Published online Apr 14, 2013. doi: 10.3748/wjg.v19.i14.2234
Revised: January 9, 2013
Accepted: February 2, 2013
Published online: April 14, 2013
Processing time: 158 Days and 18.3 Hours
AIM: To evaluate the expression of epithelial markers of colorectal carcinogenesis in patients with long-term ulcerative colitis (UC) and primary sclerosing cholangitis (PSC) before and after transplantation.
METHODS: Eight patients with UC and PSC prior to liver transplantation (PSC-UC), 22 patients with UC after liver transplantation for PSC (OLT), 9 patients with active ulcerative colitis without PSC (UCA), 7 patients with UC in remission (UCR) and 10 controls (N) underwent colonoscopy with multiple biopsies. Specimens were analysed histologically and semi-quantitatively immunohistochemically for p53, Bcl-2 and cyclooxygenase-2 (COX-2) markers. Statistical analysis was performed by Kruskal-Wallis and Fisher’s exact tests.
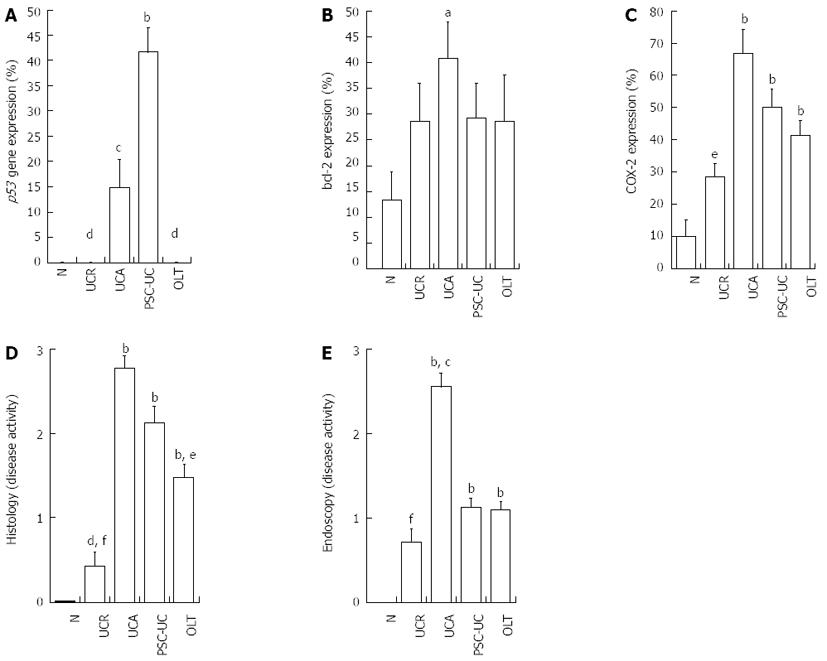
RESULTS: PSC-UC had a statistically significantly higher expression of p53 in the nondysplastic mucosa as compared to OLT, UCA, UCR and N (P < 0.05). We also found a statistically significant positive correlation between the incidence of PSC and the expression of p53 (P < 0.001). UCA had a higher p53 expression as compared to UCR. OLT had a significantly lower expression of p53 as compared with PSC-UC (P < 0.001). Bcl-2 had a significant higher bcl-2 expression as compared with controls. No difference in COX-2 expression between PSC-UC, UCR and UCA was found. UCA had higher COX-2 expression as compared to UCR. We also found a statistically significant positive correlation between the expression of COX-2 and p53. Patients after liver transplantation for PSC had a statistically significantly lower expression of the p53 compared with PSC-UC (P < 0.001). PSC-UC had the same inflammatory endoscopic activity as OLT and UCR when evaluated with the Mayo score.
CONCLUSION: Our study shows that the nondysplatic mucosa of UC patients with PSC is characterised by a higher expression of the tumour suppressor gene p53, suggesting a higher susceptibility of cancer. This p53 overexpression correlates with the presence of PSC whilst it is not present in patients with UC after liver transplantation for PSC.
- Citation: Wohl P, Hucl T, Drastich P, Kamenar D, Spicak J, Honsova E, Sticova E, Lodererova A, Matous J, Hill M, Wohl P, Kucera M. Epithelial markers of colorectal carcinogenesis in ulcerative colitis and primary sclerosing cholangitis. World J Gastroenterol 2013; 19(14): 2234-2241
- URL: https://www.wjgnet.com/1007-9327/full/v19/i14/2234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i14.2234
Patients with ulcerative colitis (UC) have an increased risk of colorectal cancer (CRC). Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease often associated with inflammatory bowel disease (IBD)[1]. PSC patients have a greater risk of potential malignant impact[1]. IBD may be diagnosed at any time during the course of PSC but, in most cases, IBD is recognised first[2]. Although both diseases run distinct courses with no direct relationship between their severities there are some features that distinguish patients with PSC and UC. Some authors have even suggested that PSC with UC represents a distinct disease phenotype[3,4].
The increased risk of CRC is associated with long-term UC, the activity of the disease, the extent of the disease, the presence of PSC and the family incidence of CRC[5,6]. In a population-based Swedish study, the cumulative incidence of CRC in UC patients with PSC was 33% at 20 years[7]. Moreover, dysplasia and cancer in patients with a combined diagnosis of PSC-UC have recently been found even in patients with a shorter duration of the disease[8]. Liver transplantation for PSC in UC patients has also been shown as a risk factor for CRC[9-12]. However, some reports have not yet confirmed this data[2,13].
Colitis-associated carcinoma (CAC) has several distinguishing clinical features when compared with sporadic colorectal carcinoma (SCC). CAC progresses to invasive adenocarcinoma from flat and nonpolypoid dysplasia more frequently than SCC. CAC may also be multifocal, likely due to the broad field-effect of mucosal inflammation contributing to the development of neoplasia[14]. Standard colonoscopy is thus insufficient in detecting flat dysplasia and regenerative changes in colonic mucosa. Consequently, multiple biopsies or advanced endoscopic techniques such as chromoendoscopy, narrow band imaging or autofluorescence have been used[14,15]. Recently, it has been indicated that early detection of premalignant changes in the nondysplastic mucosa of UC by immunohistochemistry and polymerase chain reaction methods might be possible[8] Epithelial histopathological markers of colorectal carcinogenesis, which have thus far been utilised especially in advanced dysplastic changes, may also now have clinical impact in nondysplastic mucosa[8]. To the best of our knowledge, PSC-UC patients at present have not been studied in the context of histopathological markers in nondysplastic mucosa.
The tumour suppressor gene p53 is a 53 kDa nuclear protein involved in the control of the cell cycle, apoptosis and the maintenance of genomic stability[16-18]. p53 plays an active role in both DNA repair and the induction of apoptosis[19]. It is mutated in a variety of cancers including colorectal carcinoma[20-22]. Abnormal p53 expression, detected by immunohistochemistry, is often used as a marker of p53 mutation and thus found in dysplastic or cancerous tissue. Surprisingly, high p53 expression has been found in chronic UC patients with severe disease without cancer[14,23]. Interestingly, alterations of p53 were reported to occur early in the carcinogenesis of CAC compared to SCC, where they seem to be a late event. Bcl-2 is an important antiapoptotic gene. Some of the effects of p53 may be at least partially mediated by the downregulation effect on bcl-2. Bcl-2 has been shown to be overexpressed in SCC; however, its role in CAC is uncertain.
Cyclooxygenase-2 (COX-2) is an important inflammatory mediator which might play a role in the pathophysiologic processes of inflammatory bowel disease and the development of neoplasia as well[24]. COX-2 is induced upon cellular activation by hormones, proinflammatory cytokines, growth factors and tumour promoters[25]. COX-2 overexpression occurs early in UC-associated neoplasia and the COX-2 increase cannot be explained only by inflammatory activity alone[26].
Liver disease (PSC) might influence colonic mucosa by an unknown mechanism. One of the possible explanations of this mechanism is bile acid.
The aim of this study was to evaluate the expression of epithelial markers of colorectal carcinogenesis (p53, bcl-2, COX-2) in UC patients with or without the presence of PSC and after liver transplantation for PSC and correlate this expression with clinical and histopathological parameters.
Eight patients with UC and PSC without liver transplantation (PSC-UC), 22 patients with UC after liver transplantation for PSC (OLT), 9 patients with active ulcerative pancolitis (UCA), 7 patients with UC in remission (UCR) and 10 controls (N) were included into the study (Table 1). UC activity was evaluated by the endoscopic Mayo score (0-remission, 1-mild, 2-moderate, 3-severe). The diagnosis of PSC was confirmed by ERCP or MR-CP and liver biopsy. All subjects gave their informed consent with the study protocol which had been reviewed and approved by the local ethics committee. The study was performed in accordance with the Helsinki Declaration and Title 45, Code of Federal Regulations, Part 46, Protection of Human Subjects.
| n | Age (yr) | Sex (M/F) | Duration of UC (yr) | PSC | Duration after OLT (yr) | Histology | Endoscopy score (Mayo) | |
| N | 10 | 52.2 ± 14.09 | 4/6 | 0 | No | 0 | 0 | 0 |
| UCR | 7 | 41.57 ± 13.35 | 3/4 | 9.57 ± 1.59 | No | 0 | 0.42 ± 0.4 | 0.71 ± 0.45 |
| UCA | 9 | 45.88 ± 17.62 | 5/4 | 9.56 ± 2.41 | No | 0 | 2.7 ± 0.4 | 2.5 ± 0.49 |
| PSC-UC | 8 | 37.12 ± 6.8 | 5/3 | 8.75 ± 1.56 | Yes (n = 8) | 0 | 2.1 ± 0.59 | 1.12 ± 0.33 |
| OLT | 22 | 43.33 ± 12.11 | 11/11 | 12.4 ± 5.24 | No | 5.19 ± 2.61 | 1.4 ± 0.49 | 1.09 ± 0.29 |
All patients underwent a colonoscopy with a standard white light endoscope. All UC patients, regardless of PSC diagnosis, that were included into the study suffered pancolitis. Biopsies were taken from the entire colon in 10cm intervals (approximately 40 samples). Neither dysplasia nor cancer was detected. Semiquantitative evaluation of p53, bcl-2 and COX-2 immunoreactivity was performed independently by two the hispathologists (Eva H, Eva S) in a blinded fashion. There was a general agreement between these observers. For the few discrepancies, a second evaluation was undertaken to find an agreement. Biopsies were analysed histologically and semi-quantitatively immunohistochemically for p53, bcl-2 and COX-2 with a scoring scale comparable to other studies[19,21,26,27]. The expression of antigens was analysed on 4 μm thick sections by a two-step indirect immunoperoxidase method. Slides were deparaffinised in xylene and rehydrated in graded ethanol. After deparaffinisation and rehydratation, the slides were cooked in a microwave oven (buffers used for antigen retrieval are listed in Table 2). Endogenous peroxidase was blocked by 0.3% H2O2 in 70% methanol for 30 min. Next, the specimens were incubated with a primary antibody for 30 min. The antibody was detected by incubation with a secondary antibody (Histofine Simple Stain MAX PO, Nichirei, Japan) for 30 min and incubation with Dako Liquid DAB+ Substrate-Chromogen System (DakoCytomation, Denmark). Afterwards, the specimens were counterstained with Haematoxylin and mounted in Entellan (Merck, Germany). Monoclonal antibodies (Ab) used in this study are listed in Table 2. p53 was evaluated in the intranuclear region, whereas bcl-2 and COX-2 were examined by imunohistochemistry in colonic cytoplasmatic region of the epithelial cells.
| Specificity | Origin | Company | Antigen retrieval | Dilution of antibodies |
| bcl-2 Oncoprotein | Mouse | DakoCytomation, Denmark | Buffer EDTA, pH 8 | 20 × |
| p53 | Mouse | DakoCytomation, Denmark | Tris/EDTA, pH 9 | 40 × |
| COX-2 | Mouse | Cayman, Michigan, United States | Citrate buffer, pH 6 | 40 × |
The immunohistochemistry scoring scale was based on the evaluation of the percentage of staining of positive cells, 0, no staining, 1+, mild 1%-32% of epithelial cell population, 2+, moderate from 33% to 66% of cell population and 3+, the highest staining from 67% to 100%. A positive result was considered as staining of more than 33% of the epithelial cells. Staining intensity was evaluated as weak, moderate and strong. Histological and endoscopical disease activity (Mayo score, also known as the Mayo Clinic Score and the Disease Activity Index) were evaluated (0-no inflammation, 1, mild, 2, medium and 3, severe inflammation)[28,29].
The data were evaluated using a robust Kruskal-Wallis test followed by Dunn’s multiple comparison with Bonferroni correction. The relationships between positivity in epithelial markers was evaluated by Fisher’s exact test (P value < 0.05 was considered significant). The relationships between the continuous variables were evaluated using Spearman´s correlation.
PSC-UC had a significantly higher expression of p53 in the nondysplastic mucosa as compared to OLT, UCA, UCR and N (P < 0.05) (Figure 1A). We also found a statistically significant positive correlation between the presence of PSC and the expression of p53 (P < 0.001) (Table 3). UCA had a higher p53 expression as compared to UCR (P < 0.05). Correlation between p53 expression and duration of UC did not reach significance (r = -0.014, P = 0.917, n = 55).
| PSC- | PSC+ | Row total | COX-2- | COX-2+ | Row total | |
| p53- | 43 (78.18) | 0 (0) | 43 (78.18) | 34 (61.82) | 5 (9.09) | 39 (70.91) |
| p53+ | 4 (7.27) | 8 (14.55) | 12 (21.82) | 9 (16.36) | 7 (12.73) | 16 (29.09) |
| Column total | 47 (85.45) | 8 (14.55) | 55 (100) | 43 (78.18) | 12 (21.82) | 55 (100) |
UCA had a significantly higher bcl-2 expression as compared to controls (Figure 1B).
The expression of COX-2 did not differ in PSC-UC as compared to OLT, UCA and UCR. UCA had a higher COX-2 expression as compared to UCR (P < 0.05) (Figure 1C). We also found a statistically significant positive correlation between the expression of COX-2 and p53 (P < 0.05) (Table 3).
PSC-UC, UCA and OLT did not significantly differ in histological disease activity. However, their histological activity was significantly higher when compared with UCR and N (P < 0.001) (Figure 1D). PSC-UC had the same inflammatory endoscopic activity as OLT and UCR when evaluated with the Mayo score but this activity was lower when compared with UCA (P < 0.05) (Figure 1E).
Patients after liver transplantation for PSC had a statistically significantly lower expression of the p53 gene compared with PSC-UC. These two groups of patients did not differ in the other tested parameters (bcl-2, COX-2, histology and endoscopy) (Table 4).
The presence of PSC in UC patients is generally considered as a risk factor for colorectal cancer. However, comprehension of the specific mechanisms involved in CAC pathogenesis in PSC patients remains limited. The role of colonic mucosal markers such as p53, bcl-2 and COX-2 based on immunohistochemistry evaluation in PSC-UC patients has not yet been reported.
Our study shows that PSC-UC is characterised by a higher expression of the tumour suppressor gene p53 in nondysplastic mucosa as compared with OLT, UCA, UCR and controls which suggests a higher neoplastic potential of PSC-UC. Moreover, we found a statistically significant positive correlation between the incidence of PSC and p53 expression. The observed expression of p53 is driven mainly by inflammation while it did not correlate either with histological or endoscopical activity. Surprisingly, we found a lower p53 expression in OLT when compared to PSC-UC. To our knowledge, this finding has not been previously described in the literature and suggests the hypothesis that liver disease (PSC) is associated by an unknown mechanism with increased expression of p53 in the intestinal mucosa. In addition, p53 expression correlates with higher COX-2 expression suggesting that inflammation may contribute to the amount of p53 gene expression. On the other hand, the expression of COX-2 did not differ between PSC-UC and OLT, UCA and UCR. This finding might advance the hypothesis that the COX-2 mediated inflammatory pathway could play a similar role in PSC-UC and UC patients irrespective of the presence of PSC. We have also confirmed the previously described higher expression of p53 and COX-2 in the active disease[23,30,31].
The importance of the p53 tumour suppressor gene in PSC associated carcinogenesis has been demonstrated for hepatobiliary malignancies including cholangiocarcinoma and CAC without PSC[17,32,33]. Increased p53 gene expression in the colonic mucosa in UC patients has been reported; however, patients with PSC have not been evaluated in these studies[14,15,17,29,34-38]. Our data clearly show a positive correlation between the presence of PSC and the level of expression of p53 in the intestinal nondysplastic mucosa of UC patients. These results thus support the hypothesis that PSC plays a role in UC associated colorectal carcinogenesis. We suggest that this happens, at least in part, through the overexpression of p53.
Alterations in the p53 gene predispose to colonocytes dysplasia[25]. Mutations of the p53 gene seem to occur at an early stage in CAC carcinogenesis compared to it being a late event in CRC[19,27]. Previously, we confirmed this by showing p53 overexpression in nondysplastic mucosa in a disease with high risk of cancer development. There is an ongoing debate in the literature whether p53 alterations can occur in nondysplatic epithelium. Patients with longstanding UC without dysplasia showing p53 overexpression may develop neoplasia 5 times more likely than those without[17]. Other studies reported p53 mutations in nondysplastic epithelium in patients with or without colorectal cancer[35,39]. In contrast, p53 was found only in dysplastic mucosa by others[25,36-37]. Accordingly, p53 expression clearly preceded dysplasia. It also appeared earlier in the course of the disease than previously reported[38]. However, we did not confirm this data in our study. One explanation is the small number of the tested group. In addition, the early expression of p53 in nondysplastic mucosa might make it a high risk marker of premalignant epithelium. The hypothesis of a p53 driven carcinogenesis in PSC-UC is further supported by the fact that we found a difference between p53 expression in PSC-UC patients and in patients after liver transplantation. Surprisingly, we found no p53 expression in the OLT group. Liver transplantation may contribute to the reduction of p53 gene expression by eliminating the causative liver disease (PSC?). Liver transplantation could thus be viewed as having a temporary protective effect in the CAC pathogenesis. However, this finding needs to be verified in further studies. The mechanism of this phenomenon remains unknown. It would be interesting to see whether p53 expression diminishes in the same patients following transplantation or whether it comes back as in the case of the recurrence of PSC. In a 6 year follow up of our tested group, we observed no PSC recurrence. Another factor that may contribute to the different expression is the use of immunosuppressive therapy in patients after transplantation.
The mechanisms involved in the pathogenesis of CAC may be different in patients with PSC as compared to UC alone[10,40]. The effects of hepatobiliary factors may be one explanation. Bile acids play an important role in PSC-UC[1,2,17,40]. Secondary bile acids have been shown to result in hyperproliferation and thus play a role in PSC-UC and CAC pathogenesis[1,2,17]. Reduction of the incidence of CAC was achieved in PSC-UC with the use of ursodeoxycholic acid[41,42]. Unfortunately, the effects of ursodeoxycholic acid cannot be judged from our study as all our patients with PSC, prior or after transplantation, received it.
The inflammatory theory is still considered to be important in the process of colorectal carcinogenesis in UC[14,22]. The mechanisms of COX-2 driven carcinogenesis are still not fully understood, though studies suggest that an increased expression of COX-2 as a consequence of inflammation reduces apoptosis and increases angiogenesis[26,31]. In our study, we confirmed higher COX-2 expression in UCA compared to UCR. PSC-UC did not differ in the expression of COX-2 when compared with OLT, UCA and UCR. However, PSC-UC was identical in histological inflammatory activity to UCA, but had a higher activity in comparison to UCR despite similar COX-2 expression. The COX-2 expression thus did not fully correlate with histological inflammatory activity alone. Interestingly, in the study of Agoff et al[26], COX-2 overexpression occurred early in UC-associated neoplasia; however, the cancer risk increase could not be explained solely by inflammatory activity alone. In their study, overall neoplastic change explained the majority of the variation in COX-2 expression, whereas inflammatory activity explained only 11%[26].
Bcl-2 is considered as an important antiapoptotic gene which is in reciprocal relation with p53[43]. Ilyas et al[34] have shown that bcl-2 plays an important role in UC associated carcinogenesis. We found a higher bcl-2 expression in UCA as compared to controls. Inflammation could be one possible explanation. In contrast to p53, no association with the presence of PSC was observed. We also did not find negative regulation of bcl-2 and p53 as previously described in breast cancer or adenomas. Thus, the impact of bcl-2 on colorectal cancer pathogenesis of PSC-UC based on our findings is still unclear.
We could also suggest, as other authors have, that PSC-UC might be a subgroup of UC[3,4,44]. PSC-UC is characterised by the same histological inflammatory activity as UCA but differs from UCR and N. PSC-UC had a higher p53 expression as compared to UCA, UCR, OLT and N; however, no difference between these groups was observed in COX-2 expression. PSC-UC thus shows signs of both UCA and UCR characteristics. Because of the known mild clinical course of PSC-UC as compared to UC alone, it may be underdiagnosed with unfavourable clinical consequences. Accordingly, regular colonoscopy has been recommended for all PSC patients. For that reason, p53 overexpression might be a useful predictor of potential carcinogenesis of colorectal mucosae in PSC-UC patients. In addition, according to our study, routine clinical and endoscopic indexes of colitis without PSC (e.g., Mayo, UCDAI) cannot be used in PSC-UC. The presence of PSC in patients with UC should be taken into account especially in clinical and experimental studies.
Our study has several limitations. We investigated only a small group of subjects and used the immunohistochemical method for detection of mucosal markers. It should be noted, however, that immunohistochemical investigations and mutation analysis rely on samples of mucosa obtained by colonoscopic biopsy and thus are subject to the same sampling error[27]. In addition, we may have missed some non-sense mutations resulting in a truncated protein[23]. We also did not detect dysplasia in any of our patients. However, it might have been interesting to compare the expression of these markers in nondysplastic and dysplastic mucosa. Moreover, it would have been better to analyse the same patients with PSC and UC before and after OLT. This was not possible since the PSC-UC patients had not yet undergone OLT, but these patients will be included into a subsequent study.
In conclusion, PSC-UC was characterised by a higher expression of the tumour suppressor gene p53 in nondysplatic mucosa explaining, at least in part, the higher neoplastic potential of PSC-UC. Furthermore, this overexpression was not present in UC patients who underwent liver transplantation for PSC. The expression of p53 thus correlated with the presence of PSC, suggesting a carcinogenic effect of the liver disease on colonic mucosa. The presence of p53 expression in nondysplastic mucosa may support its use as a marker of increased susceptibility to cancer that may enable detection of premalignant epithelium. It may be the use of epithelial markers of carcinogenesis which may in the future be used to better predict the risk preneoplastic lesions and CAC in UC patients with PSC and after liver transplantation. Our results need to be verified in larger future studies.
The presence of primary sclerosing cholangitis (PSC) in ulcerative colitis (UC) patients is generally considered a risk factor for colorectal cancer. However, comprehension about the specific mechanisms involved in colitis associated carcinoma (CAC) pathogenesis in PSC patients remains limited. The aim of this study was to evaluate the expression of epithelial markers of colorectal carcinogenesis in patients with long-term UC and PSC before and after transplantation.
CAC has several distinguishing clinical features when compared with sporadic colorectal carcinoma (SCC). CAC progresses to invasive adenocarcinoma from flat and nonpolypoid dysplasia more frequently than SCC. CAC may also be multifocal likely due to the broad field-effect of mucosal inflammation contributing to the development of neoplasia. Standard colonoscopy is thus insufficient in detecting flat dysplasia and regenerative changes in colonic mucosa. Recently, it has been indicated that early detection of premalignant changes in the nondysplastic mucosa of UC by immunohistochemistry and polymerase chain reaction methods might be possible. Epithelial histopathological markers of colorectal carcinogenesis, which have thus far been utilised especially in advanced dysplastic changes, may also now have clinical impact in nondysplastic mucosa. The role of colonic mucosal markers such as p53, bcl-2 and cyclooxygenase-2 (COX-2) based on immunohistochemistry evaluation in UC and PSC prior to liver transplantation (PSC-UC) patients has not yet been reported.
Data clearly show a positive correlation between the presence of PSC and the level of expression of p53 in the intestinal nondysplastic mucosa of UC patients. These results thus support the hypothesis that PSC plays a role in UC associated colorectal carcinogenesis. The authors suggest that this happens, at least in part, through the overexpression of p53.
Because of the known mild clinical course of PSC-UC as compared to UC alone, it may be underdiagnosed with unfavourable clinical consequences. Accordingly, regular colonoscopy has been recommended for all PSC patients. For that reason, p53 overexpression might be a useful predictor of potential carcinogenesis of colorectal mucosae in PSC-UC patients. In addition, according to their study, routine clinical and endoscopic indexes of colitis without PSC (e.g., Mayo, UCDAI) cannot be used in PSC-UC. The presence of PSC in patients with UC should be taken into account especially in clinical and experimental studies. The presence of p53 expression in nondysplastic mucosa may support its use as a marker of increased susceptibility to cancer that may enable detection of premalignant epithelium. It may be the use of epithelial markers of carcinogenesis which may in the future be used to better predict the risk preneoplastic lesions and CAC in UC patients with PSC and after liver transplantation.
The study shows that the nondysplatic mucosa of UC patients with PSC is characterised by a higher expression of the tumour suppressor gene p53, suggesting a higher susceptibility of cancer. This p53 overexpression correlates with the presence of PSC, whilst it is not present in patients with UC after liver transplantation for PSC.
P- Reviewer Ignazio M S- Editor Jiang L L- Editor A E- Editor Zhang DN
| 1. | Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48 Suppl 1:S38-S57. [PubMed] |
| 2. | Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [PubMed] |
| 4. | Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, Poupon R, Seksik P. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497-3503. [PubMed] |
| 5. | Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727-2737. [PubMed] |
| 6. | Lindberg BU, Broomé U, Persson B. Proximal colorectal dysplasia or cancer in ulcerative colitis. The impact of primary sclerosing cholangitis and sulfasalazine: results from a 20-year surveillance study. Dis Colon Rectum. 2001;44:77-85. [PubMed] |
| 7. | Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522-525. [PubMed] |
| 8. | Navaneethan U, Venkatesh PG, Lashner BA, Remzi FH, Shen B, Kiran RP. Temporal trends in colon neoplasms in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2012;6:845-851. [PubMed] |
| 9. | Vera A, Gunson BK, Ussatoff V, Nightingale P, Candinas D, Radley S, Mayer A, Buckels JA, McMaster P, Neuberger J. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Karlsen TH, Schrumpf E, Boberg KM. Primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2010;24:655-666. [PubMed] |
| 11. | Loftus EV, Aguilar HI, Sandborn WJ, Tremaine WJ, Krom RA, Zinsmeister AR, Graziadei IW, Wiesner RH. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis following orthotopic liver transplantation. Hepatology. 1998;27:685-690. [PubMed] |
| 12. | MacLean AR, Lilly L, Cohen Z, O’Connor B, McLeod RS. Outcome of patients undergoing liver transplantation for primary sclerosing cholangitis. Dis Colon Rectum. 2003;46:1124-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Drastich P, Wohl P, Kamenar D, Trunecka P, Spicak J. Risk of colorectal cancer in patients with ulcerative colitis after orthotopic liver transplantation for primary sclerosing cholangitis-a single center experience. JCC. 2012;6:s69. |
| 14. | Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [PubMed] |
| 16. | Harris AL. Mutant p53--the commonest genetic abnormality in human cancer? J Pathol. 1990;162:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Lashner BA, Shapiro BD, Husain A, Goldblum JR. Evaluation of the usefulness of testing for p53 mutations in colorectal cancer surveillance for ulcerative colitis. Am J Gastroenterol. 1999;94:456-462. [PubMed] |
| 18. | Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ilyas M, Talbot IC. p53 expression in ulcerative colitis: a longitudinal study. Gut. 1995;37:802-804. [PubMed] |
| 20. | Sato A, MacHinami R. p53 immunohistochemistry of ulcerative colitis-associated with dysplasia and carcinoma. Pathol Int. 1999;49:858-868. [PubMed] |
| 21. | Kinra SLP, Mehta CA, Rai LGR. Study of p53 and bcl-2 Oncoproteins in Ulcerative Colitis with Dysplasia. MJAFI. 2005;2:125-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [PubMed] |
| 23. | Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333-3337. [PubMed] |
| 24. | Paiotti AP, Artigiani Neto R, Forones NM, Oshima CT, Miszputen SJ, Franco M. Immunoexpression of cyclooxygenase-1 and -2 in ulcerative colitis. Braz J Med Biol Res. 2007;40:911-918. [PubMed] |
| 25. | Romero M, Artigiani R, Costa H, Oshima CT, Miszputen S, Franco M. Evaluation of the immunoexpression of COX-1, COX-2 and p53 in Crohn’s disease. Arq Gastroenterol. 2008;45:295-300. [PubMed] |
| 26. | Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol. 2000;157:737-745. [PubMed] |
| 27. | Brüwer M, Schmid KW, Senninger N, Schürmann G. Immunohistochemical expression of P53 and oncogenes in ulcerative colitis-associated colorectal carcinoma. World J Surg. 2002;26:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [PubMed] |
| 29. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [PubMed] |
| 30. | Laurent C, Svrcek M, Flejou JF, Chenard MP, Duclos B, Freund JN, Reimund JM. Immunohistochemical expression of CDX2, β-catenin, and TP53 in inflammatory bowel disease-associated colorectal cancer. Inflamm Bowel Dis. 2011;17:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Jackson LM, Wu KC, Mahida YR, Jenkins D, Hawkey CJ. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47:762-770. [PubMed] |
| 32. | Ahrendt SA, Rashid A, Chow JT, Eisenberger CF, Pitt HA, Sidransky D. p53 overexpression and K-ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. J Hepatobiliary Pancreat Surg. 2000;7:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44:1055-1065. [PubMed] |
| 34. | Ilyas M, Tomlinson IP, Hanby AM, Yao T, Bodmer WF, Talbot IC. Bcl-2 expression in colorectal tumors: evidence of different pathways in sporadic and ulcerative-colitis-associated carcinomas. Am J Pathol. 1996;149:1719-1726. [PubMed] |
| 35. | Holzmann K, Weis-Klemm M, Klump B, Hsieh CJ, Borchard F, Gregor M, Porschen R. Comparison of flow cytometry and histology with mutational screening for p53 and Ki-ras mutations in surveillance of patients with long-standing ulcerative colitis. Scand J Gastroenterol. 2001;36:1320-1326. [PubMed] |
| 36. | Claessen MM, Schipper ME, Oldenburg B, Siersema PD, Offerhaus GJ, Vleggaar FP. WNT-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance. Cell Oncol. 2010;32:303-310. [PubMed] |
| 37. | Wong NA, Mayer NJ, MacKell S, Gilmour HM, Harrison DJ. Immunohistochemical assessment of Ki67 and p53 expression assists the diagnosis and grading of ulcerative colitis-related dysplasia. Histopathology. 2000;37:108-114. [PubMed] |
| 38. | Rapozo DC, Grinmann AB, Carvalho AT, de Souza HS, Soares-Lima SC, de Almeida Simão T, de Paiva D, Abby F, Albano RM, Pinto LF. Analysis of mutations in TP53, APC, K-ras, and DCC genes in the non-dysplastic mucosa of patients with inflammatory bowel disease. Int J Colorectal Dis. 2009;24:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Alkim C, Savas B, Ensari A, Alkim H, Dagli U, Parlak E, Ulker A, Sahin B. Expression of p53, VEGF, microvessel density, and cyclin-D1 in noncancerous tissue of inflammatory bowel disease. Dig Dis Sci. 2009;54:1979-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Gnewuch C, Liebisch G, Langmann T, Dieplinger B, Mueller T, Haltmayer M, Dieplinger H, Zahn A, Stremmel W, Rogler G. Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World J Gastroenterol. 2009;15:3134-3141. [PubMed] |
| 41. | Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Herszényi L, Farinati F, Miheller P, Tulassay Z. Chemoprevention of colorectal cancer: feasibility in everyday practice? Eur J Cancer Prev. 2008;17:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-1805. [PubMed] |
| 44. | Jørgensen KK, Grzyb K, Lundin KE, Clausen OP, Aamodt G, Schrumpf E, Vatn MH, Boberg KM. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis. 2012;18:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |