Published online Apr 14, 2013. doi: 10.3748/wjg.v19.i14.2162
Revised: November 27, 2012
Accepted: December 22, 2012
Published online: April 14, 2013
Processing time: 167 Days and 20.7 Hours
AIM: To investigate the effects of the probiotic Bifidobacterium longum BB536 on the health management of elderly patients receiving enteral feeding.
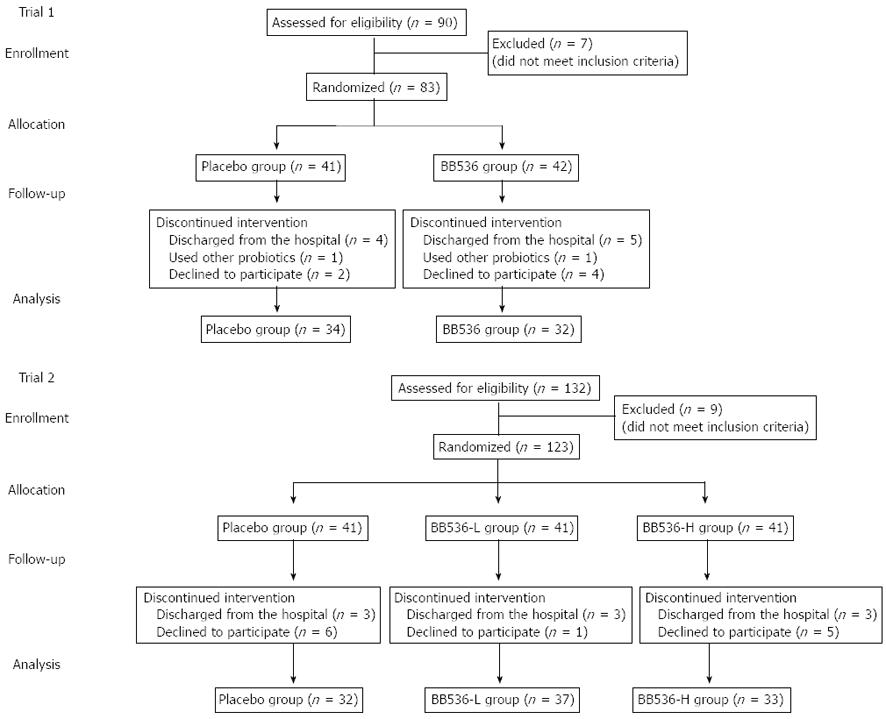
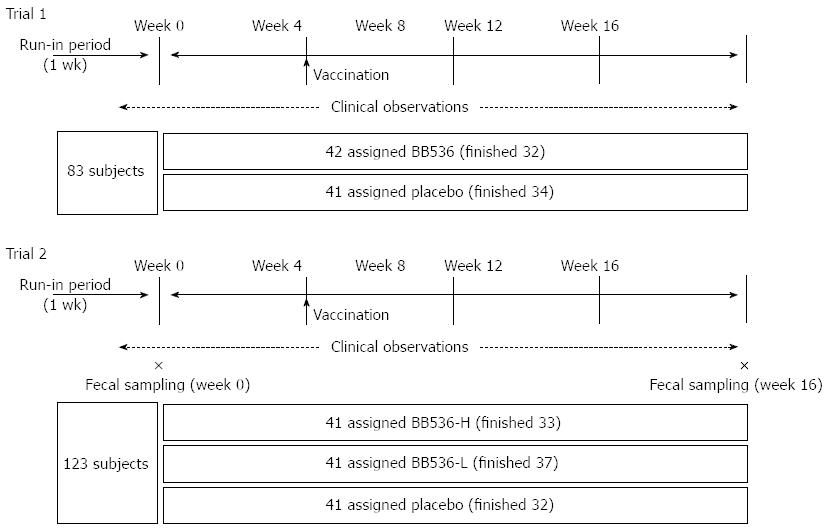
METHODS: Two double-blind, placebo-controlled trials were performed with long-term inpatients receiving enteral tube feeding at Kitakyushu Hospital Group, Fukuoka, Japan. BB536 was administered as BB536-L and BB536-H powders that contained approximately 2.5 × 1010 and 5 × 1010 cfu of BB536, respectively. In the first trial, 83 patients (age range: 67-101 years) were randomized into 2 groups that received placebo (placebo group) or BB536-H (BB536 group) powders. In the second trial, 123 patients (age range: 65-102 years) were randomized into 3 groups, and each group received placebo (placebo group), BB536-L (BB536-L group), or BB536-H (BB536-H group) powders. Each patient received the study medication for 16 wk after 1 wk of pre-observation. Fecal samples were collected from each patient prior to and after the intervention during Trial 2. Clinical observations included body temperature, occurrence of infection, frequency of defecation, and fecal microbiota.
RESULTS: No significant changes were observed in the frequency of defecation for either treatment in Trial 1. However, a significant change was noted in the BB536-L group (P = 0.0439) in Trial 2 but not in the placebo or BB536-H groups. Subgroup analyses based on the frequency of defecation for each patient during the pre-observation period for both trials revealed significant increases in bowel movements in patients with a low frequency of defecation and significant decreases in the bowel movements of patients with a high frequency of defecation during the intervention period in the BB536 groups. The combination of Trials 1 and 2 data revealed a modulatory effect of BB536 ingestion on the changes in bowel movements. Significantly increased bowel movements were observed in patients in the low frequency subgroup with significant intergroup differences (P < 0.01). Significantly decreased bowel movements were observed in patients in the high subgroup, but no significant intergroup differences were observed compared with the placebo group. BB536 ingestion increased the prevalence of normally formed stools. BB536 intake also significantly (P < 0.01) increased the cell numbers of bifidobacteria in fecal microbiota, and significant intergroup differences were observed at week 16. No adverse events were reported in any group.
CONCLUSION: Our results suggest that BB536 ingestion modulated the intestinal environment and may have improved the health care of elderly patients receiving enteral feeding.
-
Citation: Kondo J, Xiao JZ, Shirahata A, Baba M, Abe A, Ogawa K, Shimoda T. Modulatory effects of
Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding. World J Gastroenterol 2013; 19(14): 2162-2170 - URL: https://www.wjgnet.com/1007-9327/full/v19/i14/2162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i14.2162
Enteral feeding is a common method of nutritional support for patients who are unable to achieve their nutritional requirements through an oral diet alone. No accurate data exist on the number of patients who receive enteral feeding. However, the number of new patients requiring enteral feeding in 2007 was approximately 130 000 in Japan[1], and this number is expected to increase in the future. Elderly people, particularly those who are hospitalized and receiving enteral feeding, exhibit significant problems with defecation, and the consequences of constipation or diarrhea may significantly impact their quality of life[2,3]. The prevalence of constipation is generally higher in elderly individuals who reside in nursing homes or hospitals compared with elderly individuals in the community[2]. Diarrhea, which is a potential consequence of enteral feeding, is observed in 2%-95% of patients who receive this therapy[3,4].
Intestinal microbiota are the largest source of microbial stimulation in the host, and these microbiota affect mucosal and systemic immunity[5]. The composition of the intestinal microbiota in elderly people is different from that in younger adults, and the number of bifidobacteria decreases with age[6,7]. Bifidobacteria in the intestinal microbiota may exert beneficial effects in the host, such as the promotion of gut maturation and integrity, antagonism against pathogens, and immune modulation[8].
Probiotics are currently used in the prevention and treatment of disease, specifically diseases of the intestinal environment. Several studies have investigated the beneficial effects of probiotics in the management of constipation and diarrhea in elderly patients[9-11]. However, these effects may be strain-dependent, and they are not consistently observed. Therefore, further investigation is required to clarify this relationship.
The probiotic strain Bifidobacterium longum (B. longum) BB536 was originally isolated from a healthy infant, and it is used in the dairy industry as a probiotic[12]. Several studies have evaluated the effects of BB536 on the intestinal environment in healthy adults with frequent constipation[12-14]. Seki et al[15] reported that the intake of BB536-supplemented milk improved constipation and increased the prevalence of intestinal Bifidobacterium in aged individuals in a preliminary study. Moreover, BB536 intake suppresses antibiotic-induced intestinal disorders[16].
The present study investigated the efficacy of BB536 in the health care of hospitalized elderly patients receiving enteral nutrition. We performed 2 double-blind, placebo-controlled trials using a 16-wk administration of BB536 to evaluate effects on health, defecation frequency, and the bifidobacterial composition of fecal microbiota in elderly patients receiving enteral nutrition.
Subject recruitment for this study was conducted in long-stay inpatients (age > 65 years) receiving enteral tube feeding at the Kitakyushu Hospital Group (Fukuoka, Japan). The subjects or their relatives provided written informed consent. The following exclusion criteria were used: presence of diabetes, renal dysfunction, severe infectious disease, autoimmune disease, immunodeficiency, pancreatic disease, or hepatic disease prior to the start of the study. The ethics committee of the Kitakyushu Hospital Group approved all study protocols, which followed the Declaration of Helsinki.
Three types of study medications were used in the present study: placebo powder, BB536-L powder, and BB536-H powder. BB536-L and BB536-H powders contained lyophilized BB536 at doses of approximately 2.5 × 1010 and 5 × 1010 cfu, respectively, and the placebo powder contained only inactive ingredients (i.e., primarily dextrin). Each dose was supplied in an aluminum sachet (2 g), and all sachets were identical in taste and appearance.
Two trials were performed in this study, and both trials were performed using a double-blind, placebo-controlled, parallel-group design. Randomization for each group of participants was conducted using a minimization procedure to balance for gender, age, and hospital ward. The trial flows and schedules are presented in Figures 1 and 2, respectively. Routine enteral nutrition was provided to all the subjects during the trial period to maintain nutritional status. Participants, physicians, and other research staff in the study were unaware of treatment assignment. The study powder was suspended in drinking water and administered immediately after enteral feeding. The daily intake of energy and nutrients of each patient group during the trial period are summarized in Table 1. No significant differences in nutrient intake between the groups were observed.
| Group | n | Gender (M/F) | Age (yr) | Total energy (kcal/d) | Protein (g/d) | Lipid (g/d) | Carbohydrates (g/d) | Dietary fiber (g/d) | |
| Trial 1 | |||||||||
| Placebo | 32 | 9/23 | 82.7 ± 9.5 | 884.7 ± 207.2 | 37.2 ± 11.6 | 28.8 ± 9.6 | 118.8 ± 30.6 | 10.8 ± 3.5 | |
| BB536-H | 34 | 8/26 | 85.8 ± 7.3 | 917.6 ± 162.6 | 37.5 ± 7.8 | 30.2 ± 10.3 | 124.2 ± 22.5 | 10.3 ± 3.7 | |
| Trial 2 | |||||||||
| Placebo | 32 | 9/23 | 83.9 ± 7.5 | 798.1 ± 176.3 | 35.1 ± 11.9 | 24.5 ± 6.1 | 112.3 ± 31.4 | 9.6 ± 3.3 | |
| BB536-L | 37 | 9/28 | 84.4 ± 6.8 | 845.6 ± 186.9 | 37.0 ± 10.5 | 26.6 ± 9.9 | 118.0 ± 28.6 | 11.1 ± 4.6 | |
| BB536-H | 33 | 10/23 | 84.4 ± 10.1 | 854.8 ± 194.9 | 37.4 ± 10.7 | 26.0 ± 8.0 | 120.1 ± 29.9 | 10.4 ± 3.7 |
Trial 1: The first trial was performed during the winter from the end of November 2009 to the end of March 2010. This period included one week for pre-observation and 16 wk for the ingestion of study medications. A total of 83 patients were randomized into 2 groups, and each group was assigned to receive placebo (placebo group) or BB536-H powder (BB536 group) once daily.
Trial 2: The second trial was performed to confirm the results of Trial 1, investigate the dose effect of BB536, and determine any possible influences of treatment on fecal microbiota. This trial was also conducted during the winter from the end of November 2010 to the end of March 2011. The trial period included one week for pre-observation and 16 wk for study medication ingestion. A total of 123 patients were randomized into 3 groups, and each group was assigned to receive the placebo (placebo group), BB536-L (BB536-L group), or BB536-H powder (BB536-H group) twice daily. Fecal samples were collected from each patient prior to (pre-observation week) and after the intervention (week 16). Fecal samples were collected in plastic tubes, cooled immediately after collection, and stored at -20 °C until analysis.
Body temperature and the times of defecation were recorded daily. The occurrence of infection and fever and use of other medications, including antibiotics, were also recorded. A trained caregiver monitored stool characteristics during daily care, and stool form and consistency were evaluated using the Bristol Stool Form Scale. The Bristol Stool Form Scale scores range from 1 (separate hard lumps, like nuts and hard to pass) to 7 (watery, no solid pieces, entirely liquid); stools scored at 3 or 4 were considered normal stools[17].
DNA was extracted from the fecal samples as described previously[18]. Briefly, each fecal sample (20 mg) was suspended in 1.0 mL phosphate-buffered saline (PBS) and centrifuged at 14 000 ×g. The resulting pellet was washed twice with 1.0 mL PBS and resuspended in 450 μL of an extraction buffer [100 mmol/L Tris-HCl and 40 mmol/L ethylenediaminetetraacetic acid (EDTA) at pH 9.0] with 50 μL of 10% sodium dodecyl sulfate (SDS). Glass beads (300 mg, 0.1 mm diameter) and 500 μL of buffer-saturated phenol were added to the suspension, and the resulting mixture was vigorously vortexed for 30 s with a FastPrepTM FP 100A (Bio 101, Vista, CA, United States) device at a power level of 5.0. The mixture was centrifuged at 14 000 ×g for 5 min, and 400 μL of the supernatant was extracted with phenol-chloroform; 250 μL of the supernatant was precipitated with isopropanol. Purified DNA was dissolved in 200 μL of a Tris-EDTA buffer at pH 8.0.
Real-time polymerase chain reaction (PCR) was performed using an ABI PRISM® 7500 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, United States), with SYBR® Premix Ex Taq (TaKaRa Shuzo, Japan) and ROX Reference Dye II (TaKaRa Shuzo, Japan) as an internal standard. Primers for the bifidobacterial species and B. longum BB536 were used as described previously[19,20]. The amplification program consisted of 1 cycle at 94 °C for 10 s, followed by 40 cycles at 94 °C for 5 s and 60 °C for 30 s. Fluorescent products were detected at the last step of each cycle. Melting curves were obtained by heating from 60 °C to 95 °C in 0.2 °C/s increments with continuous fluorescence data collection.
Data are expressed as means ± SD or SE. Daily recorded scores for body temperature and times of defecation were averaged weekly for each individual. Changes in values from baseline (week-1) were calculated based on the weekly scores. Weekly scores or changes were further averaged every 4 wk for analysis. The frequency of each stool type was summed for the total intervention period, and the prevalence of each stool type was calculated. Cell numbers for each bacterial target are expressed as means after logarithmic transformation for each group among individuals with cell numbers that exceeded the detection limit, which was 1 × 106 per gram wet weight of feces. However, statistical analyses were conducted on cell numbers after logarithmic transformation, in which cell numbers below the detection limit were substituted with 1 × 106. For analysis of sequence differences within a group, two-sequence differences were assessed using the paired Student t-test, and multi-sequence differences were analyzed using a repeated measures analysis of variance (ANOVA), followed by Dunnett’s test for each time point against the baselines. For analysis of between group differences, two-group differences were evaluated using the Student t-test, and multi-group differences were evaluated using a non-repeated measures ANOVA, followed by the Student-Newman-Keuls test for comparisons of each group. Differences in changes from baseline between groups were evaluated using the Student t-test at each time point. P values less than 0.05 were considered statistically significant. Analyses were performed using SPSS software (Version 15.0J for Windows, Chicago, United States).
No significant differences in the baseline characteristics of patients were observed between the groups of either trial (Table 1). No significant changes in body temperature during the intervention period were observed between groups in either trial. A few patients experienced body temperatures > 38 °C and received antibiotics, but the incidence of fever was not significantly different between the groups in either trial (data not shown).
No significant changes in the frequency of defecation were observed following treatments during Trial 1 (Table 2). However, significant changes were observed in the BB536-L group in Trial 2 but not in the placebo or BB536-H groups (Table 2).
| Subgroups of subjects1 | Intervention group | Subjects(n) | Bowel movements (times/wk) | P value2 | |||||
| Week-1 | Weeks 1-4 | Weeks 5-8 | Weeks 9-12 | Weeks 13-16 | |||||
| Trial 1 | |||||||||
| Whole | Placebo | 34 | 4.88 ± 2.70 | 5.18 ± 2.57 | 5.20 ± 2.58 | 5.17 ± 2.74 | 4.8 ± 2.2 | 0.326 | |
| BB536-H | 32 | 5.53 ± 3.76 | 6.62 ± 3.83 | 6.37 ± 3.34 | 6.04 ± 3.25 | 6.0 ± 2.8 | 1.051 | ||
| Low | Placebo | 19 | 3.00 ± 0.94 | 4.07 ± 1.37a | 4.25 ± 1.94a | 4.07 ± 1.34a | 3.87 ± 1.13a | 0.002 | |
| BB536-H | 14 | 2.93 ± 0.92 | 4.41 ± 1.89b | 4.32 ± 1.85b | 4.79 ± 1.92b | 4.82 ± 1.92b | 0.001 | ||
| Normal | Placebo | 12 | 6.25 ± 1.36 | 5.46 ± 2.56 | 5.29 ± 2.07 | 5.23 ± 2.40 | 5.21 ± 2.69 | 0.564 | |
| BB536-H | 14 | 5.79 ± 1.19 | 7.02 ± 2.84 | 7.36 ± 3.16 | 6.27 ± 3.25 | 6.48 ± 2.7 | 0.340 | ||
| High | Placebo | 3 | 10.50 ± 1.00 | 11.38 ± 1.77 | 10.38 ± 0.72 | 12.5 ± 3.22 | 8.50 ± 0.58 | 0.786 | |
| BB536-H | 4 | 13.75 ± 3.77 | 12.94 ± 4.94 | 10.06 ± 3.86 | 9.63 ± 4.75 | 8.75 ± 4.20a | 0.044 | ||
| Trial 2 | |||||||||
| Whole | Placebo | 32 | 5.28 ± 3.34 | 5.02 ± 2.67 | 4.78 ± 2.54 | 4.73 ± 2.80 | 4.60 ± 2.20 | 0.563 | |
| BB536-L | 37 | 5.51 ± 4.12 | 6.10 ± 3.85 | 5.90 ± 3.43 | 5.11 ± 2.60 | 4.90 ± 3.00 | 0.044 | ||
| BB536-H | 33 | 5.91 ± 4.30 | 6.12 ± 3.89 | 6.20 ± 3.51 | 6.30 ± 3.25 | 5.60 ± 3.80 | 1.075 | ||
| Low | Placebo | 20 | 3.05 ± 0.76 | 3.73 ± 0.88 | 3.45 ± 1.05 | 3.41 ± 1.10 | 3.63 ± 1.09 | 0.387 | |
| BB536-L | 22 | 2.64 ± 1.05 | 3.69 ± 1.22b | 3.74 ± 1.42b | 3.70 ± 1.54b | 3.44 ± 1.32b | 0.001 | ||
| BB536-H | 18 | 3.00 ± 1.03 | 3.96 ± 1.33a | 4.24 ± 1.89b | 4.35 ± 2.09b | 3.83 ± 1.44a | 0.015 | ||
| Normal | Placebo | 6 | 6.83 ± 1.33 | 4.79 ± 1.16 | 5.04 ± 1.07 | 4.88 ± 1.61 | 4.79 ± 1.42 | 0.219 | |
| BB536-L | 7 | 7.00 ± 1.29 | 7.39 ± 3.15 | 7.86 ± 3.58 | 6.46 ± 1.81 | 6.61 ± 2.89 | 0.842 | ||
| BB536-H | 8 | 6.50 ± 1.41 | 6.66 ± 3.70 | 7.47 ± 3.50 | 7.03 ± 3.11 | 5.66 ± 4.18 | 1.217 | ||
| High | Placebo | 6 | 11.17 ± 0.98 | 9.54 ± 2.90 | 8.96 ± 2.54 | 9.00 ± 3.57 | 7.92 ± 2.59a | 0.149 | |
| BB536-L | 8 | 12.13 ± 2.10 | 11.59 ± 3.01 | 10.13 ± 2.12 | 7.81 ± 2.89b | 7.44 ± 4.03b | 0.006 | ||
| BB536-H | 7 | 12.71 ± 3.68 | 11.07 ± 4.08 | 9.79 ± 3.55b | 10.46 ± 3.80a | 10.25 ± 4.12a | 0.040 | ||
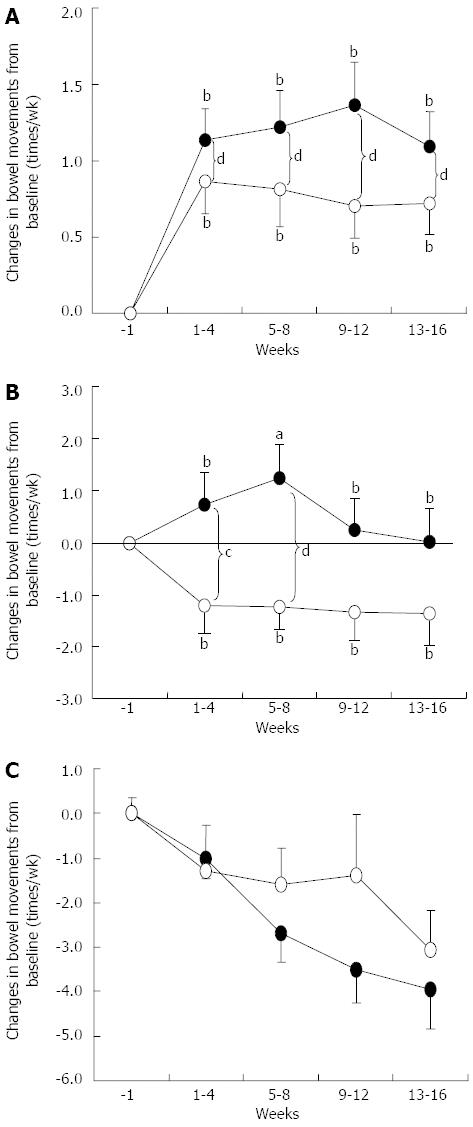
The frequency of defecation varied for each patient during the pre-observation period. Therefore, subgroup analyses were performed for patients with infrequent (low) defecation (≤ 4 times a week), normal frequency of defecation (5-9 times a week), and a high frequency of defecation (≥ 10 times a week) at baseline (week-1). We observed significant changes in the frequency of defecation in the low frequency subgroup of the placebo and BB536 groups and the high frequency subgroup of the BB536 group in Trial 1. However, no significant changes were observed in the normal frequency subgroup of either the placebo or BB536 group or the high frequency subgroup of the placebo group during treatment (Table 2). Defecation frequency increased significantly after treatment in the low frequency subgroups of both the placebo and BB536 groups, and the frequency tended to be higher (P < 0.1) in the BB536 group compared with the placebo group at weeks 13-16. In contrast, defecation frequency decreased after treatment in the high frequency subgroup of the BB536 group but not in the placebo group, and significant differences were observed at weeks 9-12 and 13-16 in the BB536 group (Table 2).
Significant changes were observed in the frequency of defecation in the low and high frequency subgroups of the BB536 group but not the placebo group in Trial 2 (Table 2). No significant changes in the normal frequency subgroups of any of the three treatment groups were observed (Table 2). Defecation frequency increased significantly after treatment in the low frequency subgroups of both the BB536-L and BB536-H groups, and a trend for a difference was noted in the BB536-H group compared with the placebo group at weeks 9-12 (P < 0.1). In contrast, defecation frequency decreased after treatment in the high frequency subgroups of both the BB536-L and BB536-H groups. Significant differences were observed at weeks 9-12 and 13-16 in the BB536-L group and weeks 5-8, 9-12, and 13-16 in the BB536-H group (Table 2).
Figure 3 summarizes the changes in defecation frequency for the three subgroups in the two trials. Defecation frequency increased significantly in the low frequency subgroup of both placebo (n = 39) and BB536 (n = 54) groups. However, the frequency was significantly higher in the BB536 group compared with the placebo group. Defecation frequency increased significantly at weeks 5-8 in the normal frequency subgroup of the BB536 group (n = 29). However, defecation frequency decreased significantly during the intervention period in the placebo group (n = 18), and significant intergroup differences were observed at weeks 1-4 and 5-8. In contrast, defecation frequency decreased during the intervention period in the high frequency subgroup at weeks 5-8, 9-12, and 13-16 for the BB536 group (n = 19) but only at weeks 13-16 for the placebo group (n = 9). However, no significant intergroup differences were observed due to the small number of patients.
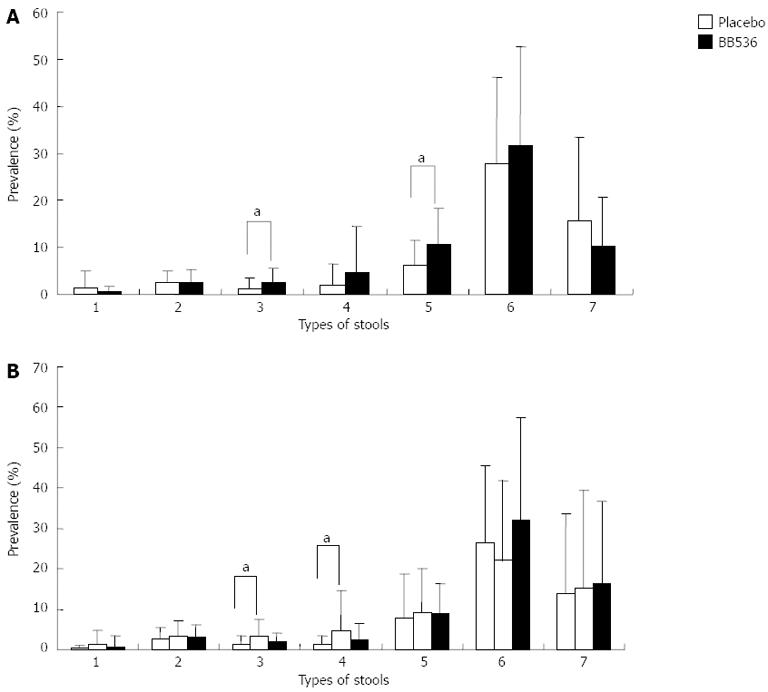
Figure 4 presents the incidence of each stool type during the intervention. A significantly higher incidence of stool type 3 (i.e., like a sausage but with cracks on its surface) and type 5 (soft blobs with clear-cut edges that could be passed easily) was observed in the BB536 group than in the placebo group in Trial 1. A significantly higher incidence of stool types 3 and 4 (like a sausage or snake, smooth and soft) was observed in the BB536-L group compared with the placebo group in Trial 2.
Real-time polymerase chain reaction analyses revealed that the cell numbers of total bifidobacteria, B. longum subsp. longum, and BB536 increased significantly after treatment in all 3 groups, and the cell numbers of these bacterial groups were significantly higher in the BB536 groups than in the placebo group (Table 3). The cell numbers of (Bifidobacterium breve) B. breve and B. longum subsp. infantis were significantly higher in the BB536-H group after treatment than before treatment. In addition, the cell numbers of (Bifidobacterium adolescentis) B. adolescentis were significantly higher in the BB536-H group than the placebo group at week 16. No differences in the cell numbers of the other dominant species of Bifidobacterium were observed after treatment.
| Species of Bifidobacterium | Period | mean (log/g) ± SD (prevalence, %) | ||
| Placebo | BB536-L | BB536-H | ||
| All Bifidobacterium | Week-1 | 8.27 ± 1.32 (57.6) | 8.68 ± 1.26 (62.2) | 8.58 ± 0.94 (36.1) |
| Week 16 | 8.41 ± 1.29 (78.8)a | 9.05 ± 0.91 (94.6)bc | 8.94 ± 0.75 (94.4)bc | |
| B. longum subsp. longum | Week-1 | 6.91 ± 0.41 (15.2) | 7.40 ± 0.88 (27) | 7.29 ± 0.9 (30.6) |
| Week 16 | 7.56 ± 0.94 (39.4)b | 8.13 ± 0.74 (94.6)bd | 8.26 ± 0.65 (91.7)bd | |
| B. adolescentis | Week-1 | ND (0) | ND (0) | 10.06 (2.8) |
| Week 16 | 6.64 ± 0.15 (36.4) | 6.57 ± 0.21 (24.3) | 6.97 ± 1.14 (25) | |
| B. catenulatum | Week-1 | 11.12 ± 2.24 (9.1) | 12.27 ± 3.01 (8.1) | 12.88 ± 1.2 (5.6) |
| Week 16 | 9.08 ± 0.36 (6.1) | 8.98 ± 0.28 (8.1) | 8.74 ± 0.05 (5.6) | |
| B. breve | Week-1 | 7.91 ± 0.96 (39.4) | 8.04 ± 0.87 (48.6) | 7.84 ± 0.49 (22.2) |
| Week 16 | 7.77 ± 1.00 (57.6) | 8.34 ± 0.84 (48.6) | 7.82 ± 0.82 (50.0)b | |
| B. bifidum | Week-1 | ND (0) | 8.63 (2.7) | ND (0) |
| Week 16 | 9.2 ± 0.66 (6.1) | 7.81 ± 1.30 (5.4) | 7.44 ± 1.24 (8.3) | |
| B. longum subsp. infantis | Week-1 | 8.8 ± 0.46 (9.1) | 8.00 ± 1.12 (13.5) | 8.94 ± 0.05 (8.3) |
| Week 16 | 7.71 ± 1.56 (18.2) | 7.91 ± 1.04 (16.2) | 8.16 ± 0.97 (25)a | |
| BB536 | Week-1 | 6.42 (3) | 6.71 (2.7) | 6.92 ± 0.26 (5.6) |
| Week 16 | 6.98 ± 0.77 (24.2)a | 7.97 ± 0.70 (89.2)bd | 8.13 ± 0.63 (91.7)bd | |
The present results revealed obvious effects of BB536 therapy vs placebo in the normalization of defecation frequency in patients who exhibited low and high frequencies of defection. BB536 administration increased the incidence of close-to-normal stools (types 3-5, Figure 3), which is consistent with the results for defecation frequency. BB536 administration also increased the cell population of bifidobacteria in the microbiota of elderly patients.
The pathogenesis of constipation and diarrhea are multifactorial, and the definition of constipation and diarrhea requires the presence of clinical symptoms and changes in the frequency of defecation[21]. A careful diagnosis was not possible in the present study because the stools were monitored during daily care. Therefore, we could not classify low or high defecation frequencies as constipation or diarrhea, respectively. However, a defecation frequency ≤ 4 times per week may be considered mild constipation[22]. The present results suggested a modulatory effect of BB536 in the improving of bowel movements in individuals with a low and high frequency of defecation, which normalized the frequency of defecation.
The effects of BB536 on fecal microbiota were investigated in Trial 2. We focused on the types of Bifidobacteria that are the major components of intestinal microbiota in humans and provide beneficial effects to human health[23]. The number of intestinal bifidobacteria decreases with age[6,7,24]. The populations of bifidobacteria in feces increased significantly after probiotic ingestion in the present study. These results confirmed previous findings that the ingestion of yogurt containing BB536 increased the population of bifidobacteria in healthy adults with a tendency toward constipation[13,14]. The administered strain was the primary contributor to this increase in the bifidobacterial microbiota population. However, increases in the cell numbers of B. breve and B. adolescentis were also observed in the BB536-H group. These results suggest the potential of BB536 administration in the modulation of the intestinal environment, which enhanced the proliferation of endogenous bifidobacterial species.
Trial 2 was performed to confirm the results of Trial 1 (i.e., the beneficial effects on defecation frequency) and investigate the dose effect of BB536. We confirmed the effect of BB536 ingestion on defecation frequency in both trials. However, no significant differences in defecation frequency, stool types, or fecal microbiota were observed between the BB536-L and BB536-H groups, likely because the dose of the probiotic was only doubled. Further studies are required to investigate the dose response of BB536 using a broader dose range.
The present study had several strengths, including randomized treatment allocation, use of placebo controls, assessment of dose effect, evaluation using two successive studies, and evaluation of fecal microbiota during the study. This study also had several limitations, as previously discussed for other probiotic strains[25]. The results presented herein are applicable only to B. longum BB536 and cannot be generalized to other probiotic strains or products. Caution should be exercised in extrapolating these study outcomes to individuals with chronic and/or severe gastrointestinal complications. Another limitation may be the mild effect of the treatment compared with other therapies, such as prokinetics and laxatives, particularly when cost-effectiveness is considered. However, the clinical implications of prokinetic agents are controversial[26]. In contrast, probiotics are considered to be generally safe. Furthermore, as shown in the present study, B. longum BB536 showed a modulatory effect in improving the bowel movements of patients receiving enteral feeding whose bowel movements and frequency were not normal, i.e., patients having either constipation or diarrhea. Such effects would contribute to an improved quality of life in the patients and a decreased burden of care for nurses or caregivers. In addition, although an immunoprotective effect was not observed in the present study because no patient experienced influenza infection during the study period, other studies have suggested immune-modulating and anti-infectious effects of BB536[28,29]. In the present study, we found that probiotic ingestion increased bifidobacteria in the microbiota. In addition, several studies have demonstrated the effects of administration of BB536 in eliminating harmful bacteria[13,14,30]. Based on these findings, we consider that this probiotic may represent an alternative strategy in the treatment of gastrointestinal disorders and health management in the elderly.
In conclusion, the present findings revealed that the 16-wk long-term ingestion of the probiotic BB536 strain modulated bowel movements and normalized defecation frequency in elderly patients receiving enteral feeding. BB536 administration also significantly increased the population of bifidobacteria in the intestinal microbiota. No adverse effects were associated with the ingestion of BB536. Overall, these results suggest that BB536 ingestion may improve health care in the elderly.
We thank the patients and their guardians for their cooperation and the medical staff and attending physicians for their participation.
Elderly individuals, particularly patients who are hospitalized and receiving enteral nutrition, exhibit significant problems in defecation, which may impact on quality of life due to constipation or diarrhea. The development of novel therapeutic strategies is necessary to treat these patients more effectively, and probiotics are increasingly used as one alternative in the management of constipation.
Several studies have investigated the beneficial effects of probiotics in the management of constipation and diarrhea in elderly patients. However, these effects may be strain-dependent, and they are not consistently observed. Therefore, further investigation is required to clarify this relationship. The present study investigated the efficacy of a probiotic Bifidobacterium strain in the health management of hospitalized elderly patients receiving enteral nutrition in two double-blind, placebo-controlled trials following a 16-wk administration of BB536.
Authors demonstrated effects of Bifidobacterium longum BB536 therapy vs placebo in the normalization of defecation frequency in patients who exhibited low and high frequencies of defection and increased the cell population of bifidobacteria in fecal microbiota.
The results of the present clinical trials suggest that the ingestion of the probiotic Bifidobacterium BB536 is an alternative strategy for the treatment of gastrointestinal disorders in the elderly.
This is a formal good study of double-blind, placebo-controlled trials. The authors should discuss if probiotics should become part of regular EN in the elderly. In conclusion, I think that this had a good study design with interesting results for therapy.
P- Reviewer Dormann J S- Editor Gou SX L- Editor Cant MR E- Editor Zhang DN
| 1. | Takezako Y, Kajii E. [National study on acceptance by Japanese nursing homes of patients with feeding tubes]. Nihon Ronen Igakkai Zasshi. 2010;47:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Bharucha AE. Constipation. Best Pract Res Clin Gastroenterol. 2007;21:709-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Cataldi-Betcher EL, Seltzer MH, Slocum BA, Jones KW. Complications occurring during enteral nutrition support: a prospective study. JPEN J Parenter Enteral Nutr. 1983;7:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 196] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | DeMeo M, Kolli S, Keshavarzian A, Borton M, Al-Hosni M, Dyavanapalli M, Shiau A, Tu N, Frommel T, Zarling E. Beneficial effect of a bile acid resin binder on enteral feeding induced diarrhea. Am J Gastroenterol. 1998;93:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Macfarlane GT, Cummings JH. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? West J Med. 1999;171:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 709] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 8. | Blum S, Schiffrin EJ. Intestinal microflora and homeostasis of the mucosal immune response: implications for probiotic bacteria? Curr Issues Intest Microbiol. 2003;4:53-60. [PubMed] |
| 9. | Tanaka R, Shimosaka K. [Investigation of the stool frequency in elderly who are bed ridden and its improvements by ingesting bifidus yogurt]. Nihon Ronen Igakkai Zasshi. 1982;19:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Riezzo G, Orlando A, D’Attoma B, Guerra V, Valerio F, Lavermicocca P, De Candia S, Russo F. Randomised clinical trial: efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation--a double-blind, controlled, crossover study. Aliment Pharmacol Ther. 2012;35:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Zaharoni H, Rimon E, Vardi H, Friger M, Bolotin A, Shahar DR. Probiotics improve bowel movements in hospitalized elderly patients--the PROAGE study. J Nutr Health Aging. 2011;15:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Xiao JZ. Bifidobacterium longum BB536. Hanbook of Probiotics and Prebiotics, 2nd ed. New Jersey: John Wiley & Sons 2009; 488-491. |
| 13. | Ogata T, Nakamura T, Yaeshima T, Takahashi S, Fukuwatari Y, Ishibashi N, Fujisawa T, Iino H. Effect of Bifidobacterium longum BB536 administration on the intestinal environment, defecation frequency and fecal characteristics of human volunteers. Bioscience Microflora. 1997;16:53-58. |
| 14. | Yaeshima T, Takahashi S, Matsumoto N, Ishibashi N, Hayasawa H, Iino H. Effect of yogurt containing Bifidobacterium longum BB536 on the intestinal environment, fecal characteristics and defecation frequency: A comparison with standard yogurt. Bioscience Microflora. 1997;16:73-77. |
| 15. | Seki M, Igarashi M, Fukuda Y, Shimamra S, Kawashima T, Ogata K. The effect of Bifidobacterium cultured milk on the “Regularity” among an aged group. J Jap Soc Nutr Food Sci. 1978;34:379–387. |
| 16. | Colombel JF, Cortot A, Neut C, Romond C. Yoghurt with Bifidobacterium longum reduces erythromycin-induced gastrointestinal effects. Lancet. 1987;2:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | O’Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300:439-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 382] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Odamaki T, Xiao JZ, Iwabuchi N, Sakamoto M, Takahashi N, Kondo S, Iwatsuki K, Kokubo S, Togashi H, Enomoto T. Fluctuation of fecal microbiota in individuals with Japanese cedar pollinosis during the pollen season and influence of probiotic intake. J Investig Allergol Clin Immunol. 2007;17:92-100. [PubMed] |
| 19. | Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Gianotti L, Morelli L, Galbiati F, Rocchetti S, Coppola S, Beneduce A, Gilardini C, Zonenschain D, Nespoli A, Braga M. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol. 2010;16:167-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Bliss DZ, Guenter PA, Settle RG. Defining and reporting diarrhea in tube-fed patients--what a mess! Am J Clin Nutr. 1992;55:753-759. [PubMed] |
| 22. | Sairanen U, Piirainen L, Nevala R, Korpela R. Yoghurt containing galacto-oligosaccharides, prunes and linseed reduces the severity of mild constipation in elderly subjects. Eur J Clin Nutr. 2007;61:1423-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Mitsuoka T, Kaneuchi C. Ecology of the bifidobacteria. Am J Clin Nutr. 1977;30:1799-1810. [PubMed] |
| 24. | Benno Y, Endo K, Mizutani T, Namba Y, Komori T, Mitsuoka T. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl Environ Microbiol. 1989;55:1100-1105. [PubMed] |
| 25. | Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Pfab F, Nowak-Machen M, Napadow V, Fleckenstein J. Alternatives to prokinetics to move the pylorus and colon. Curr Opin Clin Nutr Metab Care. 2012;15:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Bloch F, Thibaud M, Dugué B, Brèque C, Rigaud AS, Kemoun G. Laxatives as a risk factor for iatrogenic falls in elderly subjects: myth or reality? Drugs Aging. 2010;27:895-901. [PubMed] |
| 28. | Namba K, Hatano M, Yaeshima T, Takase M, Suzuki K. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci Biotechnol Biochem. 2010;74:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Akatsu H, Iwabuchi N, Xiao JZ, Matsuyama Z, Kurihara R, Okuda K, Yamamoto T, Maruyama M. Clinical Effects of Probiotic Bifidobacterium longum BB536 on Immune Function and Intestinal Microbiota in Elderly Patients Receiving Enteral Tube Feeding. JPEN J Parenter Enteral Nutr. 2012;Epub ahead of print. [PubMed] |
| 30. | Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao JZ. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |