement, peaking at the arterial phase (Figure 1). Color ultrasonography showed that his liver had a normal appearance, but there was a 3.9 cm × 3.9 cm mass with inhomogeneous echo in his right hemiliver with unclear boundaries and weak blood flow signal within the mass (Figure 2). He underwent surgery, which revealed the mass located in segment VI. Intraoperative ultrasonography showed that the mass was 4.0 cm × 3.8 cm × 3.8 cm in size, and had a dark and soft appearance, with irregular edges (Figure 3). No abnormality was observed in any other abdominal organs. Rapid pathological examination on frozen sections suggested the possibility of a hepatic hemangioma, although pathological assessment confirmed PH (Figure 4). The operation was successful, and after being treated with anti-infection agents, the patient had a good recovery.
At gross inspection, the peliotic lesions in a PH liver look like “Swiss cheese slices”. Microscopically, there are two types of peliosis: “parenchymal peliosis” and “phlebectatic peliosis”[1], the main difference being whether the cavities are lined by endothelium or fibrotic tissue. The patient described here had parenchymal peliosis.
The pathogenesis of PH remains unknown, and pathogenic factors vary. Roughly they can be divided into three categories: drug-related, autoimmune and infectious. PH has been associated with the use of hormones and immunosuppressive medications, especially α-alkyl steroid hormones and thiopurine[2]. Autoimmune factors are those associated with secondary immunodeficiency caused by certain potential consumptive diseases, such as tuberculosis, hematological malignancies[3,4], acquired immunodeficiency syndrome[5], immune deficiency after transplantation[6], and hepatocellular carcinoma (HCC)[7]. Infectious factors include Bartonella infection, which leads to cat-scratch disease[8,9]. PH has also been observed in dogs infected with Bartonella[10]. Despite the absence of symptoms of cat-scratch disease, the patient’s dog-bite experience suggests the possibility of Bartonella infection.
PH is usually caused by autoimmune disorders triggered by endogenous and exogenous risk factors. These autoimmune disorders can induce primary dysfunction in the endothelial cells of the liver sinus, as well as hepatocyte necrosis. This causes angiectasis and hyperemia, producing multiple blood-filled cystic spaces in the liver parenchyma.
Clinical development of PH is not apparent, and it is difficult to detect at its onset. The diagnosis of PH is based on pathological examination and liver imaging. Imaging can disclose either intrahepatic space-occupying or diffuse lesions. MRI scans of the patient reported here showed a mass in the anterior segment of the right liver, approximately 3.8 cm × 3.1 cm in size, with irregular boundaries, that showed a weak T1 and a strong T2 signal, resulting in inhomogeneous enhancement. In addition, the diffusion weighted imaging phase showed a strong signal with increasing B values. This result is consistent with those observed in other patients with PH[11].
PH shows a broad spectrum of appearances on radiography, primarily because the demonstrations of MRI scans largely depend on the blood supply to the lesions[12]. This lack of specificity can easily result in a misdiagnosis of other hypervascular tumors[13]. In addition, PH and HCC may occur together[7]. Therefore, only pathologic findings are considered the gold standard for the diagnosis of PH.
PH is a benign vasogenic lesion, which usually can be cured by removal of the mass or part of the liver. No patient has shown recurrence or metastasis. Its special pathological structure nonetheless makes patients prone to liver rupture and hemorrhage[14] or death due to hepatic failure. The incidence of PH is relatively low, but it has high risks. Thus, it should be aggressively treated once diagnosed, in order to prevent complications. Treatment should be tailored based on the location and extent of the lesion, the damage to liver function, and whether or not serious amalgamative complications can occur.
Because of its insidious onset and insufficient specific clinical manifestations, the diagnosis of PH is often delayed, making treatment more difficult. Most patients are not diagnosed until PH develops into diffuse lesions accompanied by severe liver failure, at which point liver transplantation is life-saving[15]. The treatments for diffuse lesions without severe damage to liver function typically include finding and removing the cause (i.e., treating the primary disease, controlling infection, or stopping any associated drugs), assisted by medicines that protect liver function. Liver transplantation is indicated, however, for patients with severely diffuse lesions accompanied by hepatic failure. If focal lesions are diagnosed by laparoscopy, biopsy or fine-needle aspiration and the cause has been clearly determined, the treatment usually consists of removing the cause and closely monitoring the patient. Patients with ruptured lesions and bleeding should be promptly treated by transcatheter super-selective embolization or even surgical hemostasis (if necessary)[16,17]. If the cause is unknown or conservative treatment is ineffective, surgery should be performed. Although the lesion was focal in the patient described in this report, its space-occupying nature could not be clearly estimated. The patient had no liver function abnormality (Child-Pugh grade A) and no relevant basic disease or medical history. However, the patient had mild anemia that might be caused by hemorrhage of the lesion. Since surgical removal can effectively prevent complications such as abdominal bleeding, and can result in a clear diagnosis to guide further treatments, we decided to perform a precise hepatectomy. The long-term therapeutic effects remain to be determined.
P- Reviewer He JY S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L
| 1. | Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int. 2005;149:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Kleger A, Bommer M, Kunze M, Klaus J, Leithaeuser F, Wegener M, Adler G, Dikopoulos N. First reported case of disease: peliosis hepatis as cardinal symptom of Hodgkin’s lymphoma. Oncologist. 2009;14:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Tsirigotis P, Sella T, Shapira MY, Bitan M, Bloom A, Kiselgoff D, Levin M, Libster D, Abdul Hai A, Gesundheit B. Peliosis hepatis following treatment with androgen-steroids in patients with bone marrow failure syndromes. Haematologica. 2007;92:e106-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Perkocha LA, Geaghan SM, Yen TS, Nishimura SL, Chan SP, Garcia-Kennedy R, Honda G, Stoloff AC, Klein HZ, Goldman RL. Clinical and pathological features of bacillary peliosis hepatis in association with human immunodeficiency virus infection. N Engl J Med. 1990;323:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 169] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Izumi S, Nishiuchi M, Kameda Y, Nagano S, Fukunishi T, Kohro T, Shinji Y. Laparoscopic study of peliosis hepatis and nodular transformation of the liver before and after renal transplantation: natural history and aetiology in follow-up cases. J Hepatol. 1994;20:129-137. [PubMed] |
| 7. | Hoshimoto S, Morise Z, Suzuki K, Tanahashi Y, Ikeda M, Kagawa T, Mizoguchi Y, Sugioka A. Hepatocellular carcinoma with extensive peliotic change. J Hepatobiliary Pancreat Surg. 2009;16:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Nadal D, Zbinden R. [Illnesses caused by Bartonella. Cat-scratch disease, bacillary angiomatosis, bacillary peliosis hepatis, endocarditis]. Internist (Berl). 1996;37:890-894. [PubMed] |
| 9. | Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428-438. [PubMed] |
| 10. | Kitchell BE, Fan TM, Kordick D, Breitschwerdt EB, Wollenberg G, Lichtensteiger CA. Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med Assoc. 2000;216:519-523, 517. [PubMed] |
| 11. | Yekeler E, Dursun M, Tunaci A, Cevikbas U, Rozanes I. Diagnosing of peliosis hepatis by magnetic resonance imaging. J Hepatol. 2004;41:351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kim EA, Yoon KH, Jeon SJ, Cai QY, Lee YW, Yoon SE, Yoon KJ, Juhng SK. Peliosis hepatis with hemorrhagic necrosis and rupture: a case report with emphasis on the multi-detector CT findings. Korean J Radiol. 2007;8:64-69. [PubMed] |
| 13. | Cohen GS, Ball DS, Boyd-Kranis R, Gembala RB, Wurzel J. Peliosis hepatis mimicking hepatic abscess: fatal outcome following percutaneous drainage. J Vasc Interv Radiol. 1994;5:643-645. [PubMed] |
| 14. | Karger B, Varchmin-Schultheiss K, Fechner G. Fatal hepatic haemorrhage in a child-peliosis hepatis versus maltreatment. Int J Legal Med. 2005;119:44-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Hyodo M, Mogensen AM, Larsen PN, Wettergren A, Rasmussen A, Kirkegaard P, Yasuda Y, Nagai H. Idiopathic extensive peliosis hepatis treated with liver transplantation. J Hepatobiliary Pancreat Surg. 2004;11:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Suzuki S, Suzuki H, Mochida Y, Hirai H, Yoshida T, Ide M, Tani M, Shimura T, Morinaga N, Ishizaki M. Liver hemorrhage due to idiopathic peliosis hepatis successfully treated with hepatic artery embolization. Int Surg. 2011;96:310-315. [PubMed] |
| 17. | Omori H, Asahi H, Irinoda T, Takahashi M, Kato K, Saito K. Peliosis hepatis during postpartum period: successful embolization of hepatic artery. J Gastroenterol. 2004;39:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |