Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1890
Revised: August 29, 2012
Accepted: September 28, 2012
Published online: March 28, 2013
Processing time: 242 Days and 2.2 Hours
Accurate determination of the presence and degree of fibrosis in liver is of great importance, because the prognosis and management strategies for chronic liver disease depend mainly on these factors. To date, liver biopsy (LB) remains the “gold standard” for assessing the severity of liver fibrosis; however, LB is often limited by its invasiveness, sampling error, and intra/inter-observer variability in histological interpretation. Furthermore, repeated LB examinations within a short time interval are indeed ineligible in a real clinical practice. Thus, due to the pressing need for non-invasive surrogates for liver fibrosis, transient elastography (TE), as a novel ultrasound based technology, has allowed a noninvasive measurement of liver stiffness and has gained in popularity over recent years. In the past few years, additional roles for transient TE beyond the initial purpose of a non-invasive surrogate for LB have included the prediction of the most two critical consequences of fibrosis progression: the development of portal hypertension-related complications and hepatocellular carcinoma. This indicates that the role of transient TE is not merely limited to reducing the need for LB, but transient TE can enable the establishment of tailored management strategies by providing more detailed prognostic information. In particular, under the concept in which the clinical course of liver fibrosis is dynamic and bidirectional, especially when appropriate intervention is commenced, transient TE can be used to track the dynamic changes in fibrotic burden during antiviral or antifibrotic treatment. This review discussed extended applications of transient TE in prediction of the development of real clinical endpoints from a longitudinal perspective.
- Citation: Kim BK, Fung J, Yuen MF, Kim SU. Clinical application of liver stiffness measurement using transient elastography in chronic liver disease from longitudinal perspectives. World J Gastroenterol 2013; 19(12): 1890-1900
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1890
The prognosis and management of chronic liver disease (CLD) depend mainly on the amount and progression of liver fibrosis, which is defined as the excessive accumulation of extracellular matrix proteins, resulting from chronic liver insults[1,2]. The initiation of its deposition is an important phase of CLD. As liver fibrosis eventually progresses without appropriate intervention, this process will lead to architectural change of the liver, followed by deterioration of liver function and hemodynamics, complications due to portal hypertension, and an increased tendency for hepatocarcinogenesis[3].
Thus, accurate determination of the presence and degree of liver fibrosis is of paramount importance in choosing treatment strategies, evaluating responses to treatment and the risks of developing liver-related complications, and predicting prognosis in patients with CLD. To assess the severity of liver fibrosis, liver biopsy (LB) remains the “gold standard”. However, LB is often limited by its invasiveness and rare, but serious, complications, including bleeding, pneumothorax, and procedure-related death[4,5]. Moreover, repeated LB examinations within a short time interval are impractical. Additionally, concerning the reliability of pathological examinations, not only sampling error inherent in the percutaneous approach, but also intra- and inter-observer variability in histological interpretation may still occur[6]. Even if the LB is performed by an experienced physician and interpreted by an expert pathologist, it has an up to 20% error rate in disease staging[7,8].
Ideally, a method of evaluating liver fibrosis should accurately determine the presence of significant fibrosis, and be readily available, highly reproducible, and widely applicable to liver diseases of various etiologies. Although LB does not fulfil all these criteria, it has remained the gold standard, likely due to the absence of a better alternative. Recently, liver stiffness measurement using transient elastography (TE) was introduced as a promising non-invasive method for assessment of liver fibrosis[9-15]. In many studies, TE proved to be a reliable and accurate surrogate for LB in terms of prediction of significant fibrosis or cirrhosis[8,16-19]. In a large-scale meta-analysis including 50 studies, the mean areas under the receiver operating characteristic curves (AUROCs) for the diagnosis of significant fibrosis and cirrhosis were 0.84 and 0.94, respectively, with optimal cutoff values of 7.6 and 13.1 kPa, respectively[20].
Most studies to date have focused on assessing the performance of TE, reflected by AUROC, from a cross-sectional perspective, with reference to histological fibrosis. However, because LB as a reference standard is imperfect, it may have only limited clinical implications in terms of increasing the AUROC of TE to 1 (i.e., perfect concordance with LB). Thus, additional roles for TE, namely prediction of long-term prognosis of the disease and monitoring clinical courses, have recently begun to attract attention. This indicates that the role of TE is not merely limited to lessening the frequency of unnecessary LB, but TE can also enable establishment of tailored management strategies by providing more detailed prognostic information[21]. In this regard, the “classical” end-points of “static” liver fibrosis in recent cross-sectional studies on TE are shifting to the “real and solid” end-points of the development of clinical events related to liver fibrosis progression, including hepatic decompensation, hepatocellular carcinoma (HCC), or liver-related death in a longitudinal study from a prospective cohort with long-term follow-up. Additionally, the performance of non-invasive methods is being judged and compared from this viewpoint.
In this article, we reviewed recent studies that focused on the prognostic value of TE for prediction of clinical end-points related to liver fibrosis progression, such as decompensation events, HCC development, or liver-related death, from a longitudinal perspective.
The development of portal hypertension is a common consequence of fibrosis progression, leading to the formation of esophageal and gastric varices responsible for variceal bleeding, and other severe complications, such as portosystemic encephalopathy, spontaneous bacterial peritonitis and sepsis[22-24]. Measurement of the hepatic venous pressure gradient (HVPG) is the gold standard for portal hypertension assessment in patients with cirrhosis; however, it is invasive and is routinely available only in experienced centers[25-29]. Although TE was initially proposed for assessment of liver fibrosis, a good correlation between TE values and HVPG has been reported, as well as the presence of esophageal varices, suggesting that it may be a valuable tool for the non-invasive evaluation of portal hypertension[30-32]. Subsequent studies have investigated correlations between TE values and the hepatic decompensation due to increased portal hypertension. A significant correlation between TE values and portal hypertension, expressed as the HVPG, was reported by Vizzutti et al[33] suggesting that TE may reflect a progressive rise in portal pressure due primarily to increased hepatic vascular resistance, caused by fibrillar extracellular matrix accumulation. Based on this concept, Foucher et al[34] first reported that cutoff values of 27.5, 37.5, 49.1, 53.7 and 62.7 kPa had > 90% negative predictive values for the presence of large esophageal varices (stage 2/3), Child-Pugh score B or C, past history of ascites, HCC and esophageal bleeding, respectively.
As variceal bleeding is a life-threatening complication of portal hypertension, the relationship between TE values and the presence of esophageal varices has been investigated in several studies[35-40]. All demonstrated a significant correlation between TE values and the presence of esophageal varices and that TE values could predict the presence of large varices (more than grade 2)[38,40]. Table 1 summarizes reports of the relationship between TE values and esophageal varices[33,38,40-44].
| Ref. | No. of patients (etiology) | Endpoints | AUROC | Cutoffs (kPa) | Sensitivity | Specificity | PPV | NPV |
| Vizzutti et al[33] | 47 (HCV) | EV | 0.76 | 17.6 | 90% | 43% | 77% | 66% |
| Castéra et al[38] | 70 (HCV) | EV | 0.84 | 21.5 | 76% | 78% | 68% | 84% |
| Large EV | 0.87 | 30.5 | 77% | 85% | 56% | 94% | ||
| Kazemi et al[40] | 165 (CLD) | EV | 0.83 | 13.9 | 95% | 43% | 57% | 91% |
| Large EV | 0.84 | 19.0 | 91% | 60% | 48% | 95% | ||
| Bureau et al[41] | 89 (CLD) | EV | 0.85 | 21.1 | 84% | 71% | NA | NA |
| Large EV | 0.76 | 29.3 | 81% | 61% | NA | NA | ||
| Pritchett et al[42] | 211 (CLD) | EV | 0.74 | 19.5 | 76% | 66% | 56% | 82% |
| Large EV | 0.76 | 19.8 | 91% | 56% | 91% | 55% | ||
| Nguyen-Khac et al[43] | 183 (CLD) | Large EV | 0.76 | 48.0 | 73% | 73% | 44% | 90% |
| 58 (HCV/HBV) | Large EV | 0.73 | 19.8 | 89% | 55% | 27% | 97% | |
| 103 (alcohol) | Large EV | 0.77 | 47.2 | 85% | 64% | 44% | 93% | |
| Malik et al[44] | 124 (CLD) | EV | 0.85 | 20.0 | NA | NA | 80% | 75% |
Although TE can predict the presence of esophageal varices and consequently assist in selection of candidates for endoscopic screening or prophylactic treatment, several issues remain unresolved. First, the cutoff values (range, 13.9-21.5 kPa) and performance of TE varied (AUROC range, 0.76-0.85) among studies[38-40]. Second, from data currently available, diagnostic performances of TE are acceptable for the prediction of esophageal varices, but far from satisfactory for screening cirrhotic patients without endoscopy confidently. Thus, Kim et al[45] recently proposed a novel prediction model [liver stiffness-spleen diameter to platelet ratio score (LSPS)] to address this issue, achieving higher accuracy using TE values and other parameters simultaneously that reflect portal hypertension as constituent variables. Overall, this model had excellent diagnostic accuracy for the prediction of high-risk esophageal varices (HEV, AUROC = 0.953; negative predictive value 94.7%, positive predictive value 93.3%).
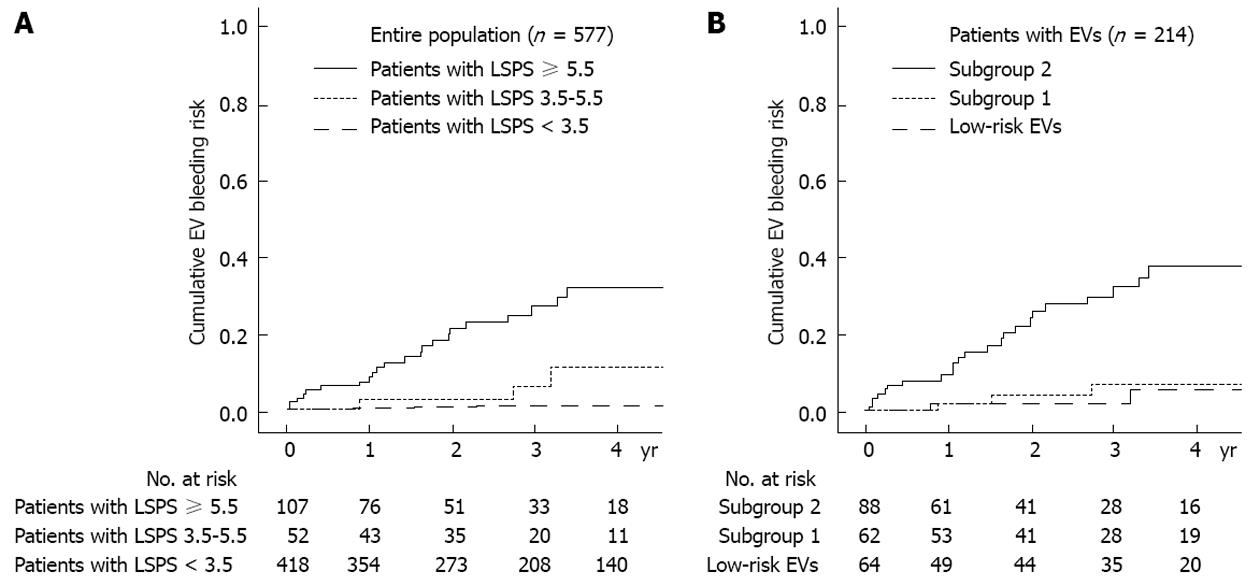
Beyond this cross-sectional analysis, a subsequent study by the same group recently showed that LSPS can be a reliable predictor of the development of variceal bleeding[20]. In this prospective, longitudinal study analyzing 577 patients with hepatitis B virus-related cirrhosis, those with LSPS ≥ 5.5 had higher cumulative incidences of esophageal variceal bleeding during the follow-up period and LSPS score ≥ 6.5 was an independent risk factor of variceal bleeding among those with HEV, indicating that further prophylactic treatment such as endoscopic ligation in addition to a non-selective beta-blocker should be considered in these high-risk patients (Figure 1). In a similar context, Kim et al[46] stratified the risk of hepatic decompensation, such as ascites, hepatic encephalopathy, variceal hemorrhage, and deterioration of liver function to Child-Pugh class B or C, based upon three classes of TE values (TE value < 13, 13-18 and ≥ 18 kPa) in histologically proven hepatitis B virus-related cirrhosis with well-preserved liver function and no history of decompensation. In a multivariate analysis, patients with a TE value of 13-18 kPa [hazard ratio (HR), 4.547; P = 0.044] and ≥ 18 kPa (HR, 12.446; P < 0.001) showed independently higher risks than patients with TE value < 13 kPa.
Another promising area for the application of TE, other than portal hypertension-related decompensation events, is the prediction of HCC development. Unless HCC is diagnosed at an early stage, a poor prognosis is expected due to the limited treatment options[47-51]. Thus, early prediction of HCC development is of great importance, especially in high-risk patients. Among traditional risk factors, advanced liver fibrosis and cirrhosis is known to have a close association with risk of HCC development[47]. Thus, assessment of the severity of liver fibrosis at a given time point with subsequent monitoring of liver fibrosis progression by serial check-up is essential for effective and optimized surveillance strategies for the early detection of HCC[3].
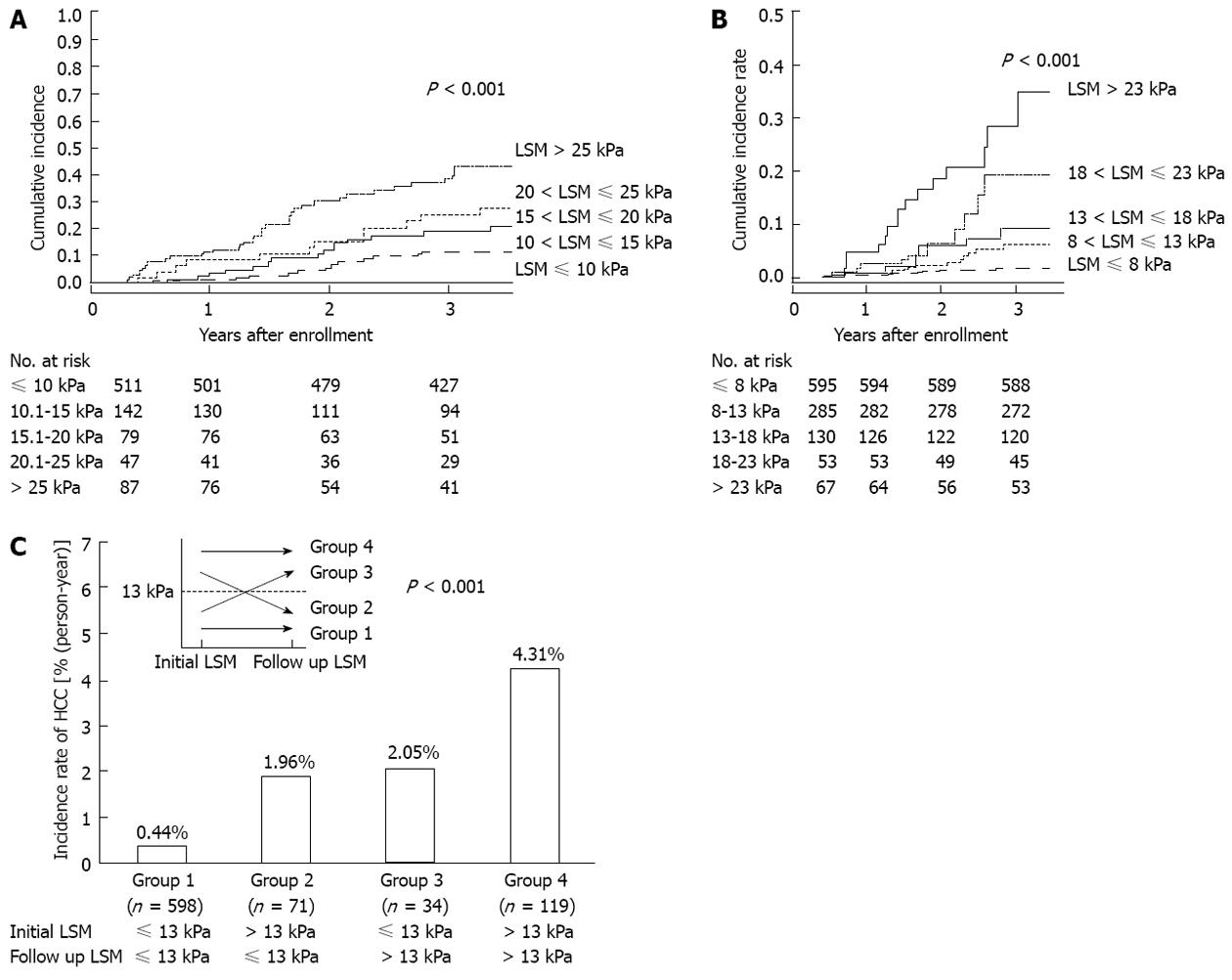
Recently, several Asian studies have investigated the clinical role of TE in the noninvasive prediction of HCC development[52-56]. The first large prospective cohort study of 866 Japanese patients with chronic hepatitis C (CHC) tested whether TE can predict the future development of HCC[52]. During a mean follow-up of 3 years, 77 patients developed HCC. By multivariate analysis, together with age, male gender, and clinical cirrhosis, stratified TE value was identified as an independent risk factor for HCC development, with relative risks of 16.7, 20.0, 25.6 and 45.5 for TE values of 10-15, 15-20, 20-25 and > 25 kPa, respectively, vs an TE value of < 10 kPa as the reference and the cumulative incidence of HCC showed a step-wise increase according to stratified TE value (Figure 2A). Despite there being no histological analysis in relation to TE values and inclusion of patients with high alanine aminotransferase (ALT) levels [> 5× upper limit of normal (ULN)] both of which can attenuate the accuracy of TE, this study confirmed that severity of liver fibrosis, reflected by higher TE values, was closely associated with higher risk of HCC development and suggested a clinical role for TE in a longitudinal setting using HCC development as a solid clinical endpoint. Interestingly, in this study, even patients with not so high level of TE (10-15 kPa) were still more subject to HCC development with an adjusted HR of 16.7, compared to those with a TE value < 10 kPa.
Another large Korean cohort study with 1130 patients with chronic hepatitis B (CHB) also confirmed the longitudinal role of TE on HCC development[53]. Together with age, male gender, heavy alcohol consumption, lower serum albumin, and HBeAg positivity, stratified TE value was identified as an independent risk factor for HCC development, with relative risks of 3.07, 4.68, 5.55 and 6.60 for liver stiffness measurement (LSM) values of 8-13, 13-18, 18-23 and > 23 kPa, respectively, when compared with a LSM value of < 8 kPa as a reference (Figure 2B). In contrast to the Japanese study[52], several additional issues were further analyzed in this Korean study. First, when the diagnosis of cirrhosis showed discordant results between TE-based and clinical-based criteria, patients with cirrhosis based on TE were at a higher risk of HCC development than those with cirrhosis based on clinical criteria, indicating the superiority of TE for diagnosis of compensated liver cirrhosis. Second, patients with TE values below the cutoff level for cirrhosis, 8-13 kPa, had a higher relative risk of HCC development than those with LSM values < 8 kPa. Although this finding should be validated in large prospective studies, the issue of expansion of the high-risk group for HCC surveillance to include those with significant fibrosis was raised by this study. Furthermore, when patients with available follow-up TE values were analyzed, the risk of HCC development changed according to the pattern of the changes in TE values, suggesting a potential role for serial measurements of TE as a dynamic monitoring tool for risk estimation of HCC development (Figure 2C). However, other confounding factors including lack of histological information, insensitive HBV DNA tests, and heterogeneity in antiviral treatment should be noted when interpreting these results. Recently, Chon et al[56] compared the performance of various noninvasive fibrosis prediction methods [aspartate aminotransferase-to-platelet ratio index (ARRI), age-spleen-to-platelet ratio index (ASPRI), TE, LSPS, P2/MS and FIB-4] for prediction of HCC development in patients with CHB and concluded that TE and LSPS showed the best performance (AUROC = 0.789 and 0.788, respectively). Using multivariate analyses, TE and LSPS were identified as independent predictors of HCC development.
In another study[54] from Hong Kong, which followed up 528 patients with HBeAg negative CHB for a median length of 35 mo and identified seven patients with HCC development, the cumulative incidence of HCC was higher in patients with TE values ≥ 10 kPa than those with TE values < 10 kPa (9% vs 0%, respectively; P < 0.001), and the cumulative liver-related mortality was also higher in patients with TE values < 10 kPa compared with those with TE values ≥ 10 kPa (4% vs 0%, respectively; P < 0.001). By multivariate analysis, only TE value was significantly associated with HCC development and liver-related mortality.
Similarly, Kim et al[55] investigated the prognostic role of TE in predicting the development of overall liver-related events (LREs), defined as development of HCC, hepatic decompensation, or liver-related mortality, among 128 patients with CHB showing histologically advanced liver fibrosis (≥ F3) and high viral loads (HBV DNA ≥ 2000 IU/mL) before starting nucleos(t)ide analogs. When the study population was stratified into two groups using the optimal cutoff value (19 kPa), patients with TE values > 19 kPa were at significantly greater risk for LRE development than those with TE values ≤ 19 kPa (HR, 7.176; P = 0.001). Moreover, the incidence of LREs was similar in patients with F3 and F4 (22.2% vs 13.6%; P = 0.472); however, it differed significantly between patients with TE values ≤ 19 kPa and those with TE values > 19 kPa (6.9% vs 44.4%; P < 0.001), indicating the superior performance of TE to that of histology in prediction of LRE development.
Apart from predicting HCC development, the application of TE was validated in a study by Vergniol et al[57], in which 1457 patients with CHC were followed up; 5-year survival outcomes worsened as TE values increased. The prognostic values of TE were demonstrated to be statistically significant (P < 0.0001) after adjustment for other important factors, including treatment response, patient age, and estimates of necroinflammatory grade. For example, the 5-year overall survival was 96% in patients with TE value < 9.5 kPa, and 47% in patients with TE value > 40 kPa.
Overall, TE has shown the potential for a clinical role in predicting the development of portal hypertension-related hepatic decompensation and/or HCC and, in part, demonstrated superior performance to histology and other noninvasive tools[41,58-63]. This is most likely due to the wider dynamic range of TE values in the evaluation of liver cirrhosis. In fact, as the stage of ‘‘cirrhosis’’ has to date been defined by histopathological evidence of one or two qualitative categories (METAVIR stage F4 or ISHAK S5-S6), or more generally by the presence of so-called ‘‘regenerative’’ or ‘‘cirrhotic nodules’’, an interval scale cannot be used in this setting[64-66]. However, the degree of liver fibrosis may vary widely among patients in this category, and the risk of hepatic decompensation and HCC may not be uniform. Thus, in this regard, because TE value, expressed in kPa as a continuous variable, has a wide dynamic range within the cirrhotic stage from the cutoff level from non-cirrhosis (15-17 kPa) to the upper measurement limit of present devices (75 kPa), it would seem to be a more reasonable tool for detailed prognostication.
Because TE values show significant correlations with portal hypertension and HCC development, prediction of postoperative short-term outcomes, such as hepatic insufficiency, and long-term outcomes, such as recurrence or liver-related death using TE has been tested in several pilot studies[67-69]. Although further studies are required to validate these results, TE may facilitate stratification of patients undergoing curative resection according to different prognoses.
In the first place, Kim et al[67] investigated whether preoperative TE values could predict the development of postoperative hepatic insufficiency after curative resection of HCC. In this study, multivariate analyses revealed that a TE value > 25.6 kPa was the only predictor of postoperative insufficiency. The AUROC of 25.6 kPa was higher than that of indocyanine green R15, which is a popular method for assessment of preoperative functional reserve liver function (0.824 vs 0.620, respectively). Similar results were obtained in a subsequent investigation by the same group[68]. In this study, the performance of TE was superior to that of diffusion-weighted magnetic resonance imaging, which has also been shown to be a noninvasive fibrosis prediction tool for the assessment of liver fibrosis and the prediction of postoperative hepatic insufficiency.
Another issue is prediction of HCC recurrence after curative resection, that is, de novo recurrence in the background liver with fibrotic burden, using preoperative TE. In an analysis of 133 patients who underwent preoperative TE and curative resection (HCC recurred in 62 patients), TE was selected as an independent predictor of recurrence, whereas histological fibrosis status was not[69]. In the study, patients with preoperative TE values > 13.4 kPa were at a greater risk of recurrence, with an HR of 1.925 (P = 0.010). More specifically, when recurrence was stratified into early (< 2 years) and late (≥ 2 years), TE values were significantly related to late recurrence, thus supporting the hypothesis. These results suggest that preoperative TE could reveal the potential influence of liver fibrosis on recurrence and explain multicentric carcinogenesis in a fibrotic liver. However, more data are needed to clarify this issue.
Recently, the concept of “cirrhosis” has changed from static and uncompromisingly progressive to rather dynamic and bidirectional, especially when treatment against the causative agent of tissue damage (i.e., antiviral agents against CHB or CHC and antifibrotic agents) can be introduced successfully at this stage of the disease. The ideal approach to evaluate histological outcomes during antiviral therapy, such as fibrosis regression and necroinflammation stabilization, is serial LB examinations. However, this is impractical, primarily due to the inherent invasiveness of LB. Instead, because of the ease, safety, and rapidity of TE, it may be useful for monitoring the dynamic changes in liver fibrosis during antiviral or antifibrotic treatment. Indeed, several studies have reported the clinical usefulness of TE for monitoring potential fibrosis regression during antiviral treatment in patients with CHC and CHB[57,70-77].
Kim et al[71] analyzed 41 patients with CHB who received antiviral treatment using nucleos(t)ide analogs. To prevent the confounding effect of high ALT, patients with high ALT levels more than 2× ULN, were excluded. Although ALT levels did not show a statistically significant change during the first 12 or 24 mo of antiviral treatment, TE values decreased significantly, indicating potential fibrosis regression due to prolonged antiviral treatment. Indeed, fibrosis regression and stabilization of necroinflammation was noted in two patients with available paired LBs. Enomoto et al[70] reported the changes in LSM values during the first 12 mo of entecavir treatment in 20 patients. Median TE values decreased significantly from 11.2 to 7.8 kPa after 12 mo of treatment, and serum fibrosis markers, such as PIIINP and type IV collagen 7S domain, also decreased significantly. In one patient with available paired LBs, histological fibrosis regression and stabilization of necroinflammation were noted. Although these studies suggest a role for TE for monitoring fibrosis regression due to prolonged antiviral treatment, the short duration of observation and small sample sizes with paired biopsies are major limitations of these studies.
Recently, data regarding a longer antiviral treatment duration (more than 3 years) have become available[72-74,78]. Fung et al[72] reported a significant decline in TE values from baseline after subsequent ALT normalization with 3-year treatment (n = 110, 7.8 to 6.1 kPa; P = 0.002). In this study, independent factors associated with a significant decline in TE value of ≥ 1 kPa included antiviral therapy and ALT levels at the follow-up time point. Another study by Andersen et al[78] also noted significant declines in TE values after a median antiviral treatment duration of 50.5 mo (n = 66), and concluded that prolonged antiviral treatment in patients with CHB resulted in significant declines in TE values, suggesting regression of fibrosis in a majority of patients with advanced fibrosis or cirrhosis.
Likewise, for patients with CHC, changes in TE values during antiviral treatment have been investigated in several studies. Two prospective studies by Vergniol et al[57] and Ogawa et al[75] demonstrated that patients with CHC showing sustained virological responses to pegylated interferon-ribavirin combination therapy had significantly reduced TE values at the end of follow-up. Moreover, Ogawa et al[75] reported that patients with non-sustained virological responses, but with a biochemical response, showed a greater reduction in TE values than did those with a non-biochemical response. Subsequent studies reported similar results, suggesting that changes in TE values during antiviral treatment in patients with CHC may represent alterations in the severity of liver fibrosis[76,77]. However, it should be further confirmed whether the favorable changes in LSM values during or after antiviral treatment does have a significant influecne on the long-term prognosis such as disease-specific survival in patients with CHC.
Taken together, TE value seems to decrease during and after antiviral therapy. However, without paired histological results through repeated LB, whether the reduction in TE values is closely correlated with regression of liver fibrosis or improvement in necroinflammatory scores remains unclear. To clarify this, Lim et al[73] investigated patterns of TE values among patients who were treated with entecavir. In all subjects, the median TE value at baseline was 15.1 (range, 5.6-75.0) kPa and decreased significantly, to 8.8 (range, 3.0-33.8) kPa after 12 mo of therapy, and a decrease in TE values correlated significantly with increase in albumin, decrease in bilirubin, decrease in ALT level, and decrease in aspartate aminotransferase levels (all P < 0.05). However, among 15 patients with available paired LBs, decreases in TE values were correlated significantly with improved necroinflammatory scores, but not with fibrosis regression. Similarly, Wong et al[74] insisted that the decline in absolute TE values during antiviral treatment did not reflect the change in histologically assessed liver fibrosis, probably due to the confounding influence of ALT reduction caused by antiviral treatment.
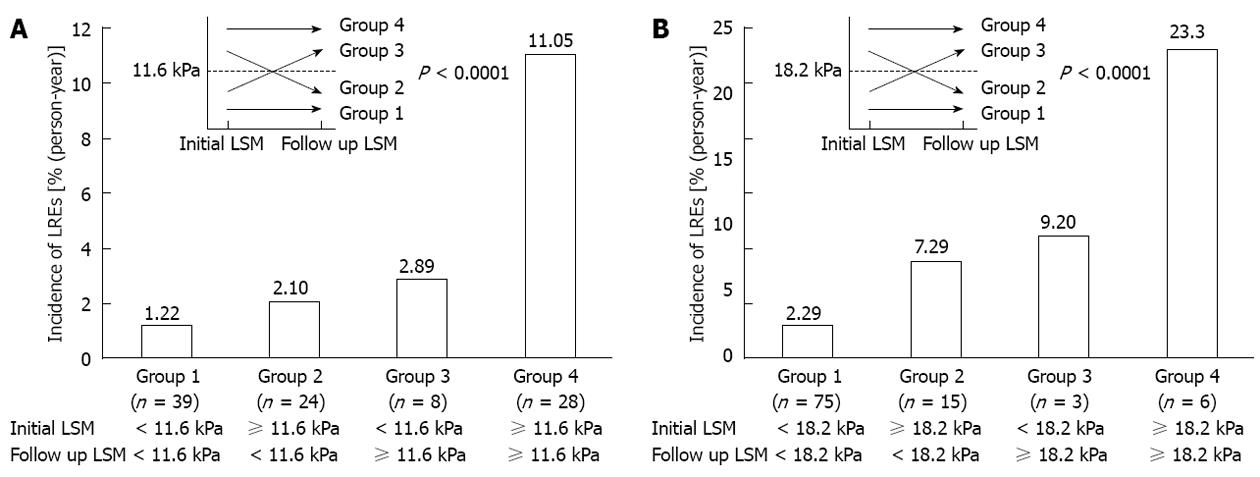
However, regardless of whether TE values during antiviral treatment are due to fibrosis regression, activity stabilization, or both, changes in TE value during antiviral treatment can be translated into the overall response of chronically diseased liver to antiviral treatment from the viewpoint of its long-term clinical implications. Thus, it is more logical to investigate whether the decline in TE value can be used as a favorable predictor of long-term prognosis. Encouraging results were recently published by Jung et al[53] suggesting that the change in TE values in patients with CHB showed a significant correlation with differential future risk of HCC development. Additionally, Kim et al[79] insisted that changes in TE values were significantly associated with the difference risk of liver-related event occurrence, such as hepatic decompensation, HCC development, and LREs (Figure 3). This would suggest that the assessment of overall background liver status using TE may be an important end-point in the management of CHB and prediction of long-term outcomes. Further research is needed to evaluate the reproducibility of such findings in independent populations.
Although TE has demonstrated reliable diagnostic accuracy with excellent inter-observer and intra-observer agreement, additional space-occupying tissue abnormalities, such as edema and inflammation, cholestasis, and congestion may interfere with TE, regardless of the degree of liver fibrosis, because the liver is wrapped in a distensible, but non-elastic, envelope (Glisson’s capsule)[80].
First, the extent of histological necroinflammatory activity has been shown to influence TE results in patients with viral hepatitis, resulting in an overestimation of TE values that increases in parallel with the degree of necroinflammatory score[81-85]. Consistent with these results, a risk of overestimation of TE values has been reported in cases of ALT flares in patients with acute viral hepatitis or CHB[86-92]. Thus, in such subjects, TE examinations should be delayed until ALT levels have stabilized. In this regard, several studies have investigated the optimal period (3 to 6 mo) for restoration of the reliability of TE values in patients with acute flares[88,91,93,94]. Furthermore, even mild to moderate elevalation in ALT can be associated with higher liver stiffness values, and may cause discrepancies between TE results and the actual underlying fibrosis. Apart from necroinflammation, extrahepatic cholestasis[95] and congestive heart failure[96-98] may also contribute to the overestimation of TE.
Additionally, the performance of TE may be limited in patients with a high body mass index (BMI), narrow intercostal space, or ascites[9]. Although TE reproducibility has been shown to be excellent in terms of inter-observer and intra-observer agreement, a high BMI (> 28 kg/m2) and waist circumference were significantly associated with TE failure[99]. These results emphasize the need for adequate operator training and for technological improvements in specific patient populations, such as those with non-alcoholic fatty liver disease. For this, a new TE probe (the XL probe) was recently introduced to lessen the TE failure rate in obese patients; however, its efficacy should be further validated[100].
Over the past decade, significant progress has been made in the non-invasive assessment of liver fibrosis in patients with CLD. Of the methods now available, TE appears to be an excellent tool for assessing liver fibrosis, particularly for diagnosis of cirrhosis, and also has prognostic value from a longitudinal perspective. Although TE cannot completely obviate the need for invasive tests, such as LB, endoscopic examination for identification of varices, or HVPG, it represents an important non-invasive tool, enabling more efficient and tailored management strategies for patients with CLD (Table 2). We hope that other researchers will evaluate the usefullness of other similar techniques such as the measurement of spleen stiffness in comparison or in combination with TE in the future.
| Clinical setting | Role of TE |
| Prediction of portal hypertension | TE with platelet counts and spleen size complementary to HVPG |
| Prediction of esophageal varices | TE with platelet counts and spleen size complementary to endoscopy |
| Prediction of developing esophageal variceal bleeding | TE with platelet counts and spleen size |
| Prediction of developing portal hypertension-related complications | TE with platelet counts and spleen size |
| Prediction of developing hepatocellular carcinoma | TE |
| Prediction of developing postoperative hepatic insufficiency after surgical resection | TE |
| Prediction of developing recurrence of hepatocellular carcinoma after curative resection | TE |
| Monitoring of fibrotic burden during antiviral treatment | TE |
The authors thank Mr. Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, South Korea, for his help with the figures.
P- Reviewer Atta HM S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Jang JW. Current status of liver diseases in Korea: liver cirrhosis. Korean J Hepatol. 2009;15 Suppl 6:S40-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc. 2007;82:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 4. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [PubMed] |
| 5. | Jin SY. Role of liver biopsy in the assessment of hepatic fibrosis--its utility and limitations. Korean J Hepatol. 2007;13:138-145. [PubMed] |
| 6. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 7. | Abraldes JG, Araujo IK, Turón F, Berzigotti A. Diagnosing and monitoring cirrhosis: Liver biopsy, hepatic venous pressure gradient and elastography. Gastroenterol Hepatol. 2012;35:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1071] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 9. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1936] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 10. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 11. | Stauber RE, Lackner C. Noninvasive diagnosis of hepatic fibrosis in chronic hepatitis C. World J Gastroenterol. 2007;13:4287-4294. [PubMed] |
| 12. | Kang JK, Cheong JY, Cho SW, Cho JH, Park JS, Kim YB, Kim DJ, Hwang SG, Yang JM, Park YN. Liver stiffness measurement for the diagnosis of hepatic fibrosis in patients with chronic viral hepatitis. Korean J Hepatol. 2007;13:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Jung HS, Kim YS, Kwon OS, Ku YS, Kim YK, Choi DJ, Kim JH. Usefulness of liver stiffness measurement for predicting the presence of esophageal varices in patients with liver cirrhosis. Korean J Hepatol. 2008;14:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kim SG, Kim YS, Jung SW, Kim HK, Jang JY, Moon JH, Kim HS, Lee JS, Lee MS, Shim CS. The usefulness of transient elastography to diagnose cirrhosis in patients with alcoholic liver disease. Korean J Hepatol. 2009;15:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kim SM, Sohn JH, Kim TY, Roh YW, Eun CS, Jeon YC, Han DS, Oh YH. Comparison of various noninvasive serum markers of liver fibrosis in chronic viral liver disease. Korean J Hepatol. 2009;15:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Scott DR, Levy MT. Liver transient elastography (Fibroscan): a place in the management algorithms of chronic viral hepatitis. Antivir Ther. 2010;15:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, Gazzard B, Nelson M. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Han KH, Yoon KT. New diagnostic method for liver fibrosis and cirrhosis. Intervirology. 2008;51 Suppl 1:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther. 2009;30:557-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Kim BK, Kim do Y, Han KH, Park JY, Kim JK, Paik YH, Lee KS, Chon CY, Ahn SH. Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol. 2011;106:1654-1662, 1730. [PubMed] |
| 21. | Kim SU, Han KH, Ahn SH. Non-invasive assessment of liver fibrosis: time to move from cross-sectional studies to longitudinal ones. J Gastroenterol Hepatol. 2010;25:1472-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Addley J, Tham TC, Cash WJ. Use of portal pressure studies in the management of variceal haemorrhage. World J Gastrointest Endosc. 2012;4:281-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. [PubMed] |
| 24. | Suk KT, Kim CH, Park SH, Sung HT, Choi JY, Han KH, Hong SH, Kim DY, Yoon JH, Kim YS. Comparison of hepatic venous pressure gradient and two models of end-stage liver disease for predicting the survival in patients with decompensated liver cirrhosis. J Clin Gastroenterol. 2012;46:880-886. [PubMed] |
| 25. | Keiding S, Vilstrup H. Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis. Scand J Gastroenterol. 2002;37:960-964. [PubMed] |
| 26. | Rincón D, Lo Iacono O, Tejedor M, Hernando A, Ripoll C, Catalina MV, Salcedo M, Matilla A, Senosiain M, Clemente G. Prognostic value of hepatic venous pressure gradient in patients with compensated chronic hepatitis C-related cirrhosis. Scand J Gastroenterol. 2012;Aug 8; Epub ahead of print. [PubMed] |
| 27. | Pineda JA, Recio E, Camacho A, Macías J, Almodóvar C, González-Serrano M, Merino D, Tellez F, Ríos MJ, Rivero A. Liver stiffness as a predictor of esophageal varices requiring therapy in HIV/hepatitis C virus-coinfected patients with cirrhosis. J Acquir Immune Defic Syndr. 2009;51:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Wadhawan M, Dubey S, Sharma BC, Sarin SK, Sarin SK. Hepatic venous pressure gradient in cirrhosis: correlation with the size of varices, bleeding, ascites, and child’s status. Dig Dis Sci. 2006;51:2264-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Kim MY, Baik SK, Suk KT, Yea CJ, Lee IY, Kim JW, Cha SH, Kim YJ, Um SH, Han KH. Measurement of hepatic venous pressure gradient in liver cirrhosis: relationship with the status of cirrhosis, varices, and ascites in Korea. Korean J Hepatol. 2008;14:150-158. [PubMed] |
| 30. | Hobolth L, Bendtsen F, Møller S. Indications for portal pressure measurement in chronic liver disease. Scand J Gastroenterol. 2012;8-9:887-892. [PubMed] |
| 31. | Bañares R, Catalina MV, Ripoll C, Rincón D. Prognostic markers in patients who have recovered from an acute variceal bleeding: role of HVPG measurement. Dis Markers. 2011;31:165-169. [PubMed] |
| 32. | Seijo S, Reverter E, Miquel R, Berzigotti A, Abraldes JG, Bosch J, García-Pagán JC. Role of hepatic vein catheterisation and transient elastography in the diagnosis of idiopathic portal hypertension. Dig Liver Dis. 2012;44:855-860. [PubMed] |
| 33. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 527] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 34. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 955] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 35. | Cho SB, Park KJ, Lee JS, Lee WS, Park CH, Joo YE, Kim HS, Choi SK, Rew JS, Kim SJ. Comparison of terlipressin and octreotide with variceal ligation for controlling acute esophageal variceal bleeding--a randomized prospective study. Korean J Hepatol. 2006;12:385-393. [PubMed] |
| 36. | Youn KH, Kim DJ. Prevention of variceal bleeding and measurement of hepatic vein pressure gradient. Korean J Hepatol. 2006;12:464-468. [PubMed] |
| 37. | Seo YS, Um SH, Hyun JJ, Kim YH, Park S, Keum BR, Kim YS, Jeen YT, Lee HS, Chun HJ. A prospective study comparing the efficacy of early administration of terlipressin and somatostatin for the control of acute variceal bleeding in patients with cirrhosis. Korean J Hepatol. 2006;12:373-384. [PubMed] |
| 38. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 39. | Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, Grando-Lemaire V, Trinchet JC, Beaugrand M. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther. 2008;28:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 269] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 41. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 42. | Pritchett S, Cardenas A, Manning D, Curry M, Afdhal NH. The optimal cut-off for predicting large oesophageal varices using transient elastography is disease specific. J Viral Hepat. 2011;18:e75-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL. Noninvasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res. 2010;34:1146-1153. [PubMed] |
| 44. | Malik R, Lai M, Sadiq A, Farnan R, Mehta S, Nasser I, Challies T, Schuppan D, Afdhal N. Comparison of transient elastography, serum markers and clinical signs for the diagnosis of compensated cirrhosis. J Gastroenterol Hepatol. 2010;25:1562-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim do Y. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 46. | Kim BK, Park YN, Kim do Y, Park JY, Chon CY, Han KH, Ahn SH. Risk assessment of development of hepatic decompensation in histologically proven hepatitis B viral cirrhosis using liver stiffness measurement. Digestion. 2012;85:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Kudo M. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75 Suppl 1:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [PubMed] |
| 49. | Frenette C, Gish R. Targeted systemic therapies for hepatocellular carcinoma: clinical perspectives, challenges and implications. World J Gastroenterol. 2012;18:498-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 51. | Maggs JR, Suddle AR, Aluvihare V, Heneghan MA. Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1113-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 53. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 54. | Fung J, Lai CL, Seto WK, Wong DK, Yuen MF. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat. 2011;18:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Kim SU, Lee JH, Kim do Y, Ahn SH, Jung KS, Choi EH, Park YN, Han KH, Chon CY, Park JY. Prediction of liver-related events using fibroscan in chronic hepatitis B patients showing advanced liver fibrosis. PLoS One. 2012;7:e36676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Chon YE, Jung ES, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Jung KS, Kim SU. The accuracy of noninvasive methods in predicting the development of hepatocellular carcinoma and hepatic decompensation in patients with chronic hepatitis B. J Clin Gastroenterol. 2012;46:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Vergniol J, Foucher J, Castéra L, Bernard PH, Tournan R, Terrebonne E, Chanteloup E, Merrouche W, Couzigou P, de Lédinghen V. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol. 2012;18:163-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Poca M, Puente A, Graupera I, Villanueva C. Prognostic markers in patients with cirrhosis and portal hypertension who have not bled. Dis Markers. 2011;31:147-154. [PubMed] |
| 60. | Robic MA, Procopet B, Métivier S, Péron JM, Selves J, Vinel JP, Bureau C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 61. | Pesce A, Scilletta R, Branca A, Nigro L, Montineri A, Larocca L, Fatuzzo F, Castaing M, Puleo S. Does transient elastography (FibroScan®) have a role in decision making in hepatocellular carcinoma? HPB (Oxford). 2012;14:403-408. [PubMed] |
| 62. | Motosugi U, Ichikawa T, Koshiishi T, Sano K, Morisaka H, Ichikawa S, Enomoto N, Matsuda M, Fujii H, Araki T. Liver stiffness measured by magnetic resonance elastography as a risk factor for hepatocellular carcinoma: a preliminary case-control study. Eur Radiol. 2013;23:156-162. [PubMed] |
| 63. | Masuzaki R, Tateishi R, Yoshida H, Arano T, Uchino K, Enooku K, Goto E, Nakagawa H, Asaoka Y, Kondo Y. Assessment of disease progression in patients with transfusion-associated chronic hepatitis C using transient elastography. World J Gastroenterol. 2012;18:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 64. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 65. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 848] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 66. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3784] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 67. | Kim SU, Ahn SH, Park JY, Kim do Y, Chon CY, Choi JS, Kim KS, Han KH. Prediction of postoperative hepatic insufficiency by liver stiffness measurement (FibroScan((R))) before curative resection of hepatocellular carcinoma: a pilot study. Hepatol Int. 2008;2:471-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Kim SU, Kim YC, Choi JS, Kim KS, Choi GH, Choi JS, Park JY, Kim do Y, Ahn SH, Choi EH. Can preoperative diffusion-weighted MRI predict postoperative hepatic insufficiency after curative resection of HBV-related hepatocellular carcinoma? A pilot study. Magn Reson Imaging. 2010;28:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Jung KS, Kim SU, Choi GH, Park JY, Park YN, Kim do Y, Ahn SH, Chon CY, Kim KS, Choi EH. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan®). Ann Surg Oncol. 2012;19:4278-4286. [PubMed] |
| 70. | Enomoto M, Mori M, Ogawa T, Fujii H, Kobayashi S, Iwai S, Morikawa H, Tamori A, Sakaguchi H, Sawada A. Usefulness of transient elastography for assessment of liver fibrosis in chronic hepatitis B: Regression of liver stiffness during entecavir therapy. Hepatol Res. 2010;40:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Kim SU, Park JY, Kim do Y, Ahn SH, Choi EH, Seok JY, Lee JM, Park YN, Chon CY, Han KH. Non-invasive assessment of changes in liver fibrosis via liver stiffness measurement in patients with chronic hepatitis B: impact of antiviral treatment on fibrosis regression. Hepatol Int. 2010;4:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Fung J, Lai CL, Wong DK, Seto WK, Hung I, Yuen MF. Significant changes in liver stiffness measurements in patients with chronic hepatitis B: 3-year follow-up study. J Viral Hepat. 2011;18:e200-e205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Lim SG, Cho SW, Lee YC, Jeon SJ, Lee MH, Cho YJ, Kim SS, Kim YB, Seok JY, Cheong JY. Changes in liver stiffness measurement during antiviral therapy in patients with chronic hepatitis B. Hepatogastroenterology. 2011;58:539-545. [PubMed] |
| 74. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chu SH, Chan FK, Sung JJ, Chan HL. On-treatment monitoring of liver fibrosis with transient elastography in chronic hepatitis B patients. Antivir Ther. 2011;16:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Ogawa E, Furusyo N, Toyoda K, Takeoka H, Maeda S, Hayashi J. The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha-2b and ribavirin. Antiviral Res. 2009;83:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Wang JH, Changchien CS, Hung CH, Tung WC, Kee KM, Chen CH, Hu TH, Lee CM, Lu SN. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan. J Gastroenterol Hepatol. 2010;25:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Arima Y, Kawabe N, Hashimoto S, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Kobayashi K, Ichino N. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol Res. 2010;40:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Andersen ES, Weiland O, Leutscher P, Krarup H, Westin J, Moessner B, Konopski Z, Frigstad SO, Kjær M, Christensen PB. Low liver stiffness among cirrhotic patients with hepatitis B after prolonged treatment with nucleoside analogs. Scand J Gastroenterol. 2011;46:760-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Kim SU, Oh HJ, Park YN, Park JY, Kim do Y, Ahn SH, Chon CY, Han KH. Sub-classification of cirrhosis and prediction of liver-related events using fibroscan in patients with HBV-related cirrhosis. J Hepatol. 2012;56:s34. [DOI] [Full Text] |
| 80. | Bae RC, Cho HJ, Oh JT, Lee EK, Heo J, Shin KY, Park SY, Jeong MK, Jeon SW, Cho CM. Clinical factors influencing liver stiffness as measured by transient elastography (Fibroscan) in patients with chronic liver disease. Korean J Hepatol. 2010;16:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 82. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 83. | Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 84. | Taylor-Robinson SD, Cobbold JF, Thomas HC. Liver stiffness measurements in acute hepatitis B: implications for clinical practice. Eur J Gastroenterol Hepatol. 2010;22:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Lee da M, Moon EJ, Hwang JA, Lee MS, Cheong JY, Cho SW, Kim YB, Kim DJ, Hwang SG, Yang JM. Factors associated with liver stiffness in chronic liver disease. Korean J Hepatol. 2009;15:464-473. [PubMed] |
| 86. | Viganò M, Massironi S, Lampertico P, Iavarone M, Paggi S, Pozzi R, Conte D, Colombo M. Transient elastography assessment of the liver stiffness dynamics during acute hepatitis B. Eur J Gastroenterol Hepatol. 2010;22:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 88. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Chan HL. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2009;24:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 90. | Kim SU, Kim JK, Park YN, Han KH. Discordance between liver biopsy and Fibroscan® in assessing liver fibrosis in chronic hepatitis b: risk factors and influence of necroinflammation. PLoS One. 2012;7:e32233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Fung J, Lai CL, But D, Hsu A, Seto WK, Cheng C, Wong DK, Yuen MF. Reduction of liver stiffness following resolution of acute flares of chronic hepatitis B. Hepatol Int. 2010;4:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 93. | Oliveri F, Coco B, Ciccorossi P, Colombatto P, Romagnoli V, Cherubini B, Bonino F, Brunetto MR. Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol. 2008;14:6154-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Park H, Kim SU, Kim D, Kim do Y, Ahn SH, Han KH, Chon CY, Park JY. Optimal time for restoring the reliability of liver stiffness measurement in patients with chronic hepatitis B experiencing acute exacerbation. J Clin Gastroenterol. 2012;46:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 463] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 96. | Colli A, Pozzoni P, Berzuini A, Gerosa A, Canovi C, Molteni EE, Barbarini M, Bonino F, Prati D. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology. 2010;257:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 97. | Hopper I, Kemp W, Porapakkham P, Sata Y, Condon E, Skiba M, Farber L, Porapakkham P, Williams TJ, Menahem S. Impact of heart failure and changes to volume status on liver stiffness: non-invasive assessment using transient elastography. Eur J Heart Fail. 2012;14:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 98. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 99. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 100. | de Lédinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, Le Bail B, Choi PC, Chermak F, Yiu KK. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |